Please click here to login first
The N doping led to the formation of a localized state near the valence band of the oxides, which is essential for the tuning of the bandgap energy of ternary metal oxides (TMOs).
- ternary metal oxides
- photocatalysis
- N doping
1. Introduction
Energy is a critical issue related to all trades and professions. Therefore, it is an indispensable factor in influencing the economy and developing modern society for the whole world. Stepping into 21st century, the sharp increasing global energy consumption leads to one of the biggest challenges, the energy crisis, because of the fast expansion of industry and growth of the world population. Under such a circumstance, one of the Sustainable Development Goals (SDGs) of the 2030 Agenda has been proposed to ensure access to affordable, reliable, sustainable, and modern energy. It is urgent and imperative to significantly limit fossil fuel consumption and explore alternatives to meet the global issue and preserve the opportunities for future generations to live in dignity and prosperity. Nowadays, various renewable and green energy, such as solar, wind, tides, waves, and geothermal heat, have been developed. Moreover, efficient utilization of renewable energy is also one of the most important issues for realizing a green and sustainable society. Solar energy is the most abundant source of renewable energy available in most parts of the world. It is necessary to capture solar energy and transform it into other usable chemical energy that can be transported and stored. However, its technically available potential and efficiency should be improved to meet the needs of the energy supply. Besides, in the 21st century, we are confronting enormous environmental issues, including hazardous wastes, toxic heavy metals, and contaminated groundwater, which are closely related to human health. Applied solar energy to construct the environmental pollutants in air and water is a green and sustainable approach, which is beneficial for both water and energy crises. Technologies based on solar energy are also crucial for reducing carbon emissions on a global scale. However, some technical barriers, such as low efficiency, energy storage, conversion efficiency, and high cost, should be addressed and improved in the future. To this end, the development of novel and functional materials is a promising approach to apply to energy and environmental issues. Semiconductor materials have attracted considerable attention due to their versatile physicochemical properties. This material has been applied in a wide field, especially as photocatalysts. Semiconductors as photocatalysts with their unique electronic structure offer the possibility of utilizing naturally available and renewable solar energy as a light source for photochemical waste remediation, thereby making the process green and sustainable. Nowadays, extensive research is being conducted to develop and produce large-scale functional photocatalytic materials with the assistance of cutting-edge approaches to detect and eliminate hazardous contaminants in the environment.
Since water photolysis was discovered by Fijishima et al. [1] with TiO2 as a photocatalyst for the production of O2 and H2 from water, many kinds of semiconductors have been fabricated and applied as photocatalysts, especially for the widely studied oxide materials. It is accepted that the properties of materials greatly changed according to their synthesis process, chemical components, morphologies, surface modification, elements doping and the formation of composites etc. [2,3,4,5,6,7]. The simple binary metal oxides or metal-free semiconductors, such as TiO2, ZnO, WO3 and C3N4, etc., have been widely studied as photocatalysts to understand the fundamental principle, the improvement of photocatalytic efficiency and the expansion in other potential applications [8,9,10,11,12,13]. The ternary metal oxides (designated as AxByOz) with more flexible band structures possess great potential to be applied as photocatalysts. A wide range of ternary metal oxides (TMOs) have been fabricated, and their photocatalytic activity related to morphology, electronic, optical properties should be further investigated. The different constituent elements in the AxByOz composition provide multiple choices to modify the materials with tunning physical and chemical properties for an enhancement of photocatalytic performance.
The efficiency of the photocatalytic processes strongly depends on the properties of the photocatalysts, including (I) the surface area of the material, which influences the adsorption phenomena, and (II) the morphology of the material, on which the electron-hole recombination rate depends. However, the poor separation probability of the photogenerated charge carriers in photocatalytic process severely limits the practical applicability of this green and appealing photocatalytic technology. In addition, some electron-hole pairs will be captured by the defects in the bulk or on the surface of the semiconductor, which deactivate the photocatalytic performance. Therefore, the main obstacle in the field of photocatalysis is to improve the separation efficiency of charge carriers in photocatalysts in the process of environmental cleanup. Up to now, numerous TMOs have been investigated as photocatalysts for application in environmental purification. Many researchers are focused on the development of novel and efficient TMOs. However, the shortcomings of insufficient light absorption and fast recombination of photogenerated charge carriers are obstacles for the practice applications. To this end, a variety of approaches have been proposed to promote the efficiency of photocatalysts, such as heteroatom doping, facet and defect engineering, fabrication of heterojunctions and so on.
Doping with foreign atoms is one of the facilitating and practical approaches to altering the electrical and optical properties and the photocatalytic activity of semiconductors. Usually, some intermediate energy level can be introduced in the forbidden band by doping, which leads to narrowing the bandgap, extending the light-responsive ability, and enhancing the efficiency of charge carriers’ separation. There are many strategies for doping, such as metal doping, non-mental doping, self-doping, co-doping, and so on. Among these methods, anion doping is one of the most promising approaches to enhance photocatalytic performance by the interaction between nonmental dopants and O 2p electrons in the valence band [14]. The non-metal dopants, such as carbon (C) [15], nitrogen (N) [16], sulfur (S) [17], and fluorine (F) [18], etc., have attracted a great deal of attention. Typically, doping with C and N is widely used since these non-metallic elements have an advantage in narrowing the band gap. In 2001, Asahi et al. reported that the N doping of TiO2 can increase the visible light photocatalytic activity by sputtering the anatase TiO2 in an N2 (40%)/Ar gas mixture followed by annealing in N2 gas [19]. A significant red shift in the absorption spectra can be achieved by N doping compared to other anionic dopants [20]. Theoretical simulations of the electronic band structures of anatase TiO2 doped with C, N, F, P, or S substitutional dopants indicated that N doping was the most effective in narrowing the bandgap by overlapping energy states between N 2p and O 2p [19]. Nakamura and coworkers put forward the mechanism for the enhancement of N doping. TiO2 doped with nitrogen resulted in a mid-gap level (N 2p) above the top of the valence band (O 2p), which reduced the energy gap required for charge separation [21]. During the photoexcitation, the electrons were excited and migrated from the initial valence band into the mid-band gap energy level, leaving holes in the valence band. As a result, the N-doped TiO2 was introduced with visible-light responsive photocatalytic activity, and the produced impurity level was also beneficial for the separation of charge carriers. Besides the binary metal oxides, N doping is also effective in TMOs to achieve bandgap narrowing, such as N-doped SrSnO3 and La2Ti2O7 [22,23]. N doping in TMOs resulted in the formation of vacancies that acted as preeminent electron capture centers, which could lower the recombination velocity of photo-induced carriers and enhance the photocatalytic degradation performance. However, compared with the N doping in binary metal oxide, the relatively less investigation of N doping and other non-metal doping in TMOs. The detailed experimental investigation of N doping in TMOs should be further conducted towards versatile applications. In addition to N doping, TMOs interaction with N sometimes led to phase transformation to corresponding oxynitrides via ammonolysis reaction under the NH3 atmosphere. The oxynitrides or some N contained materials with a strong ability to absorb visible light have also been reported effective for the photocatalytic degradation of toxic compounds and offer a wide range of potential applications [24,25]. The narrow bandgap and tunable band position of oxynitride have also attracted much attention in many fields.
2. The Influence of N
TMOs as photocatalysts are greatly influenced by the active sites and the composition, which can dramatically increase the energy conversion or selectivity of catalysts. A common method for changing the active sites is atom doping. Although it is easier to achieve cation doping by the addition of stable metal cationic salts, anions doping is also effective in tuning the lattice and electronic structure of the catalysts, thus inducing various functionality. As one of the widely applied anions, the N can alter the structure and electronic structure of the TMOs, which could further lead to the different physical and chemical properties, such as the bandgap energy, the Fermi level, the localized electronic state, electrical conductivity and so on [26]. Asahi et al. first noted that the valence band maximum of TiO2 shifted upward by substitutional N doping to form mixing of N 2p and O 2p states, which was indispensable for visible light absorption and high photocatalytic activity [19]. Since then, plenty of works have demonstrated the prominent effect of N doping in narrowing the bandgap of the oxides by different doping methods with various N sources [23,27,28,29]. N2 gas mixed with Ar gas was reported as N source to prepare N-doped TiO2 thin film. Further calcination under N2 gas led to crystallized TiO2 with rutile and anatase phases, which was responsive to visible light [19]. In addition, heat treatment under NH3 gas or with urea is also widely applied to dope N into metal oxides [30,31,32]. Liu et al. have reported a red TiO2 by N doping under NH3 treatment. The pre-doped interstitial boron ions facilitated the substitution of O by N anions, resulting in a high visible light absorption band in B/N codoped TiO2 [28]. After ball milling of a solid mixture of ZnO and urea with different ratios, a series of the N-doped ZnO was synthesized by calcination under an air atmosphere [33]. Besides the two-steps approach to introduce N, the simple one-pot synthetic method is also an effective strategy for N doping by adding different N sources. The urea can also be used as N source in a wet chemical process. A facile microwave-assisted hydrothermal method was reported to prepare N-doped BiVO4 with different concentrations of urea in the solution [34]. In the sol-gel reverse micelle method, Na2EDTA was used as a nitrogen source to prepare N-doped TiO2 with remarkable photocatalytic activity under visible light irradiation [35]. Therefore, when various methods have been developed to introduce N anion, a better understanding of N doping amounts and doping sites is beneficial to further enhance the photocatalytic activity. This section focuses on the influence of different N doping concentrations, substitutional/interstitial doping sites, and the phase transition to form oxynitride, especially in the TMOs.
2.1. The Influence of N Doping
2.1.1. N Doping Concentration and Doping Site
The N doping led to the formation of a localized state near the valence band of the oxides, which is essential for the tuning of the bandgap energy of TMOs. One of the most important points that should be mentioned is the concentration of N doping. The bandgap usually becomes narrowed as the N doping amount increases. Although the N doping amount was widely studied in the TiO2 [36], the different doping amounts also affect the optical property, the electronic band structures as well as the photocatalytic activity of complexed N-doped TMOs.
Wang et al. reported that N-doped La2Ti2O7 with various nitrogen doping amounts showed tunable optical absorbance edge and bandgap energy (Figure 1a,b). Compared with the white color of La2Ti2O7 precursor, the N-doped samples exhibited color changed from gray to yellow as the N anions were introduced into the sample (the inset in Figure 1a). An important insight was obtained into the pre-doped N in La2Ti2O7 precursor in weaking the Ti-O bond, allowing the further introduction of N into La2Ti2O7. The DFT calculation also verified that the interaction of the N dopants would reduce the total energy of the systems [37]. The relations between N doping amount with the specific surface area indicated that the more N dopants introduced into La2Ti2O7, the smaller specific surface area could obtain (Figure 1c). This figure also showed that when the N doping amount increased from 3.5 at% to 5.74 at%, the photocatalytic activity increased until an optimal concentration was reached, which should be less than 5%. This series of N-doped La2Ti2O7 samples suggested that there was an optimum N doping amount to obtain the highest visible-light activity. The effect of the nitrogen amounts on the electronic band structures of N-doped La2Ti2O7 was investigated by theatrical calculations [37]. Two different N doping concentrations were investigated, including 1.12–1.14 at% N-doped La2Ti2O7 and 3.30–3.45 at% N-doped La2Ti2O7. Both low and high concentrations of N doping resulted in bandgap narrowing. The isolated N 2p impurity stats were formed in the bandgap at lower doping levels. The creation of localized electronic levels above the valence band was primarily responsible for the narrowing of the bandgap of N-doped La2Ti2O7. As the doping concentration increased, the localized N 2p states stretched to lower energies and integrated into the edge of the valence band. In addition to changing the amount of N doping by changing the reaction conditions, such as the reaction time, reaction temperature, and NH3 flow rate, the different N sources also affect the N doping concentration in TMOs. N-doped SrTiO3 with various N concentrations was reported with a mechanochemical approach to incorporate N into the lattice of SrTiO3 [38]. The same weight percentage of N sources (20 wt% of hexamethylenetetramine, urea, and ammonium carbonate) were mixed with SrTiO3, respectively. The different N content in these three samples explained that the effect of N precursors on the N doping concentration was different, which also determined the photocatalytic activity.
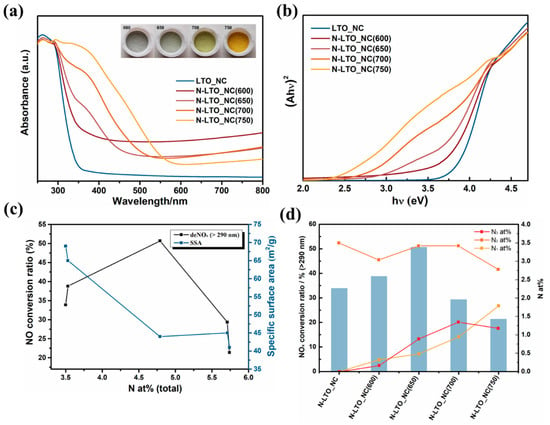
Figure 1. (a) UV–vis diffuse reflectance spectra, (b) Tauc plots of N-doped La2Ti2O7, (c) the relations of total nitrogen amount (at%) with NO conversion ratios and specific surface areas, and (d) correlation between the photocatalytic NO conversion ratio of La2Ti2O7 precursor, N-doped La2Ti2O7 under different calcination temperature (600–750 °C) and different nitrogen sites (N1: substitutional N with N-Ti-N bond, N2: substitutional N with N-Ti-O bond, and N3: interstitial N) [31] (Reproduced from Ref. [31] with permission from the Elsevier).
In addition, the nitrogen doping sites also greatly influence the bandgap energy, electronic structure, and photocatalytic activity of TMOs. In the case of N-doped La2Ti2O7, the substitutional N and interstitial N sites have been thoroughly studied [31]. The pre-doped N in La2Ti2O7 precursor was the substitutional N with N-Ti-O band. After NH3 treatment, substitutional N with N-Ti-N bond and interstitial N were introduced into N-doped La2Ti2O7 with different contents. As shown in Figure 1d, the photocatalytic deNOx activity of N-doped La2Ti2O7 was strongly related to the doping sites of the nitrogen. Although, the N-Ti-N bond (N1) and the interstitial N (N3) can possess negative and positive contribution to the visible light photocatalytic activity, respectively. The interstitial N can possess positive contributions to the visible light photocatalytic activity. For substitutional N, there was no obvious correlation between N-Ti-O substitutional N (N2) with photocatalytic activity, while substitutional N with N-Ti-N bond was not beneficial to improve the photocatalytic activity when the high concentration of N doping was applied. Jin et al. have also discussed the effect of intestinal N in MnxCo3−xO4 [39]. The analysis indicated the introduction of interstitial nitrogen changed the coordination of Mn and Co atoms and lattice distortion by the Jahn-Teller effect. The chemical structures of MnxCo3−xO4, interstitial N-MnxCo3−xO4, and substitutional N-MnxCo3−xO4 were shown in Figure 2. The DFT calculation verified the adsorption energy of aniline on the catalyst surface was negative with N doping, which was beneficial for the adsorption of aniline. Therefore, the high oxidative catalytic performance of the N-MnxCo3−xO4 was achieved. The photocatalytic activity can be greatly influenced by the electronic structure of the semiconductors, which is closely related to the light absorption ability, redox potential, and charge carrier efficiency. The DFT calculations revealed the varied electronic structure of TMOs had a close relation with different N doping sites [37]. The doping N atoms prefer to occupy the interstitial position because of the lower formation energy of interstitial N than that of substitutional N. The calculation results indicated the different N doping sites have different influences on the electronic structure of TMOs. The calculated density of states results indicated that the substitutional N at the O site effectively shifted the valence band edge upward. The introduction of O vacancy by substitutional N also reduced bandgap energy, which will be discussed in the next section.
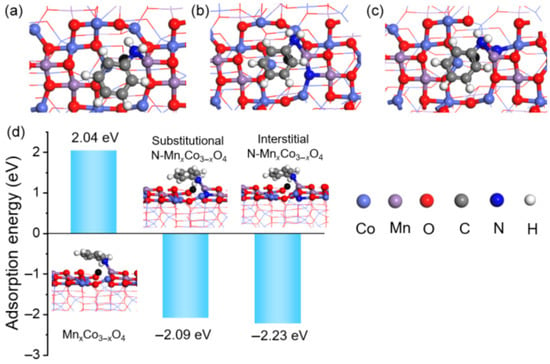
Figure 2. (a–c) the chemical structure of MnxCo3−xO4, interstitial N-MnxCo3−xO4, and substitutional N-MnxCo3−xO4, (d) the DFT calculation of adsorption energy of aniline on the surface of MnxCo3−xO4, interstitial N- MnxCo3−xO4, and substitutional N- MnxCo3−xO4 [39] (Reproduced from Ref. [39] with permission from the Springer).
2.1.2. Defects Induced by N Doping
Defect engineering is an important approach to modulating the physical and chemical properties, such as the surface energy and electronic band structure, therefore efficiently improving the photocatalytic activity of TMOs. Defects in semiconductor photocatalysts have a great impact on the carrier concentration and interface reaction, which can affect the efficiency of the photocatalytic reaction. In general, the reactivity and selectivity of the photocatalysts can be achieved by tuning different kinds of defects. Defects in semiconductors can be classified into four basic divisions based on different dimensions, including zero-dimensional point defects (vacancy and doping), one-dimensional line defects (screw or edge dislocation), two-dimensional planar defects (grain or twin boundary), and three-dimensional volume defects (lattice disorder and void) [40]. However, the defects are deemed to be recombination centers for photogenerated charge carriers and scattering centers for electron and hole traveling, both of which are detrimental to photocatalytic activity. With the development of defect engineering, the positive contribution of defects on photocatalysts with controlled defects can be recognized. The formation of vacancy was reported to enhance the photocatalytic activity and promote the migration of charge carriers. Kim et al. demonstrated that the introduction of oxygen vacancies into BaSnO3 could reduce both the recombination of charge carriers and bandgap energy. The precisely controlled bandgap energy should be attributed to the controllable induced oxygen vacancies [41]. The oxygen vacancies, especially on the surface, can be considered as shallow donors, which are beneficial for the adsorption and reaction in the photocatalytic process. The formation of ample oxygen vacancies caused by interstitial N played a significant role in N-MnxCo3−xO4 to promote the surface mobility of oxygen species and enhance aerobic oxidation reaction [39]. The formation of oxygen vacancy may also introduce reduced metal ions due to the charge compensation, for example, Ti3+ in N-doped La2Ti2O7. The formed Ti3+ could be oxidized to induce O2−• radicals, which was essential for photocatalytic activity [42]. The electron paramagnetic resonance (EPR) showed that the neutral paramagnetic Nb• species in bulk produced from the complexed oxidation in the N doping process played a key role in the photocatalytic activity by reacting with O2 to produce O2−• [42,43]. Therefore, it is necessary to investigate the influence of defects in regard to the concentration of charge carriers, transfer dynamics, interface reaction, and band structure [44]. It is important to notice that the growth of crystal facets is always accompanied by the generation of defects. The formation of a specific crystal facet is beneficial for high reactivity due to the favorable surface atomic structures and surface electronic structures [45,46]. The synergic effects of crystal facet and crystal defect can further facilitate the charge transfer and improve the photocatalytic performance of semiconductors, which could lead to a new horizon in the design of photocatalysts for practical applications.
2.2. Phase Transformation by N Interaction
Although the fact that element doping can induce visible light absorption of the traditional semiconductor photocatalysts, the localized levels generated by impurities may act as recombination sites for charge carriers, which may reduce the photocatalytic activity of the doped materials. In recent years, precise control of the bandgap and electrical/optical properties of semiconductor materials to obtain an optimal balance between optical absorption and redox potentials can be achieved by a phase transformation from TMOs [47,48,49]. Introduction of N into oxides also leads to the formation of oxynitride or nitride materials [50,51]. Many researchers have focused on the fabrication of oxynitride by topochemical ammonolysis reaction from transition metal oxides to adjust the energy band structure, such as (Ga1−xZnx)(N1−xOx) [52], (Zn1+xGe)(N2Ox) [53], ATaO2N (A = Ca, Sr, Ba) [54], NaxLa1−xTaO1+2xN2−2x [55] and so on. These oxynitride materials have been demonstrated to exhibit enhanced photocatalytic activity by modulating the band structures.
(Ga1−xZnx)(N1−xOx), a solid solution of GaN and ZnO, has been used as a visible-light responsive photocatalyst for organic pollutants degradation [56] and water splitting [57]. As shown in Figure 3a,b, different absorption edges were observed in (Ga1−xZnx)(N1−xOx) due to the various O/N ratios [58]. The interaction of N with Ga2O3 under NH3 atmosphere resulted in the topochemical transition to form (Ga1−xZnx)(N1−xOx) with visible light absorption ability. The bandgap energy of (Ga1−xZnx)(N1−xOx) showed a decreasing trend with an increasing O/N ratio. The narrowed bandgap should be originated from the p-d repulsion between N 2p/O 2p and Ga 3d states. Besides, the (Ga1−xZnx)(N1−xOx) solid solution with wurtzite structure was also reported to form by phase transformation from spinel ZnGa2O4 [59]. A broad range of compositions of (Ga1−xZnx)(N1−xOx) solid solution showed varied absorption edges and bandgap energy (Figure 3c), which were effective for visible-light-driven water oxidation. The varied composition range and band structure could be achieved by different reaction conditions under NH3 treatment. Therefore, the obtained sample showed interesting physical and chemical properties for photocatalytic activity. The interaction with N via ammonolysis reaction also resulted in the phase transformation of other TMOs, such as Zn2GeO4 to (Zn1+xGe)(N2Ox) [60], La2Ti2O7 to LaTiO2N [61], Ba2Ta2O7 to BaTaO2N [62], etc.
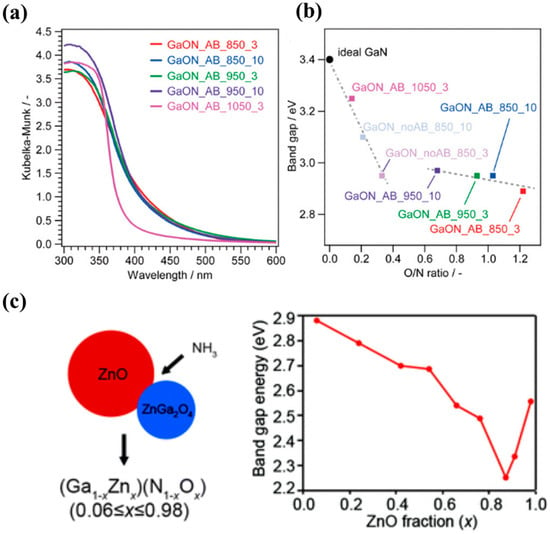
Figure 3. (a) UV-vis DRS spectra of the (Ga1−xZnx)(N1−xOx) samples. (b) Relationship between the O/N ratio and the band gap of (Ga1−xZnx)(N1−xOx), [58] and (c) the relations between bandgap energies and ZnO fraction (x) in (Ga1−xZnx)(N1−xOx) solid solutions [59] (Reproduced from Refs. [58,59] with permission from the RSC).
 Encyclopedia
Encyclopedia
