Plant secondary metabolites were reported to inhibit carbohydrate metabolizing enzymes, possess kinase activating capacity, thereby affecting all the metabolic pathways of carbohydrate, lipid and protein, and can intervene in the insulin-signaling pathway, inflammatory response, and oxidative stress and restore molecular aberrations leading to insulin resistance and glucose intolerance.
- diabetes
- secondary metabolites
- alkaloids
- flavonoids
- coumarins
- insulin signal
1. Introduction
Phytomedicine is a plant-based medicine, and it is one of the subsets of complementary and alternative medicinal (CAM) therapies [6][1].The World Health Organization (WHO) defined herbal medicine as the knowledge, skills and practices based on the views and experiences related to different cultures used in the preservation of health and the prevention, diagnosis, improvement, or treatment of illness [2,7][2][3].Traditional herbal medicines are plant-derived substances with minimal or no industrial processing that have been used to treat illness within local or regional healing conventionalisms [2].
Published research has proven that several secondary metabolites demonstrate hypoglycemic activity in vivo and in vitro, and usually, they affect multiple targets, proteins and enzymes.Alkaloids, phenols, anthocyanin, flavonoids, saponins, tannins, terpenes and coumarins were found to elicit a significant influence on diabetes [9,10][4][5].
Phlorizin is a natural dihydrochalcone found in a number of fruitful trees, mainly the Malus genus.It produces renal glycosuria and blocks intestinal and renal glucose reabsorption by inhibiting the sodium-glucose symporters located there [11,12][6][7].Recently, ertugliflozin arrived as the newest synthetic GLT-2 (glucose transporter-2) inhibitor to receive FDA approval for the treatment of diabetes [13,14][8][9].
This review highlights the proposed secondary metabolites mechanism-based action targeting various metabolic pathways involved in glucose metabolism in humans.The summarized data from in vivo andin vitrobioassays of phytochemicals will lead future research towards developing effective antidiabetics with low toxicity.
2. Mechanisms Involved in Glucose Metabolism and Homeostasis
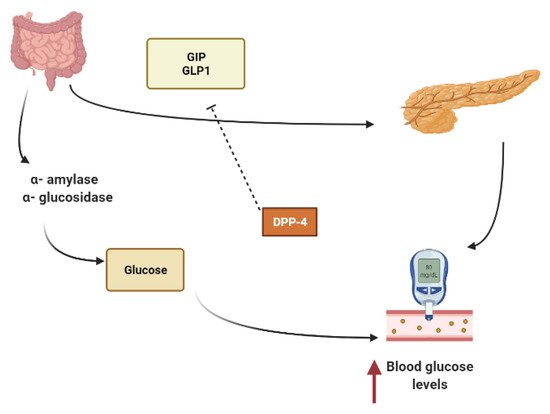
Starch is mainly converted to glucose by the action of α-amylase that hydrolyzes macromolecules into oligoglucans, and α-glucosidase further degrades oligoglucans into absorbable glucose molecules at the brush border of the small intestine and is then absorbed through the glucose transporters sodium-dependent glucose cotransporter 1 (SGLT1) and glucose transporter 2 (GLUT2)[15,16][10][11].Several phytoconstituents are known to suppress the activity of α-amylase, α-glucosidase and to inhibit intestinal absorption of glucose, inhibiting postprandial hyperglycemia and keeping the concentration of glucose in the blood constant after a meal [15,17][10][12].
Other secondary metabolites can modulate the secretion of glucagon-like peptide-1 (GLP1), inhibiting dipeptidyl peptidase-4 (DPP4) from extending the effect of GLP1.In L-cells of the small intestine, basal serum GLP1 is released in response to nutrients loads (Figure 1).It promotes insulin secretion and stimulates the hypothalamus gland to induce postprandial satiety and to inhibit glucagon secretion.GLP1 has a very short half-life because it is hydrolyzed by dipeptidyl peptidase-4 (DPP4)[18,19,20][13][14][15].
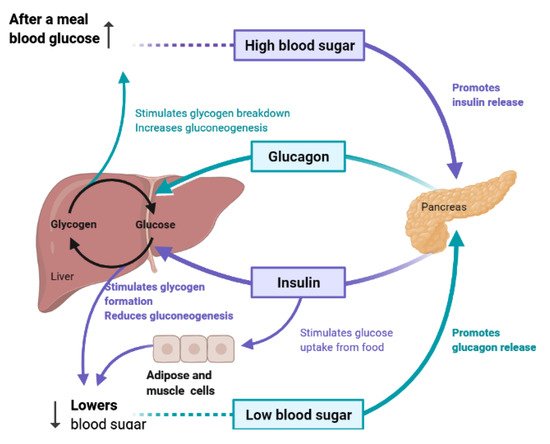
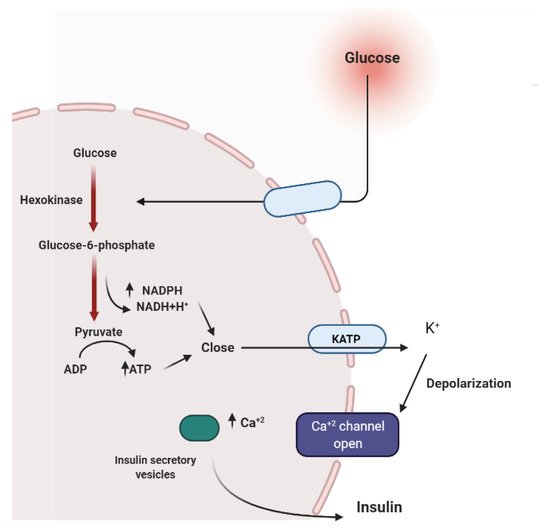
To maintain constant blood glucose levels, various body organs, including the pancreas, liver, intestine, adipose and muscle tissues with various hormones and neuropeptides, work together.The pancreas plays a crucial role in glucose homeostasis by secreting insulin and its opponent glucagon [21][16].The increased circulating glucose is sensed by pancreatic β-cells and subsequently glucose influx into the cells via GLUT2, an insulin-independent transporter (Figure 2).Glucose induces insulin secretion from β-cells via the closure of ATP-gated potassium channels and activation of voltage-gated calcium channels [16,21,22,23][11][16][17][18].
Several medicinal plants constituents can affect insulin secretion via closing the ATP-sensitive potassium channel (KATP), acting on the Ca2+channels.Others can decrease insulin degradation by inhibiting insulinase or by possessing cAMP phosphodiesterase inhibitory activity (Figure 3) [22][17].
3. Liver and Glucose Homeostasis
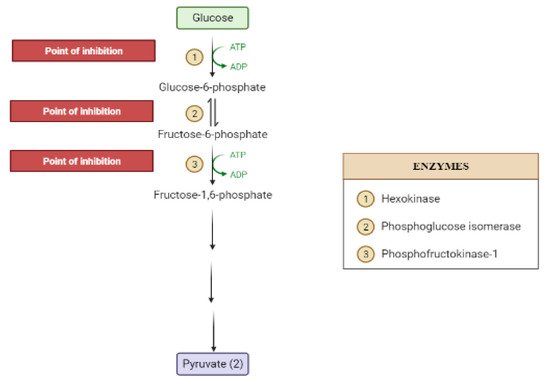
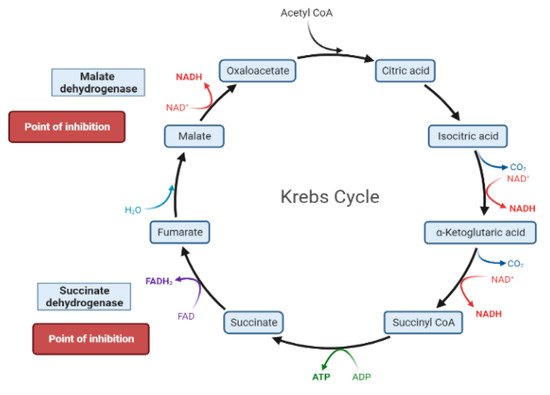
The liver is the chief organ in maintaining glucose homeostasis through controlling various pathways, including glycogenesis, glycogenolysis, glycolysis and gluconeogenesis [29][19].During feeding conditions, glucose is a primary fuel source across multiple fuel sources across numerous body tissues by eliciting ATP molecules during hydrolysis and the Krebs cycle.The increase in glucose uptake in hepatocytes promotes glycolysis and lipogenesis to generate triglycerides as storage forms of fuel [22,29][17][19].Glycolysis can be regulated through the reactions catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase (Figure 5andFigure 6).Moreover, there are seven enzymes involved in this Krebs cycle, of which only two enzymes, succinate dehydrogenase and malate synthase, can be regulated.Several plant secondary metabolites (discussed below) can regulate and affect these enzymes except for the pyruvate kinase enzyme [22,30,31][17][20][21].
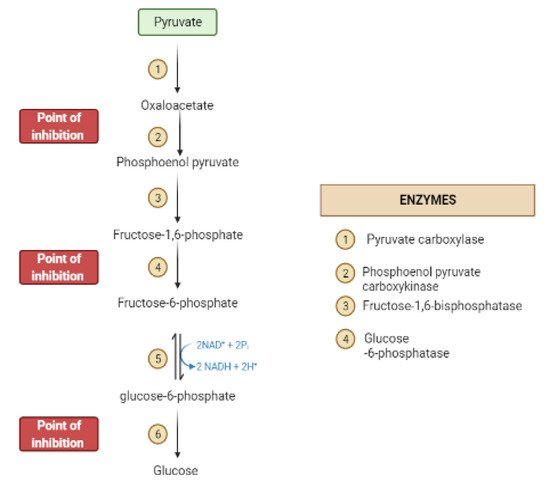
During the fasting state, the liver is the leading factory for glucose production through gluconeogenesis and glycogenolysis.The main regulatory enzymes in the gluconeogenesis pathway include glucose 6-phosphatase (G6Pase), fructose 1, 6-bisphosphatase (Fbpase1), PC (pyruvate carboxylase), and phosphoenolpyruvate carboxykinase (PEPCK), all can be inhibited by plant secondary metabolites (Figure 7)[29,30][19][20].
Glycogen synthesis from unused glucose is a multistep process carried out by the enzyme glycogen synthase in the liver.Secondary metabolites can affect the process through glycogen synthase.In addition, glycogenolysis is inhibited through glycogen phosphorylase (Figure 8) [22][17].
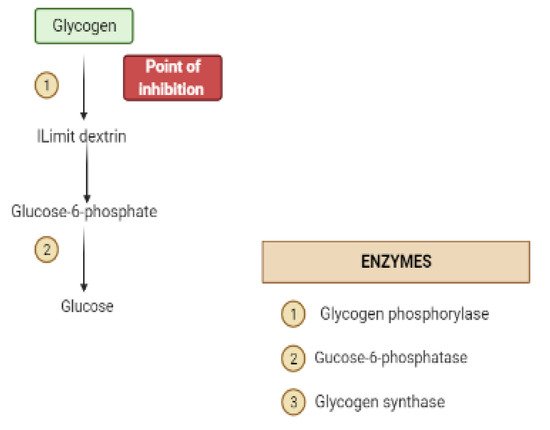
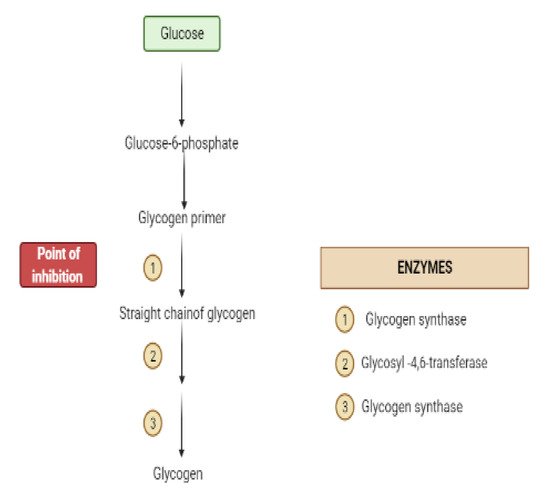
4. Obstacles for Insulin Signal Transduction and Insulin Effects
The increase in the free fatty acids is connected to alteration in the diacylglycerol (DAG)/protein kinase C (PKC) pathway.The rise in DAG activates PKC-θ, -β2 and –δ that phosphorylates IRS1, which interferes with insulin-stimulated phosphorylation of IRS1, thus inhibiting insulin signaling.It is believed that targeting PKC is beneficial in treating type 2 diabetes as it will address the secretory defect promoting insulin secretion [33,34][22][23].
Inflammatory mediators (such as TNF-α, Il-6) can impair insulin and promote serine phosphorylation of IRS-1 impairing insulin signaling or can cause degradation of IRS.It can reduce GLUT-4 expression, decreasing glucose entry to cells and cause inflammation-induced nitric oxide release that suppresses PI3K–Akt pathway [23][18].It is well-known that inflammation is a crucial cause for diabetes type-2.Relieving inflammation by secondary metabolites can improve diabetes [35][24].
Oxidative stress has been linked to diabetes.It comprises insulin secretion and insulin action.It activates NF-κB and JNK, IRS degradation, suppresses GLUT-4 expression and translocation, activates inflammatory responses [36,37][25][26].Plant secondary metabolites can stimulate mitochondrial metabolism and/or decrease mitochondrial dysfunction through targeting sirtuin 1 activators (SIRT1) and PPAR α [37][26].
A dependent relationship exists between insulin and mitochondria.Insulin release depends on mitochondrial ATP production, and mitochondrial fusion depends on insulin.Mitochondria is a major source of free radicals.However, overproduction of free radicals causes mitochondrial dysfunction [37,38][26][27].
References
- Verma, S.; Gupta, M.; Popli, H.; Aggarwal, G. Diabetes mellitus treatment using herbal drugs. Int. J. Phytomed. 2018, 10, 1–10.
- Dar, R.A.; Shahnawaz, M.; Rasool, S.; Qazi, P.H. Natural product medicines: A literature update. J. Phytopharmacol. 2017, 6, 340–342.
- Bhardwaj, S.; Verma, R.; Gupta, J. Challenges and future prospects of herbal medicine. Int. Res. Med. Health Sci. 2018, 1, 12–15.
- Chauhan, D.S.; Gupta, P.; Pottoo, F.H.; Amir, M. Secondary metabolites in the treatment of diabetes mellitus: A paradigm shift. Curr. Drug Metab. 2020, 21, 493–511.
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44.
- Ehrenkranz, J.R.; Lewis, N.G.; Ronald Kahn, C.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38.
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The bioavailability, extraction, biosynthesis and distribution of natural dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962.
- Kovacich, N.; Chavez, B. Ertugliflozin (steglatro): A new option for SGLT2 inhibition. Pharm. Ther. 2018, 43, 736.
- Triantakonstanti, V.V.; Mountanea, O.G.; Papoulidou, K.-E.C.; Andreou, T.; Koftis, T.V.; Gallos, J.K. Studies towards the synthesis of ertugliflozin from l-Arabinose. Tetrahedron 2018, 74, 5700–5708.
- Bharti, S.K.; Krishnan, S.; Kumar, A.; Kumar, A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100.
- Alkhalidy, H.; Wang, Y.; Liu, D. Dietary flavonoids in the prevention of T2D: An overview. Nutrients 2018, 10, 438.
- Fred-Jaiyesimi, A.; Kio, A.; Richard, W. α-Amylase inhibitory effect of 3β-olean-12-en-3-yl (9Z)-hexadec-9-enoate isolated from Spondias mombin leaf. Food Chem. 2009, 116, 285–288.
- Dominguez Avila, J.A.; Rodrigo Garcia, J.; Gonzalez Aguilar, G.A.; De la Rosa, L.A. The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules 2017, 22, 903.
- Turdu, G.; Gao, H.; Jiang, Y.; Kabas, M. Plant dipeptidyl peptidase-IV inhibitors as antidiabetic agents: A brief review. Future Med. Chem. 2018, 10, 1229–1239.
- Les, F.; Cásedas, G.; Gómez, C.; Moliner, C.; Valero, M.S.; López, V. The role of anthocyanins as antidiabetic agents: From molecular mechanisms to in vivo and human studies. J. Physiol. Biochem. 2020, 77, 1–23.
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes–A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13.
- Prabhakar, P.K.; Doble, M. A target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr. Diabetes Rev. 2008, 4, 291–308.
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161.
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218.
- Miyamoto, T.; Amrein, H. Gluconeogenesis: An ancient biochemical pathway with a new twist. Fly 2017, 11, 218–223.
- Ali, M.Y.; Zaib, S.; Rahman, M.M.; Jannat, S.; Iqbal, J.; Park, S.K.; Chang, M.S. Didymin, a dietary citrus flavonoid exhibits anti-diabetic complications and promotes glucose uptake through the activation of PI3K/Akt signaling pathway in insulin-resistant HepG2 cells. Chem. Biol. Interact. 2019, 305, 180–194.
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465.
- Schmitz-Peiffer, C.; Biden, T.J. Protein kinase C function in muscle, liver, and β-cells and its therapeutic implications for type 2 diabetes. Diabetes 2008, 57, 1774–1783.
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front. Pharmacol. 2018, 9, 423.
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662.
- Kaikini, A.A.; Kanchan, D.M.; Nerurkar, U.N.; Sathaye, S. Targeting mitochondrial dysfunction for the treatment of diabetic complications: Pharmacological interventions through natural products. Pharmacogn. Rev. 2017, 11, 128.
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577.
