Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Stilbenes are polyphenolic allelochemicals synthesized by plants, especially grapes, peanuts, rhubarb, berries, etc., to defend themselves under stressful conditions. They are exploited in medicine for their antioxidant, anti-proliferative and anti-inflammatory properties. Inflammation is the immune system’s response to invading bacteria, toxic chemicals or even nutrient-deprived conditions. It is characterized by the release of cytokines which can wreak havoc on healthy tissues, worsening the disease condition.
- stilbenes
- inflammation
- plant secondary metabolites
- resveratrol
1. Inflammation in Macrophages
In inflammation, macrophages have three major functions: antigen presentation, immunomodulation and phagocytosis, by means of cytokine and growth factor production. Macrophages are critical to inflammation’s onset, duration and termination. Deactivation of macrophages is achieved by anti-inflammatory cytokines (transforming growth factor β and interleukin 10) and cytokine antagonists, which macrophages mostly generate [1]. When stilbenes were used to treat macrophage inflammation, the following cell components were especially affected.
The NF-κB signaling pathway, as covered, is one of the best understood immune-related pathways. It is a critical regulator of autoimmune illnesses as well as of the inflammatory response to infections and malignant cells. It is crucial for innate immune responses in first-responder cells such as macrophages, because the canonical NF-κB response is significantly faster than non-canonical signaling [2][3]. The production of interleukin 6 (IL-6) and cyclooxygenase 2 (COX-2) is mediated by this pathway [2][3].
Sirt1 is a sequential post translational regulator that deacetylates proteins [4]. It promotes the generation of ROS, which causes the aberrant activation of the NLRP3 inflammasome, which will lead to inflammation [5]. NLRP3 recognizes stress and activates caspase-1, which triggers pyroptosis and cytokine release [6]. Lipopolysaccharide (LPS) is an outer-membrane component of Gram-negative bacteria. The proinflammatory portion is a glycolipid composed of a polysaccharide O-antigen, a core oligosaccharide and a highly conserved lipid A moiety. LPS activates cells of the innate immune system, such as macrophages and neutrophils, which synthesize IL-1β, TNFα, MMPs and free radicals that lead to dramatic secondary inflammation in tissues. RSV was used to treat mice which then had the brain infiltrating mononuclear cells ex vivo MOG restimulated. These produced less pro-inflammatory IL-17A and IL-6, which are characteristic cytokines in experimental autoimmune encephalomyelitis (EAE) [7]. In mouse encephalitogenic CD4+ cells, SK1 was markedly downregulated. RSV docks to the substrate binding pocket of SK1, directly inhibiting its catalytic activity [8] (Figure 3). This hinders localization to the plasma membrane, where it can access its sphingosine substrate [9]. In rat multiple sclerosis (MS) models, RSV reduced inflammation by SIRT 1 activation, a pathway that increases non-inflammatory cells in brain and peripheral tissue [10]. RSV-induced SIRT1 can also prevent the activation of T cells and NF-κB in colitis brought on by DSS (digestive tract inflammation) [11], as well as deacetylate c-jun transcription factor, which deactivates T cells in a collagen-induced arthritis model [12]. It does this by lowering the SIRT1 Michaelis constant for acetylated substrates [13], such as p53 [14]. RSV, (+)-ε-viniferin and hopeaphenol inhibited the damaging effects of DPPH radicals and prostaglandin E2 in rabbits with arthritis induced by LPS [15][16].
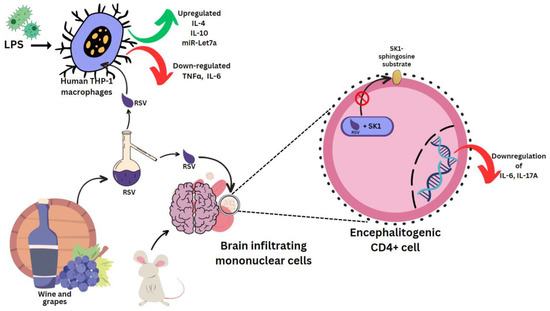
Figure 3. Effect of resveratrol on mouse and human cells. Lipopolysaccharides (LPS), clusters of differentiation 4 (CD4+) cells.
LPS was used in several experiments to induce a state of inflammation. In human THP-1 macrophages induced with LPS, RSV epigenetically regulates survival and apoptosis, and increases the anti-inflammatory IL-4 and IL-10, and miR-Let7a levels. TNFα and IL-6 were reduced [17] (Figure 3). Similar results were seen for cis- and trans-gnetin H RSV derivatives [18]. trans-resveratrol-3-O-glucoside is also a Sirt-1 activator, and along with RSV it can inhibit ICAM1 and TNFα induction. This mechanism was used in reducing inflammation in LPS-stimulated macrophages pre-treated with the extract of Sambucus ebulus L. fruit [19]. Resveratrol-4′-O-glucuronide, however, can upregulate the mRNA levels of macrophage inflammatory protein 1β (MIP-1β) [20].
Macaranga siamensis S. J. Davies contains many stilbenes unique to its species, among which macasiamenene L shows the best inhibition IC50 at a concentration of 1.8 ± 1.1 μmol/L in cell viability studies with the THP-1 cell line [21]. Meanwhile, 1 μmol/L macasiamenene F reduces TNFα from LPS-stimulated macrophages by 20%, and interferes with the DNA binding site of NF-κB. It shows anti-inflammatory properties through modulating IκBα/NF-κB signaling, by degrading IκBα. 3,3′,4,5′-tetramethoxy-trans-stilbene (3,3′,4,5′-TMS) and 3,4′,5-trimethoxy-trans-stilbene (3,4′,5-TMS) are two methoxy derivatives/analogues of RSV. RAW 264.7 cells stimulated with 1 µg/mL LPS showed enhanced NO release, with upregulated phosphorylation for MAPK pathway members p38, JNK and ERK. A 50 µM pretreatment of the cells for 4 h with 3,3′,4,5′-TMS showed significant suppression for p38 and JNK, and to a lesser extent for ERK as well. 3,4′,5-TMS could suppress expressions of all three proteins [22]. Regarding the NF-κB signaling pathway, pretreatment of 3,3′,4,5′-TMS and 3,4′,5-TMS decreased p-IKKα/β, p-IκBα, p-P65, and MDA accumulation, NF-κB p65 nuclear translocation and intracellular H2O2. Only 3,3′,4,5′-TMS showed some reversal of SIRT1 expression and decreased the O2•− production. RAW 264.7 cells differentiated in response to LPS, taking on an irregular form with pseudopodia and speeding up spreading, which could be decreased by treatment with both compounds [22].
t-ORV is able to control the NF-κB signaling pathway to decrease production of NO, TNFα, monocyte chemoattractant protein-1 (MCP-1), IL-1β, inducible nitric oxide synthase (iNOS), CXCL10 and IL-6 in a macrophage cell line and microglial cells induced with LPS [3][23]. t-ORV treatment causes the activation of all three pathways of NF-κB, MAPK and PI3K/AKT/p70S6K signaling pathways to suppress C-X-C motif chemokine ligand 10 (CXCL10), along with other pro-inflammatory cytokines [23]. Macrophages were treated with t-ORV and its glucuronide metabolites t-ORV-4′G, t-ORV-2′G, and t-ORV-3G, all of which could reduce IL-1β levels by 30–50%. However, a 4-fold concentration of glucuronide metabolites was required over the parent compound. The only compounds capable of reducing TNFα were t-ORV and t-ORV-3G. In the same way, the glucuronide metabolites of GN, namely t-GN-2′G and t-GN-3G, significantly reduced IL-1β (32–47%) and TNFα (around 65%) [24]. Moreover, GN has the ability to lower colorectal cancer cell viability and suppress platelet aggregation, platelet-collagen adhesion, and COX-1 activity [25][26].
Hopeaphenol, isohopeaphenol, PICE, and ε-viniferin significantly decreased LPS-induced TNFα and IL-1β production in RAW 264.7 cells, with isohopeaphenol being the most potent against IL-1β [27]. The expression of the TNFα induced by LPS is more significantly suppressed by PICE than RSV [28], and it also inhibits T-cell receptor signaling in primary murine splenocytes [29]. Structurally, the phenyl rings’ hydroxyl groups are important to suppress cytokines production [28]. Other properties of PICE include the inhibition of iNOS expression when bEnd.3 cells (endothelial cells isolated from brain tissue) were pre-incubated with 50 µM of the stilbene for 4 h and stimulated with 1 µg/mL LPS exposure. Only 30 min of pretreatment could reduce p38, JNK, IKKα/β, IκBα and p65 phosphorylation caused by 10 µg/mL LPS. The LPS-triggered nuclear translocation of NF-κB and ROS increase were also prevented [30]. Bavienside A treatment resulted in a significantly reduced amount of NO that LPS-induced RAW264.7 cells produced, with an IC50 value of 6.23 μM [31]. Bone marrow-derived macrophages (BMDMs) were stimulated with LPS (1 μg/mL). Here, 10 μM gigantol inhibited NO release by 47%, and decreased levels of TNFα/IL-6 as well, with as little as 1 μM as the minimum effective dose [32]. NO could be inhibited in LPS-stimulated J774.1 cells using the stilbenes found in propolis at less than 0.2% (v/v). Propolis is an animal product of bees, composed of beeswax and also plant resins, which contribute to its stilbene content. The dihydrostilbene skeletons with catechol moieties in the ethanolic extract of Senegalese propolis are the ones that show anti-inflammatory activity. PTS could inhibit the pro-inflammatory responses between RAW 264.7 macrophages and 3T3-L1 adipocytes in vitro [33]. Ampelopsin shows anti-inflammatory effects by inhibiting the NF-κB signaling cascades, phosphoinositide 3-kinase and protein kinase B in RAW264.7 cells [34]. Ampelopsin exerts anti-inflammatory properties through modulating the Toll-like receptor 4 (TLR4) pathway [35] or binding to the ryanodine receptor [36].
Stilbenes are also known to attenuate allergies. PICE suppresses the allergic inflammatory response of mast cells through regulating MAPK phosphorylation [37]. Rhapontigenin inhibits histamine release from mast cells, hyaluronidase (HYAL) activity and passive cutaneous anaphylaxis reactions [38]. A derivative of rhapontigenin, called desoxyrhapontigenin, shows anti-inflammatory properties by activating the nuclear factor erythroid 2-related factor 2/heme oxygenase-1 pathway and also in macrophages by attenuating the NF-κB and MAPK pathways [39]. Agonis flexuosa (Willd.) leaves contain the stilbenes (Z)-2,3-dihydroxystilbene-5-O-β-D-glucoside, (Z)-pinosylvin mono methyl ether and (Z)-pinosylvin-3-O-b-D-glucoside. In an in silico study conducted on the relationship between stilbenes and cellular receptors, they showed free binding energies between −11 and −31 kcal/mol when docked with human histamine H1 receptor as histamine blockers for treating allergies and inflammatory conditions. The first glycoside was also able to inhibit in vitro histamine production with an IC50 of 0.16 mM in U937 human monocytes [40].
2. Inflammation in the Liver
Liver injury accounts for 2 million deaths per year globally, with half due to cirrhosis alone [41]. The liver can also sense and respond to systemic inflammation [42]. When the complement system is activated by pathogens or antibodies, it unleashes a cascade of processes that result in inflammation. The liver is in charge of generating the majority of the complement system proteins. Specifically, C9 of the terminal complement complex C5b-9 is a known pro-inflammatory trigger [43]. The NF-κB pathway regulates COX-2 production and maintains antioxidant defenses by controlling ROS-scavenging proteins [44]. It shows antiapoptotic activity by controlling the c-Jun N-terminal kinase (JNK) cascade, thereby suppressing TNFα [45].
To model inflammation in the liver, different experiments have been conducted. CCl4 treatment is used to induce inflammation and it has myriad effects in hepatocytes. It causes upregulation of the MAPK/JNK pathway [46], JNK/cPLA2/12-LOX inflammatory pathway [47], and Akt/NF-κB pathway [48], phosphorylation of ERK1/2 and Smad [49] (Zhan et al., 2021), and mRNA expression of C3 and TCC components (Xue et al., 2020b), platelet-, and leukocyte-type 12-LOX [47]. Normally, arachidonic acid (AA) that has been esterified appears in membrane phospholipids, which, when disturbed by external stresses, becomes de-esterified and liberated by cytosolic phospholipase A2 (cPLA2). Free AA is transformed into pro-inflammatory eicosanoids such as leukotrienes (LTs) and hydroxyeicosatetraenoic acids (HETEs) by the enzymes lipoxygenases (LOX), cyclooxygenase (COX) and cytochrome P450 enzymes (CYP450). By activating neutrophils and macrophages, these eicosanoids exacerbate liver inflammation [47] (Figure 4).
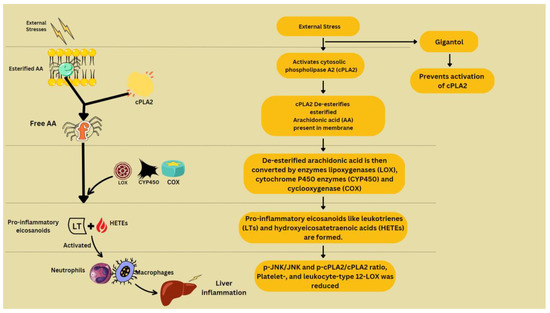
Figure 4. Arachidonic acid metabolism and contribution to inflammation.
CCl4-treated Sprague Dawley (SD) rats’ liver tissue was treated with a 300 mg/kg BW dose of PTS. This reduced the phosphorylation of ERK1/2 and Smad, and the formation of TGF-β, p-ERK1/ERK1, p-ERK2/ERK2, p-Smad1/Smad1, and p-Smad2/Smad2 proteins in the liver 2.64-, 4.36-, 2.10-, 7.47-, and 5.83-fold, respectively [49].
The signaling pathways MAPK/JNK pathway [46] and Akt/NF-κB pathway [48] are responsible for AA metabolism, which is then followed by hepatotoxicity, neutrophils infiltration and M1 polarization of macrophages. This is countered by gigantol, which also reduces the mRNA expression of C3 and TCC components, and platelet and leukocyte-type 12-LOX, responsible for oxidative and endoplasmic reticulum-mediated inflammation. By inhibiting the JNK/cPLA2/12-LOX inflammatory pathway, gigantol reduces the liver damage caused by CCl4 exposure [47]. Gigantol keeps C9 in check, and thus prevents the deposition of C5b-9 around hepatic vessels and promotes the formation of CD59b, which protects cells from complement attack in response to inflammation [32].
Subacute liver failure (SALF) is characterized by ascites and the poor regeneration of hepatocytes. As a SALF model, Wistar rats were first injected with thioacetamide (TAA), and then a mixture of 5 mg/kg trans-RSV and trans-ε-viniferin was supplemented in 3 doses. TNFα, COX-2 and iNOS were reduced and the transcription of the anti-inflammatories IL-10 and NF-κB was upregulated [44]. Steatosis is lipid buildup in the liver, leading to inflammation of the organ. This is characterized by the increase in blood alanine aminotransferase (ALT) and aspartate aminotransaminase (AST) levels. Adipose tissue fat can also generate inflammation mediators such as TNF-α, IL-6 and leptin, which can trigger liver injury [50] (Beaumont et al., 2022). ε-viniferin, found in Vitis vinifera L. grapevines, is known to reduce all of the these enzymes. To model steatosis, C57BL/6 mice were given a methionine- and choline-deficient diet, leading to lipid accumulation and then inflammation. Meanwhile, 35 and 70 mg/kg trans-2,3,5,4′-tetrahydroxystilbene-2-O-glucoside (TSG) can reduce the amounts of triglyceride, cholesterol and free fatty acids, AST and ASC, an adaptor molecule. Its inhibitory effect on IL-1β can be seen at 17.5 mg/kg and on IL-18 only at 70 mg/kg [51]. TSG also shows its medicinal effect on acetaminophen-induced liver injury induced in C57BL/6 mouse liver [52]. Pretreatment with 60–180 mg/kg TSG reduced liver injury, cell degeneration, and necrosis by reducing the production of cytokines such as IL-10, IL-6, CCL3, IL-12, IFN-γ, CCL11, IL-2, IL-3, IL-17, IL-1β, GROα/KC, and TNFα. Inflammation was also induced in mice using bicyclol. Meanwhile 40 mg/kg gigantol showed improvement in the infiltration of inflammatory cells, tumefaction, and centrilobular necrosis. The inhibitory effect of gigantol was seen against raised levels of the expression of cytokines’ mRNA (TNFα, IL-1β and IL-6) and chemokines (ICAM-1 and MCP-1) due to bicyclol [32].
ORV, RSV and MulA were used in the treatment of LPS-stimulated mice. Here, 80 mg/kg stilbenes which were intraperitoneally injected reduced MDA, ALT and AST levels. MulA showed a particularly suppressive effect on serum transaminase. Acute liver injury (ALI) comes about due to the NF-κB signaling pathway’s activity and LPS/D-GalN. MulA, followed by ORV and RSV, restrained TLR4 and MyD88, which halts the entire cascade, along with reducing amounts of IL-6 and IL-1β. Regarding the MAPK signal pathway, all three of the stilbenes could prevent the phosphorylation of p38, ERK1/2, and JNK, thereby deactivating the downstream pathway [53] (Figure 5).
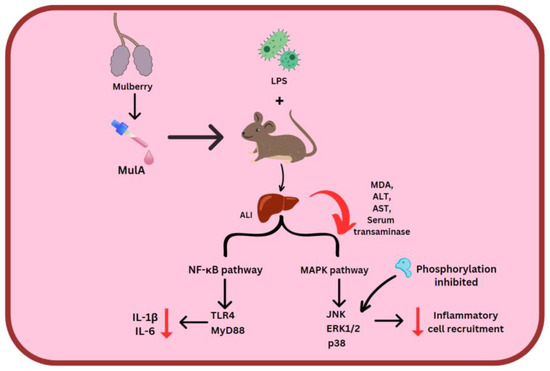
Figure 5. MulA effect on 2 inflammatory pathways. Mulberroside A (MulA), lipopolysaccharides (LPS), acute liver injury (ALI), malondialdehyde (MDA), alanine aminotransferase (ALT) and aspartate aminotransaminase (AST), Toll-like receptor 4 (TLR4), myeloid differentiation primary response 88 (MyD88).
3. Inflammation in the Cardiac Tissue
H9c2 cells derived from rat heart tissue were treated with 0.4 mM of palmitic acid, which increased the levels of IL-6, TNFα, IL-1β, Smad3 phosphorylation, and nuclear NF-κB/p65. p65, a subunit of NF-κB, binds to DNA to enhance inflammatory cytokine expression [54]. TSG is able to reverse this effect by reducing NF-κB/p65 levels and downregulating p-Smad3/Smad3 [55]. RSV and PICE, at concentrations ranging from 80 nM to 7 mM, were able to improve cell viability by 95% in H9c2 cells kept in hypoxic conditions for 48 h [56]. Meanwhile, 10–20 μM of PICE is able to inhibit the effects of COX 2 and PI3K signaling in human aortic smooth muscle cells (HASMCs) [57]. It selectively inhibits Syk, which regulates inflammation in hematopoietic cells [58]. It can also regulate iNOS and AP-1 and inhibit IL-1β, IL-18, NO, IL-8, Il-6, TNFα and PGE2. Reduction in cytokine level was observed in LPS-treated peripheral blood mononuclear cells (PBMCs) due to the action of 100 μM of 3″-methoxycochinchinenene H, a D. usambarensis F. White stem stilbene. It was found to be more potent than standard drugs [59]. The gut microbiota has a direct effect on coronary heart diseases as it generates trimethylamine (TMA), which is reduced to trimethylamine N-oxide (TMAO), and increasing concentration of which can increase the risk of atherosclerosis. The administration of stilbenes can reduce trimethylamine N-oxide by regulating the TMA-producing microbes in the gut, with resveratroloside, rhaponticin and TSG showing the best ability [60].
4. Inflammation in the Connective Tissue
Chondrocytes are specialized cells found in certain cartilage tissues such as the intervertebral discs. They are surrounded by collagenous fibers, and they produce substances which make the cartilage strong and flexible. In mouse models, the induction of inflammation causes chondrocyte activity to be hindered, resulting in a reduction in bone mass. The effects of stilbenes on inflammation are listed in Table 2.
Table 2. Effects of stilbenes on connective tissues.
| Stilbenes | Concentration | Model System | Observation | Ref. |
|---|---|---|---|---|
| Amurensin H | 4 and 8 μmol/L | IL-1β-induced rat knee chondrocytes | Blocked elevation in IL-6, IL-17, TNFα, TLR4, TRAF6, Syk phosphorylation, NO levels and iNOS expression. Decreased PGE2 and COX-2 levels. Up-regulated COL2A1 and GAG, major components of ECM | [61] |
| 20 mg/kg | MIA-induced mice | Alleviated bone wear and cartilage loss from 60 ± 13.4% to 25.5 ± 11.4%. | ||
| 3,5,4′-Trimethoxy-trans-stilbene (BTM) | 1% and 0.5% | Rabbit knee joints | Topical administration reduced inflammatory cell infiltration, decreased bone destruction and roughness, and inhibited fibrous connective tissue proliferation. Microemulsion-based hydrogel method decreased IL-1β and TNFα. |
[62] |
| Ampelopsin C | 25 μM | Human chondrocytes | Inhibits PGE2 with IC50 15.52 μM | [63] |
| Desoxyrhapontigenin | 50 mg/kg | LPS-stimulated mice and RANKL-induced osteoclastogenesis. | LPS induced trabecular separation, while bone surface and volume changes were attenuated. Anti-osteoporosis activity by inhibiting RANKL. Osteoclast formation is suppressed at an early stage by inhibiting the MAPK/AP-1 signaling pathway, ERK phosphorylation, and the expression of c-Fos and NFATc1. | [64] |
5. Inflammation in the Nephrons
The inflammatory responses seen in the kidney are usually seen as a result of hyperglycemia, causing apoptosis of podocytes due to increased ROS [65]. This induces TNFα production, due to which the glomerular permeability barrier is harmed [66][67]. Inflammatory genes including cytosolic phospholipase A2 (cPLA2) and COX-2 are increased as a result [68], as well as JNK1/2, NF-κB (p65) and ERK1/2 phosphorylation. COX then controls AA conversion to prostaglandin E2 (PGE2). NO is produced by the enzyme iNOS by using arginine and oxygen, which is activated by endotoxins or cytokines. Peroxynitrite (ONOO−, RNS) is created when NO reacts with free radicals (•O2−) that can damage the cells.
Following a 1 h pretreatment with ampelopsin C (AC), ampelopsin F (AF), or PD, rat mesangial cells were incubated with TNFα. This reduced cPLA2 protein formation but not its mRNA levels. PGE2 and the three phosphorylated signaling entities were also reduced. The RSV derivatives ampelopsin F (AF) and ampelopsin C (AC) showed an inhibitory effect on cPLA2/COX-2/PGE2 activity, even better than the parent molecule. Meanwhile, 2 μg/mL AC and AF inhibited COX-2 mRNA levels via a p38 MAPK-independent pathway [69]. PD can also control cPLA2 and COX-2. TNFα, IL-1β and PGE2 production are decreased as a result [70], as well as iNOS proteins [71]. Extracellular matrix accumulation due to high-glucose conditions is alleviated by PD in RMCs through anti-inflammatory mechanisms [72]. Diabetic nephropathy was modeled in cultured mouse podocytes (MPC5) by incubating them in a high concentration of glucose. A 48 h co-treatment with 10 μM of TSG blocked the NLRP3 inflammasome–IL-1β axis, preventing apoptosis. The Nod-like receptor protein 3 (NLRP3) inflammasome oligomerization and caspase-1 activation resulted in the release of IL-18 and IL-1β [73][74]. The amount of IL-1β was reduced when NLRP3 was knocked down. Due to TSG treatment, nephrin protein levels increased. ROS, MDA levels, caspase-3 activation, IL-1β, pro-caspase-1, caspase-1, NLRP3 and ASC were decreased [75].
6. Inflammation in the Intestine
Inflammation in the intestines is generally in the form of inflammatory bowel disorder (IBD), colitis, metabolic syndrome (MetS), etc. MetS is a debilitating systemic condition, and its inflammatory response can destroy the gut lining, resulting in insulin resistance, which hampers metabolism and hormone production [76]. Associated with the brush border cells is an abundantly available protein, aminopeptidase, or CD13, a specific cytokine receptor on the cell surface. These cytokines are members of the signaling cascade that ultimately results in inflammation. Aminopeptidase mediates the inflammatory response through G-protein-coupled receptors. By cleaving the N-terminals of cytokines, it controls their activity. Moreover, by trimming the peptides attached to MHC class II, it participates in antigen processing and the proliferation and effector function of immune-related cells [77]. Inflammation is also induced in obese conditions, as adipocytokines are produced with the increased number of adipocytes.
The effects of stilbenes on these disorders are studied using mice models and cell cultures in which inflammation has been induced. Administered RSV and PTS at 5% concentrations showed downregulation of aminopeptidase, IL-1β and TNFα on duodenal brush border membrane proteins. Only PTS could downregulate IL-6 [77]. To model obesity, the diet of C57BL/6J mice was rich in fat. The usage of 2.5% DSS induced colitis (Figure 6). The effects observed were fibroblast cell infiltration, necrotizing colitis, loss of weight and crypts, diarrhoea, and bloody stools [78]. This led to the colon shortening with an increased weight-to-length ratio [79]. PTS could prevent colitis by controlling the inflammatory response, fibrosis, and gut barrier functioning. Treatment of dietary PTS (0.005 and 0.025%) reversed these symptoms, decreased the colon weight-to-length ratio, the total number of aberrant crypt foci and aberrant crypts per colon length. It also suppressed thickening of the intestinal wall and colonic edema, and reduced the disease activity index. PTS also reduced the levels of IL-6, TNFα, IL-1β, COX-2, MMP2, TGF-β1 and p-Smad2 in the colonic mucosa, the last two of which are especially involved in fibrosis. TGF-1/Smad signaling allows fibrogenic mesenchymal cells to make more collagen after stimulation by TGF-β1 [78].
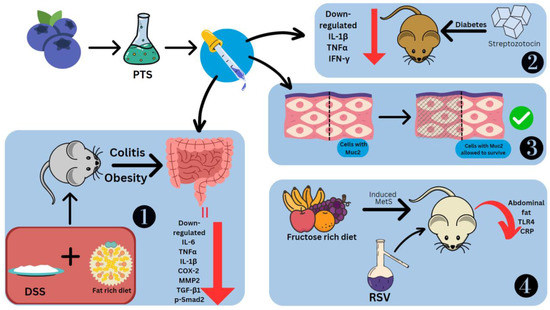
Figure 6. Effect of stilbenes in intestinal inflammation. Pterostilbene (PTS), mucin 2 (Muc2), matrix metalloproteinase-2 (MMP2), phosphorylated mothers against decapentaplegic homolog 2 (p-Smad2), metabolic syndrome (MetS), C-reactive protein (CRP). 1. PTS preventing DSS-induced colitis. 2. PTS decreasing cytokines in Streptozotocin-induced diabetic mice. 3. PTS retaining cells coated in Muc2. 4. RSV reducing Metabolic Syndrome in mice fed on fructose-rich diet.
PTS decreased TNFα, IFN-γ and IL-1β in diabetic mice models induced by streptozotocin [80]. Other effects of PTS seen in the intestine are the reduction in C/EBP homologous proteins (CHOPs), which are markers of ER stress, collagen deposition and inhibition of the loss of the E-cadherin [62]. PTS is also able to retain the cells coated with Muc2, a significant mucin that goblet cells make to protect the intestinal epithelium [78] (Figure 6). The stilbene glycoside resvebassianol A has a unique sugar unit 4-O-methyl-d-glucopyranose, which was obtained from Beauveria bassiana (Bals.-Criv.) Vuill (entomopathogenic fungus) which mediates the biotransformation of RSV. When human intestinal epithelial cells and HIEC-6 cells are stimulated with TNFα/IFN-γ, resvebassianol A inhibits IL-1β and IL-6 production. It does not show harmful effects when tested on HIEC-6 and HaCaT cells. RSV’s anti-inflammatory activity is higher at 25 μM, but resvebassianol A is safe for use even at high concentrations [81]. In Caco2 cells, derived from a colon carcinoma, RSV polymers in the 5–25 M range showed suppression of NF-ΚB. The inhibitory power was most seen in δ-viniferin, followed by trimethoxy-resveratrol, ε-viniferin, pterostilbene-transdihydrodimer and then RSV [82].
To model metabolic syndrome, Wistar rats were fed a rich fructose diet (Figure 6). They were treated with water-alcohol extraction of the V. vinifera L., which has a total stilbene content of 1.519 g/L, including trans-RSV and ε-viniferin. There was a difference in the effects of stilbenes seen with early (14th week onwards) and late (19th week onwards) treatment. Abdominal fat showed a reduction of 53.6% and 40% from the 14th and 19th weeks, respectively. Similarly, TLR4 concentration was reduced by 65.1% and 57.9%, respectively. Systemic inflammatory reaction markers, namely C-reactive proteins, which are useful prognostic indicators for cancers, showed levels that were 76.0% and 81.61% lower, respectively [83]. LPS was also used to model intestinal injury. There was a spike seen in the levels of TLR4 and NO. The former was suppressed by rhein at a concentration of 100 mg/kg body weight [84].
7. Inflammation in the Lungs
Pneumonitis is a non-infectious inflammation of the lungs. Smoking can cause buildup of cholesterol intracellularly, which can lead to the inflammation of the lungs in chronic obstructive pulmonary disease (COPD) [85]. The total leukocyte count was increased in the lungs when mice were exposed to LPS and a 250–300 ppm density of smoke daily for 1 h for 26 days. With the application of amurensin (5–20 mg/kg) or RSV (10 mg/kg) an hour before smoke exposure, a decrease in IL-6, IL-17A, IL-1β, TNFα, IFN-γ and the IFN-γ/IL-4 ratio, a restoration of Th1 bias and improved airway inflammation were observed. This was due to the stilbenes’ inhibition of p-Syk and its inflammatory factors NF-κB p65, NF-κB, and p-NF-κB [86]. Amurensin H is an anti-autophagy agent that regulates Sirt1 and FoxO3 levels, suppresses oxidative stress in CS-induced autophagy models and prevents COPD progression [87]. Upon administration of 5 mg/kg RSV encapsulated within a lipid-core nanocapsule post LPS exposure, the rise in pulmonary elastance was inhibited and there was a reduced concentration of leukocytes in the lungs. Pretreatment with RSV improved lung function, with a decrease in MDA levels and inflammatory cell infiltration, IL-6, TNFα, MCP-1, MIP-2, RANTES, macrophage inflammatory protein-1 alpha (MIP-1α) and KC chemokines. There was a restoration of the catalase level in lung tissue by reducing the phosphorylation of Akt, ERK and p38 [88]. These experiments show that when stilbenes are administered to living systems such as the lungs before exposure to compounds or factors which trigger inflammation, the sudden upshoot of the inflammation factors and accumulation of leukocytes can be reduced.
8. Inflammation in the Nervous Tissue
Neuroinflammation is an immune response seen in the spinal cord and brain, which results from the release of inflammatory factors such as ROS, cytokines, chemokines and secondary messengers, which carry messages for inflammation. These factors are mostly produced by the CNS, the glial or microglia cells and the CNS’s innate immune cells. Neurotrophins are the growth factors which are produced in vertebrate brains, and they regulate the proliferation of neural progenitors and cell death. Antioxidants which are produced by the mitochondria, along with neurotrophins, have a neuronal regenerative activity and regulate apoptosis [89]. The inflammatory cytokines produced by astrocytes and activated microglia are responsible for neuroinflammation, which results in degeneration of the blood–brain barrier, which leads to the recruitment of leukocytes and other immune cells, leading to inflammation within the brain and spinal cord.
RSV has shown positive results as an anti-neuroinflammatory agent. Its administration causes low regulation of peroxisome alpha-1 (PGC1α) and hypoxia induction factor 1 (HIF-1). These are key modulators of microglial and inflammation in the CNS. HIF-1 increases IL17 [90] and PGC1α downregulates mitochondrial antioxidant genes, resulting in increased oxidative stress and the activation of the NF-κB pathway and NK receptor (p58), making the cells more sensitive to proinflammatory cytokines [91]. RSV also decreases positive iba-1 cells, IL-6, IL-12 and IL-23 required for the dendritic cells and microglia to improve their potential as APC, which can activate T cells towards an inflammatory response. EAE studies emphasize that resveratrol can modulate or attenuate T-cell response by altering/downregulating the CD28/CTLA-4 and CD80 costimulatory pathways [92]. RSV causes T-cell apoptosis, mediated by the estrogen receptor and aryl hydrocarbon [93][94]. In a spinal cord injury model in rats, RSV promotes neuroprotection against lipid peroxidation mediated by radicals [95]. LPS activation of microglial cells produces cytokines, which results in their proliferation. RSV promotes apoptosis and drives the anti-inflammatory M2 microglial phenotype [96]. It also promotes tolerogenic dendritic cell differentiation, which has an immuno-suppressive effect [97]. RSV also inhibited LPS-induced IL-1β, TNFα, C reactive protein, IL-6, NO and MCP-1 in primary mouse astrocytes, demonstrating its highly anti-inflammatory effect [98] (Figure 7).
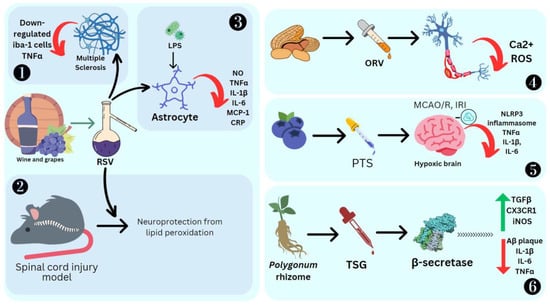
Figure 7. Effect of stilbenes on nervous system inflammation. Ionized calcium binding adaptor molecule 1 (Iba1), oxyresveratrol (ORV), middle cerebral artery occlusion/reperfusion (MCAO/R), ischemia reperfusion injury (IRI), nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 (NLRP3), trans-2,3,5,4′-tetrahydroxystilbene-2-O-glucoside (TSG), amyloid-β (Aβ), chemokine (C-X3-C motif) ligand 1 receptor (CX3CR1), reactive oxygen species (ROS). 1. RSV reducing inflammation in Multiple Sclerosis by SIRT1 activation. 2. RSV promoting neuroprotection against lipid peroxidation. 3. RSV reducing cytokines in primary mouse astrocytes stimulated by LPS. 4. ORV preventing amyloid β25−35-induced neuron damage by decreasing Ca2+ and ROS levels. 5. PTS reducing MCAO/R induced cytokines. 6. TSG preventing β-amyloid-induced plaque deposition.
BV-2 microglial cells pretreated with 10 μM each of PTS, RSV, acetyl-trans-resveratrol (ARES), ORV and TSG were subjected to LPS exposure to induce inflammation. ORV was found to exhibit stronger antioxidant activity compared to RSV due to the presence of an extra hydroxyl group. ARES, however, showed less antioxidant activity compared to RSV due to the loss of three free hydroxyl groups. Macrophage polarization to M1 is a crucial process in the pathological process mediated by the NF-κB and JAK/STATs signaling pathways, which can be downregulated by RSV [99]. These M1 macrophages are proinflammatory cells, as they secrete iNOS and TNF-α. ORV is also able to prevent amyloid β25−35-induced rat cortical neuron damage by decreasing the cytosolic Ca2+ levels, glutamate and ROS [100] (Figure 7). Ampelopsin can inhibit LPS-induced neuroinflammation through NF-κB suppression and JAK2/STAT3 signaling in microglial cells [101]. In the LPS-stimulated macrophage and microglial cell model, ε-viniferin, a dehydrodimer of resveratrol, proved to be an excellent therapeutic agent for Alzheimer’s disease (AD), as it can disaggregate amyloid deposits, leading to inflammation [102]. Furthermore, it can decrease NO production by inducing nitric oxide synthase, which is a hallmark of inflammation in tissue [103]. Nitrite buildup is a sign that NO synthase activity is present. BV2 cells were cultured with PC 12 cells induced by LPS, and it was reported that the expression of inflammation mediators such as TLR4, MyD88, and NF-κB was significantly high [104]. BV2, when treated with the stilbene isolated from Bletilla striata (Thunb.) Rchb.f., can significantly reduce the NO production and thus the cytotoxicity [104]. Stilbenoid macasiamenene was isolated from M. siamensis S. J. Davies and its anti-inflammatory effect on monocyte and microglial cells induced by LPS was studied. These studies confirmed that it can interfere with IκB/NF-κB and MAPKs/AP-1 cascade reactions towards inflammation. Pre-, co- and post-treatment of BV2 microglial cells significantly reduced the secretion of the cytokines TNFα and IL-1β, which was monitored by their gene transcription and translation processes [21].
In stroke, there is a pathogenic function of inflammation due to neuronal ischemia and reperfusion damage (IRI) [105]. PTS can attenuate the effects of inflammation by means of the suppression of Nox2-related oxidative stress and activates the NLRP3 inflammasome [106]. In cases of cerebral ischemia-reperfusion after stroke, inflammation is mediated by astrocytes, which triggers nuclear factor (NF)-κB to express and secrete proinflammatory factors. Hippocampal neuronal HT22/astrocytoma U251 cells and cerebral artery occlusion-reperfusion were treated with PTS to study inflammation. The beneficial effect of PTS was reducing the oxidative stress in cells and subsequently reducing the mediators such as TNFα, IL-1β, and IL-6, while on other hand, PTS can boost antioxidant enzymatic activities such as superoxide dismutase and glutathione peroxidase [107]. Diabetic cognitive impairment is a neurodegenerative disease. PTS can reduce chronic neuroinflammation by suppressing oxidative and carbonyl stress and glial cell activation. It also reduces dopaminergic neuronal loss. It was particularly seen to affect the TLR4/NF-κB signaling pathway [108]. Stroke due to a subarachnoid hemorrhage can injure the olfactory bulb. PICE is seen to suppress cytokines such as TNF-α and IL-6, and components of the inflammatory pathways such as NF-κB and SIRT1, in a rat model of olfactory bulb damage [109].
β-secretase, or BACE1, is a protease involved in neuronal function. β-secretase is responsible for the production of toxic β-amyloid (Aβ), which has an impact on Alzheimer’s disease etiology [110]. RSV oligomers obtained from the Paeonia suffruticosa Andrews seed coat extract had a DPPH free-radical scavenging activity and could inhibit β-secretase. Vitisinol C, scirpusin, and miyabenol C exhibited anti-aggregative activity which reduces Aβ deposition [111]. β-amyloid protein-induced neuroinflammation was prevented by administering RSV, which activated the PI3-K (phosphatidylinositol-3-phosphate kinase) signaling pathway to inhibit apoptosis [112]. PTS is a strong neuromodulator in Alzheimer’s disease, and mediates elevated peroxisome proliferator-activated receptor (PPAR)-α expression [113]. Using mice with bilateral common carotid artery occlusion (BCCAO), PTS reduced the death of hippocampus neurons and the activation of microglia, TLR4 and the downstream cytokines. There was increased TLR4 and Triad3A–TLR4 interaction ubiquitination and degradation, which could be the key anti-inflammatory target [62].
Finally, TSG was found to prevent β-amyloid-induced senile plaque deposition [114]. Mice which were orally treated with APP/PS1 + TSG for 2 months showed a significant decrease in Aβ plaque. Here, 120 mg/kg TSG affected gene regulation, where 324 genes were upregulated, including those responsible for the immune system, antigen processes, cytokine response, apoptosis and NF-κB transcription. Meanwhile, 460 genes were downregulated, some of which were responsible for chromosome segregation, cell cycle and CNS myelination (Figure 7). TSG was also found to reduce TNFα, IL-1β, and IL-6 and increase TGFβ, CX3CR1 and iNOS (Gao et al., 2020). In BV2 cells, TSG decreased the levels of IL-5, CCL4/MIP1β, IL-10, IL-1β/IL-1F2, IL-18, IL-1β, IL-2, COX-2, GM-CSF, G-CSF, iNOS, CCL5/RANTES, TNFα, IL-3 and IL-4 [115].
In C57BL/6 mice, neuroinflammation was activated by myelin oligodendrocyte peptide (MOG35–55), causing the inflammatory cell influx of Th1 and Th17 to the CNS, with inflammation caused by CD4+ T helper cells and then followed by demyelination. Here, 100 mg/kg RSV administration for 15 days lowered the level of circulating pro-inflammatory cytokines [116]. Polyphenol-pretreated mouse neurons and astrocytes cells were exposed to Aβ42 and IL-1β, which allowed for the disaggregation of Aβ42. Here, 1 μM of RSV and trans ε-viniferin could reduce Aβ42 induced TNFα by 33% and 54%, respectively, and IL-6 by 25% and 40% [117].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28093786
References
- Fujiwara, N.; Kobayashi, K. Macrophages in Inflammation. Curr. Drug. Target. Inflamm. Allergy 2005, 4, 281–286.
- Chung, K.-O.; Kim, B.; Lee, M.; Kim, Y.; Chung, H.; Park, J.; Moon, J. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J. Pharm. Pharmacol. 2010, 55, 1695–1700.
- Lee, H.; Kim, D.; Hong, J.; Lee, J.-Y.; Kim, E. Oxyresveratrol suppresses lipopolysaccharide-induced inflammatory responses in murine macrophages. Hum. Exp. Toxicol. 2015, 34, 808–818.
- Liu, T.F.; McCall, C.E. Deacetylation by SIRT1 Reprograms Inflammation and Cancer. Genes. Cancer 2013, 4, 135–147.
- Ali, M.; Gupta, M.; Wani, A.; Sharma, A.; Abdullaha, M.; Kour, D.; Choudhary, S.; Bharate, S.B.; Singh, G.; Kumar, A. IIIM-941, a Stilbene Derivative Inhibits NLRP3 Inflammasome Activation by Inducing Autophagy. Front. Pharmacol. 2021, 12.
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 7381.
- Damsker, J.M.; Hansen, A.M.; Caspi, R.R. Th1 and Th17 cells: Adversaries and collaborators. Ann. N. Y. Acad. Sci. 2010, 1183, 211–221.
- Lim, K.G.; Gray, A.I.; Anthony, N.G.; Mackay, S.P.; Pyne, S.; Pyne, N.J. Resveratrol and its oligomers: Modulation of sphingolipid metabolism and signaling in disease. Arch. Toxicol. 2014, 88, 12.
- Tian, F.; Wei, H.; Jia, T.; Tian, H. An improved highly sensitive method to determine low oxyresveratrol concentrations in rat plasma and its pharmacokinetic application. Biomed. Chromatogr. 2014, 28, 5.
- Fonseca-Kelly, Z.; Nassrallah, M.; Uribe, J.; Khan, R.S.; Dine, K.; Dutt, M.; Shindler, K.S. Resveratrol Neuroprotection in a Chronic Mouse Model of Multiple Sclerosis. Front. Neurol. 2012, 3, 84.
- Singh, U.P.; Singh, N.P.; Singh, B.; Hofseth, L.J.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-κB activation to abrogate dextran sulfate sodium-induced colitis. J. Pharmacol. Exp. Ther. 2010, 332, 3.
- Zou, T.; Yang, Y.; Xia, F.; Huang, A.; Gao, X.; Fang, D. Resveratrol Inhibits CD4+ T Cell Activation by Enhancing the Expression and Activity of Sirt1. PLoS ONE 2013, 8, e75139.
- Howitz, K.T.; Bitterman, K.; Cohen, H. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196.
- Vaquero, A.; Scher, M.; Lee, D.; Erdjument-Bromage, H.; Tempst, P.; Reinberg, D. Human SirT1 Interacts with Histone H1 and Promotes Formation of Facultative Heterochromatin. Mol. Cell 2004, 16, 93–105.
- Fuloria, S.; Sekar, M.; Khattulanuar, F.S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Subramaniyan, V.; Jeyabalan, S.; Begum, M.Y.; Chidambaram, K.; et al. Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy. Molecules 2022, 27, 5072.
- Tsai, C.-F.; Wang, K.-T.; Chen, L.-G.; Lee, C.-J.; Tseng, S.-H.; Wang, C.-C. Anti-Inflammatory Effects of Vitis thunbergii var. taiwaniana on Knee Damage Associated with Arthritis. J. Med. Food 2014, 17, 479–486.
- Song, J.; Jun, M.; Ahn, M.R.; Kim, O.Y. Involvement of miR-let7A in inflammatory response and cell survival/apoptosis regulated by resveratrol in THP-1 macrophage. Nutr. Res. Pract. 2016, 10, 377–384.
- Park, H.S.; Vick, E.J.; Gao, Y.; He, C.; Almosnid, N.M.; Farone, M.; Farone, A.L. cis- and trans-gnetin H from Paeonia suffruticosa suppress inhibitor kappa B kinase phosphorylation in LPS-stimulated human THP-1 cells. J. Ethnopharmacol. 2016, 189, 202–209.
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Kiselova-Kaneva, Y.; Galunska, B.; Nogueiras, R.; Ivanova, D. Phytochemical composition, anti-inflammatory and er stress-reducing potential of Sambucus ebulus L. Fruit extract. Plants 2021, 10, 2446.
- Schueller, K.; Pignitter, M.; Somoza, V. Sulfated and Glucuronated trans-Resveratrol Metabolites Regulate Chemokines and Sirtuin-1 Expression in U-937 Macrophages. J. Agric. Food Chem. 2015, 63, 6535–6545.
- Leláková, V.; Béraud-Dufour, S.; Hošek, J.; Šmejkal, K.; Prachyawarakorn, V.; Pailee, P.; Widmann, C.; Václavík, J.; Coppola, T.; Mazella, J.; et al. Therapeutic potential of prenylated stilbenoid macasiamenene F through its anti-inflammatory and cytoprotective effects on LPS-challenged monocytes and microglia. J. Ethnopharmacol. 2020, 263, 113147.
- Zhou, C.; Zhang, X.; Ruan, C.C.; Cheang, W.S. Two methoxy derivatives of resveratrol, 3,3′,4,5′-tetramethoxy-trans-stilbene and 3,4′,5-trimethoxy-trans-stilbene, suppress lipopolysaccharide-induced inflammation through inactivation of MAPK and NF-κB pathways in RAW 264.7 cells. Chin. Med. 2021, 16, 1–14.
- Hankittichai, P.; Lou, H.J.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Oxyresveratrol inhibits IL-1β-induced inflammation via suppressing AKT and ERK1/2 activation in human microglia, HMC3. Int. J. Mol. Sci. 2020, 21, 6054.
- Hornedo-Ortega, R.; Jourdes, M.; Da Costa, G.; Courtois, A.; Gabaston, J.; Teissedre, P.-L.; Richard, T.; Krisa, S. Oxyresveratrol and Gnetol Glucuronide Metabolites: Chemical Production, Structural Identification, Metabolism by Human and Rat Liver Fractions, and in Vitro Anti-inflammatory Properties. J. Agric. Food Chem. 2022, 70, 41.
- Remsberg, C.M.; Martinez, S.E.; Akinwumi, B.C.; Anderson, H.D.; Takemoto, J.K.; Sayre, C.L.; Davies, N.M. Preclinical Pharmacokinetics and Pharmacodynamics and Content Analysis of Gnetol in Foodstuffs. Phytother. Res. 2015, 29, 1168–1179.
- Kloypan, C.; Jeenapongsa, R.; Sri-in, P.; Chanta, S.; Dokpuang, D.; Tip-pyang, S.; Surapinit, N. Stilbenoids from Gnetum macrostachyum attenuate human platelet aggregation and adhesion. Phytother. Res. 2012, 26, 1564–1568.
- Aja-Perez, I.; Krisa, S.; Hornedo-Ortega, R.; Begoña Ruiz-Larrea, M.; Ruiz-Sanz, J.I.; Richard, T.; Courtois, A. Stilbenes at Low Micromolar Concentrations Mitigate the NO, TNF-α, IL-1β and ROS Production in LPS-Stimulated Murine Macrophages. J. Biol. Act. Prod. Nat. 2021, 11, 3.
- Son, Y.; Chung, H.T.; Pae, H.O. Differential effects of resveratrol and its natural analogs, piceatannol and 3,5,4′-trans-trimethoxystilbene, on anti-inflammatory heme oxigenase-1 expression in RAW264.7 macrophages. BioFactors 2014, 40, 138–145.
- Kim, D.-H.; Lee, Y.-G.; Park, H.-J.; Lee, J.-A.; Kim, H.J.; Hwang, J.-K.; Choi, J.-M. Piceatannol inhibits effector T cell functions by suppressing TcR signaling. Int. Immunopharmacol. 2015, 25, 285–292.
- Zhou, Y.; Khan, H.; Hoi, M.P.M.; Cheang, W.S. Piceatannol Protects Brain Endothelial Cell Line (bEnd.3) against Lipopolysaccharide-Induced Inflammation and Oxidative Stress. Molecules 2022, 27, 1206.
- Hung, N.Q.; Anh, N.T.H.; Khang, N.S.; Huong, N.T.T.; Luyenb, N.T.; Hau, D.V.; Dat, N.T. Undescribed chalcone and stilbene constituents from Lysimachia baviensis and their anti-inflammatory effect. Nat. Prod. Res. 2021, 37, 1138–1145.
- Xue, Y.-R.; Yao, S.; Liu, Q.; Peng, Z.-L.; Deng, Q.-Q.; Liu, B.; Ma, Z.-H.; Wang, L.; Zhou, H.; Ye, Y.; et al. Dihydro-stilbene gigantol relieves CCl4-induced hepatic oxidative stress and inflammation in mice via inhibiting C5b-9 formation in the liver. Acta Pharmacol. Sin. 2020, 41, 11.
- Hsu, C.L.; Lin, Y.J.; Ho, C.T.; Yen, G.C. The inhibitory effect of pterostilbene on inflammatory responses during the interaction of 3T3-L1 adipocytes and RAW 264.7 macrophages. J. Agric. Food Chem. 2013, 61, 602–610.
- Qi, S.; Xin, Y.; Guo, Y.; Diao, Y.; Kou, X.; Luo, L.; Yin, Z. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int. Immunopharmacol. 2012, 12, 278–287.
- Chang, Y.; Yuan, L.; Liu, J.; Muhammad, I.; Cao, C.; Shi, C.; Zhang, Y.; Li, R.; Li, C.; Liu, F. Dihydromyricetin attenuates Escherichia coli lipopolysaccharide-induced ileum injury in chickens by inhibiting NLRP3 inflammasome and TLR4/NF-κB signalling pathway. Vet. Res. 2020, 51, 1–12.
- Hou, L.; Jiang, F.; Huang, B.; Zheng, W.; Jiang, Y.; Cai, G.; Liu, D.; Hu, C.Y.; Wang, C. Dihydromyricetin resists inflammation-induced muscle atrophy via ryanodine receptor-CaMKK-AMPK signal pathway. J. Cell Mol. Med. 2021, 25, 21.
- Ko, Y.-J.; Kim, H.; Kim, E.; Katakura, Y.; Lee, W.; Kim, G.; Ryu, C. Piceatannol inhibits mast cell-mediated allergic inflammation. Int. J. Mol. Med. 2013, 31, 951–958.
- Kim, D.-H.; Park, E.-K.; Bae, E.-A.; Han, M.J. Metabolism of Rhaponticin and Chrysophanol 8-o-.BETA.-D-Glucopyranoside from the Rhizome of Rheum undulatum by Human Intestinal Bacteria and Their Anti-allergic Actions. Biol. Pharm. Bull. 2000, 23, 830–833.
- Choi, S.Z.; Lee, S.O.; Jang, K.U.; Chung, S.H.; Park, S.H.; Kang, H.C.; Yang, E.Y.; Cho, H.J.; Lee, K.R. Antidiabetic stilbene and anthraquinone derivatives from Rheum. undulatum. Arch. Pharm. Res. 2005, 28, 9.
- Labib, R.M.; Malak, L.G.; Youssef, F.S.; Ross, S.A. A new stilbene from Agonis flexuosa leaves and verification of its histamine release inhibitory activity using in silico and in vitro studies. S. Afr. J. Bot. 2020, 135, 384–390.
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171.
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016, 13, 267–276.
- Morgan, B.P. The membrane attack complex as an inflammatory trigger. Immunobiology 2016, 221, 6.
- Fernandes, J.C.; Schemitt, E.G.; Da Silva, J.; Marroni, N.P.; Lima, A.; Ferreira, R.B. Combination of trans-resveratrol and ε-viniferin induces a hepatoprotective effect in rats with severe acute liver failure via reduction of oxidative stress and MMP-9 expression. Nutrients 2021, 13, 3677.
- Pham, C.G.; Bubici, C.; Zazzeroni, F.; Papa, S.; Jones, J.; Alvarez, K.; Jayawardena, S.; De Smaele, E.; Cong, R.; Beaumont, C.; et al. Ferritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell 2004, 119, 649–661.
- Jang, S.; Yu, L.-R.; Abdelmegeed, M.A.; Gao, Y.; Banerjee, A.; Song, B.-J. Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015, 6, 552–564.
- Xue, Y.; Deng, Q.; Zhang, Q.; Ma, Z.; Chen, B.; Yu, X.; Peng, H.; Yao, S.; Liu, J.; Ye, Y.; et al. Gigantol ameliorates CCl4-induced liver injury via preventing activation of JNK/cPLA2/12-LOX inflammatory pathway. Sci. Rep. 2020, 10, 1–13.
- Lin, D.; Sun, Z.; Jin, Z.; Lei, L.; Liu, Y.; Hu, B.; Wang, B.; Shen, Y.; Wang, Y. Matrix Remodeling Associated 7 Deficiency Alleviates Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Front. Immunol. 2018, 9, 773.
- Zhan, J.; Hu, T.; Shen, J.; Yang, G.; Ho, C.T.; Li, S. Pterostilbene is more efficacious than hydroxystilbenes in protecting liver fibrogenesis in a carbon tetracholride-induced rat model. J. Funct. Foods 2021, 84, 104604.
- Beaumont, P.; Courtois, A.; Atgié, C.; Richard, T.; Krisa, S. Correction to: In the shadow of resveratrol: Biological activities of epsilon-viniferin. J. Physiol. Biochem. 2022, 78, 465–484.
- Han, M.; Zhang, T.; Gu, W.; Yang, X.; Zhao, R.; Yu, J. 2,3,5,4′-tetrahydroxy-stilbene-2-O-β-D-glucoside attenuates methionine and choline-deficient diet-induced non-alcoholic fatty liver disease. Exp. Ther. Med. 2018, 16, 2.
- Feng, Y.; Cui, R.; Li, Z.; Zhang, X.; Jia, Y.; Zhang, X.; Shi, J.; Qu, K.; Liu, C.; Zhang, J. Methane Alleviates Acetaminophen-Induced Liver Injury by Inhibiting Inflammation, Oxidative Stress, Endoplasmic Reticulum Stress, and Apoptosis through the Nrf2/HO-1/NQO1 Signaling Pathway. Oxid. Med. Cell Longev. 2019, 2019, 7067619.
- Jia, Y.N.; Peng, Y.L.; Zhao, Y.P.; Cheng, X.F.; Zhou, Y.; Chai, C.L.; Zeng, L.S.; Pan, M.H.; Xu, L. Comparison of the Hepatoprotective Effects of the Three Main Stilbenes from Mulberry Twigs. J. Agric. Food Chem. 2019, 67, 7245.
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium oxide nanoparticles inhibits oxidative stress and nuclear Factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61.
- Zou, Y.; Kong, M. Tetrahydroxy stilbene glucoside alleviates palmitic acid-induced inflammation and apoptosis in cardiomyocytes by regulating miR-129-3p/Smad3 signaling. Cell Mol. Biol. Lett. 2019, 24, 5.
- Boccellino, M.; Donniacuo, M.; Bruno, F.; Rinaldi, B.; Quagliuolo, L.; Ambruosi, M.; Pace, S.; De Rosa, M.; Olgaç, A.; Banoglu, E.; et al. Protective effect of piceatannol and bioactive stilbene derivatives against hypoxia-induced toxicity in H9c2 cardiomyocytes and structural elucidation as 5-LOX inhibitors. Eur. J. Med. Chem. 2019, 180, 637–647.
- Choi, K.H.; Kim, J.-E.; Song, N.R.; Son, J.E.; Hwang, M.K.; Byun, S.; Kim, J.H.; Lee, K.W.; Lee, H.J. Phosphoinositide 3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc. Res. 2010, 85, 836–844.
- Seow, C.J.; Chue, S.C.; Wong, W.S.F. Piceatannol, a Syk-selective tyrosine kinase inhibitor, attenuated antigen challenge of guinea pig airways in vitro. Eur. J. Pharmacol. 2002, 443, 189–196.
- Nchiozem-Ngnitedem, V.A.; Omosa, L.K.; Bedane, K.G.; Derese, S.; Brieger, L.; Strohmann, C.; Spiteller, M. Anti-inflammatory steroidal sapogenins and a conjugated chalcone-stilbene from Dracaena usambarensis Engl. Fitoterapia 2020, 146, 104717.
- Liudvytska, O.; Ponczek, M.B.; Ciesielski, O.; Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Balcerczyk, A.; Kolodziejczyk-Czepas, J. Rheum rhaponticum and Rheum rhabarbarum Extracts as Modulators of Endothelial Cell Inflammatory Response. Nutrients 2023, 15, 949.
- Ma, P.; Yue, L.; Yang, H.; Fan, Y.; Bai, J.; Li, S.; Yuan, J.; Zhang, Z.; Yao, C.; Lin, M.; et al. Chondroprotective and anti-inflammatory effects of amurensin H by regulating TLR4/Syk/NF-κB signals. J. Cell Mol. Med. 2020, 24, 2.
- Hu, X.B.; Kang, R.R.; Tang, T.T.; Li, Y.J.; Wu, J.Y.; Wang, J.M.; Liu, X.Y.; Xiang, D.X. Topical delivery of 3,5,4′-trimethoxy-trans-stilbene-loaded microemulsion-based hydrogel for the treatment of osteoarthritis in a rabbit model. Drug. Deliv. Transl. Res. 2019, 9, 357–365.
- Wang, K.T.; Chen, L.G.; Tseng, S.H.; Huang, J.S.; Hsieh, M.S.; Wang, C.C. Anti-inflammatory effects of resveratrol and oligostilbenes from Vitis thunbergii var. taiwaniana against lipopolysaccharide-induced arthritis. J. Agric. Food Chem. 2011, 59, 8.
- Tran, P.T.; Park, D.H.; Kim, O.; Kwon, S.H.; Min, B.S.; Lee, J.H. Desoxyrhapontigenin inhibits RANKL-induced osteoclast formation and prevents inflammation-mediated bone loss. Int. J. Mol. Med. 2018, 42, 569–578.
- Susztak, K.; Raff, A.C.; Schiffer, M.; Böttinger, E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006, 55, 894.
- Navarro, J.; Morafernandez, C. The role of TNF-α in diabetic nephropathy: Pathogenic and therapeutic implications. Cytokine Growth Factor. Rev. 2006, 17, 441–450.
- Vielhauer, V.; Mayadas, T.N. Functions of TNF and its Receptors in Renal Disease: Distinct Roles in Inflammatory Tissue Injury and Immune Regulation. Semin. Nephrol. 2007, 27, 286–308.
- Lee, I.-T.; Yang, C.-M. Inflammatory Signalings Involved in Airway and Pulmonary Diseases. Mediat. Inflamm. 2013, 2013, 791231.
- Lee, I.T.; Lin, C.F.; Huang, Y.L.; Chong, K.Y.; Hsieh, M.F.; Huang, T.H.; Cheng, C.Y. Protective mechanisms of resveratrol derivatives against TNF-α-induced inflammatory responses in rat mesangial cells. Cytokine 2019, 113, 380–392.
- Chen, L.; Lan, Z.; Lin, Q.; Mi, X.; He, Y.; Wei, L.; Lin, Y.; Zhang, Y.; Deng, X. Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem. Toxicol. 2013, 52, 28–35.
- Lee, I.T.; Lin, H.-C.; Huang, T.-H.; Tseng, C.-N.; Cheng, H.-T.; Huang, W.-C.; Cheng, C.-Y. Anti-Inflammatory Effect of Resveratrol Derivatives via the Downregulation of Oxidative-Stress-Dependent and c-Src Transactivation EGFR Pathways on Rat Mesangial Cells. Antioxidants 2022, 11, 5.
- Xie, X.; Peng, J.; Huang, K.; Huang, J.; Shen, X.; Liu, P.; Huang, H. Polydatin ameliorates experimental diabetes-induced fibronectin through inhibiting the activation of NF-κB signaling pathway in rat glomerular mesangial cells. Mol. Cell Endocrinol. 2012, 362, 183–193.
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 5963.
- Nunes, T.; De Souza, H.S. Inflammasome in intestinal inflammation and cancer. Mediat. Inflamm. 2013, 2013, 654963.
- Li, J.; Wang, B.; Zhou, G.; Yan, X.; Zhang, Y. Tetrahydroxy Stilbene Glucoside Alleviates High Glucose-Induced MPC5 Podocytes Injury Through Suppression of NLRP3 Inflammasome. Am. J. Med. Sci. 2018, 355, 588–596.
- Wang, P.-X.; Deng, X.-R.; Zhang, C.-H.; Yuan, H.-J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816.
- Gomes, M.J.C.; Kolba, N.; Agarwal, N.; Kim, D.; Eshel, A.; Koren, O.; Tako, E. Modifications in the intestinal functionality, morphology and microbiome following intra-amniotic administration (Gallus gallus) of grape (Vitis vinifera) stilbenes (resveratrol and pterostilbene). Nutrients 2021, 13, 3247.
- Fan-Jiang, P.Y.; Lee, P.S.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Pterostilbene Attenuates High-Fat Diet and Dextran Sulfate Sodium-Induced Colitis via Suppressing Inflammation and Intestinal Fibrosis in Mice. J. Agric. Food Chem. 2021, 69, 25.
- Shin, S.-H.; Song, J.-L.; Park, M.-G.; Park, M.-H.; Hwang, S.-J.; Park, K.-Y. Effects of natural raw meal (NRM) on high-fat diet and dextran sulfate sodium (DSS)-induced ulcerative colitis in C57BL/6J mice. Nutr. Res. Pract. 2015, 9, 619.
- Sireesh, D.; Ganesh, M.-R.; Dhamodharan, U.; Sakthivadivel, M.; Sivasubramanian, S.; Gunasekaran, P.; Ramkumar, K.M. Role of pterostilbene in attenuating immune mediated devastation of pancreatic beta cells via Nrf2 signaling cascade. J. Nutr. Biochem. 2017, 44, 11–21.
- Ha, S.K.; Kang, M.C.; Lee, S.; Darlami, O.; Shin, D.; Choi, I.; Kim, K.H.; Kim, S.Y. Generation of stilbene glycoside with promising cell rejuvenation activity through biotransformation by the entomopathogenic fungus Beauveria bassiana. Biomedicines 2021, 9, 555.
- Heinzl, G.C.; Iametti, S.; Mattio, L.M.; Pinto, A.; Dallavalle, S.M.D.; Capraro, J.; Scarafoni, A. Polymeric Stilbene Derivatives in Winemaking Byproducts Affect NF-kB Mediated Inflammatory Response in Caco-2 Cells. SIB Congr. 2021. Available online: https://air.unimi.it/handle/2434/871256 (accessed on 15 April 2023).
- Kubyshkin, A.; Shevandova, A.; Petrenko, V.; Fomochkina, I.; Sorokina, L.; Kucherenko, A.; Gordienko, A.; Khimich, N.; Zyablitskaya, E.; Makalish, T.; et al. Anti-inflammatory and antidiabetic effects of grape-derived stilbene concentrate in the experimental metabolic syndrome. J. Diabetes Metab. Disord. 2020, 19, 1205–1214.
- Zhang, L.; Liu, H.; Qin, L.; Zhang, Z.; Wang, Q.; Zhang, Q.; Lu, Z.; Wei, S.; Gao, X.; Tu, P. Global chemical profiling based quality evaluation approach of rhubarb using ultra performance liquid chromatography with tandem quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2015, 38, 511–522.
- Kotlyarov, S.; Kotlyarova, A. Molecular Mechanisms of Lipid Metabolism Disorders in Infectious Exacerbations of Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2021, 22, 7634.
- Fan, Y.; Zhang, Z.; Yao, C.; Bai, J.; Yang, H.; Ma, P.; Fan, Y.; Li, S.; Yuan, J.; Lin, M.; et al. Amurensin H, a derivative from resveratrol, ameliorates lipopolysaccharide/cigarette smoke-induced airway inflammation by blocking the Syk/NF-κB pathway. Front. Pharmacol. 2019, 10, 1157.
- Shi, J.; Yin, N.; Xuan, L.L.; Yao, C.S.; Meng, A.M.; Hou, Q. Vam3, a derivative of resveratrol, attenuates cigarette smoke-induced autophagy. Acta Pharmacol. Sin. 2012, 33, 888–896.
- de Oliveira, M.T.P.; Coutinho, D.d.S.; Guterres, S.S.; Pohlmann, A.R.; e Silva, P.M.R.; Martins, M.A.; Bernardi, A. Resveratrol-loaded lipid-core nanocapsules modulate acute lung inflammation and oxidative imbalance induced by lps in mice. Pharmaceutics 2021, 13, 683.
- Da Silva, T.F.; Da Silva, T.F.; Teles, S.S.D.S.; De Assis, T.S.; Junior, M.S.O. Approaches from Resveratrol Activities on Central Nervous System Inflammation. Biotechnol. Res. 2019, 5, 28–34. Available online: http://br.biomedpress.org/index.php/br/article/view/783 (accessed on 15 April 2023).
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF Transcription Factors, Inflammation, and Immunity. Immunity 2014, 41, 518–528.
- Koronowski, K.B.; Dave, K.R.; Saul, I.; Camarena, V.; Thompson, J.W.; Neumann, J.T.; Young, J.I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Induces a Novel Extended Window of Ischemic Tolerance in the Mouse Brain. Stroke 2015, 46, 2293–2298.
- Sharma, S.; Chopra, K.; Kulkarni, S.K.; Agrewala, J.N. Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin. Exp. Immunol. 2007, 147, 155–163.
- Singh, N.P.; Hegde, V.L.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol. Pharmacol. 2007, 72, 1508–1521.
- Imler, T.J.; Petro, T.M. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+IL-10+ T cells, CD4- IFN-γ+ cells, and decreased macrophage IL-6 expression. Int. Immunopharmacol. 2009, 9, 134–143.
- Ates, O.; Cayli, S.; Altinoz, E.; Gurses, I.; Yucel, N.; Kocak, A.; Yologlu, S.; Turkoz, Y. Effects of resveratrol and methylprednisolone on biochemical, neurobehavioral and histopathological recovery after experimental spinal cord injury. Acta Pharmacol. Sin. 2006, 27, 1317–1325.
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172.
- Švajger, U.; Obermajer, N.; Jeras, M. Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology 2010, 129, 525–535.
- Wight, R.D.; Tull, C.A.; Deel, M.W.; Stroope, B.L.; Eubanks, A.G.; Chavis, J.A.; Drew, P.D.; Hensley, L.L. Resveratrol effects on astrocyte function: Relevance to neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2012, 426, 112–115.
- Wang, L.; Zhao, H.; Wang, L.; Tao, Y.; Du, G.; Guan, W.; Liu, J.; Brennan, C.; Ho, C.-T.; Li, S. Effects of Selected Resveratrol Analogues on Activation and Polarization of Lipopolysaccharide-Stimulated BV-2 Microglial Cells. J. Agric. Food Chem. 2020, 68, 3750–3757.
- Ban, J.Y.; Jeon, S.-Y.; Nguyen, T.T.H.; Bae, K.; Song, K.-S.; Seonga, Y.H. Neuroprotective Effect of Oxyresveratrol from Smilacis Chinae Rhizome on Amyloid β Protein (25-35)-Induced Neurotoxicity in Cultured Rat Cortical Neurons. Biol. Pharm. Bull. 2006, 29, 2419–2424.
- Weng, L.; Zhang, H.; Li, X.; Zhan, H.; Chen, F.; Han, L.; Xu, Y.; Cao, X. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-κB and JAK2/STAT3 signaling pathways in microglia. Int. Immunopharmacol. 2017, 44, 1–8.
- Caillaud, M.; Guillard, J.; Richard, D.; Milin, S.; Chassaing, D.; Paccalin, M.; Page, G.; Bilan, A.R. Trans ε viniferin decreases amyloid deposits and inflammation in a mouse transgenic Alzheimer model. PLoS ONE 2019, 14, e0212663.
- Ha, D.T.; Long, P.T.; Hien, T.T.; Tuan, D.T.; An, N.T.T.; Khoi, N.M.; Oanh, H.V.; Hung, T.M. Anti-inflammatory effect of oligostilbenoids from Vitis heyneana in LPS-stimulated RAW 264.7 macrophages via suppressing the NF-ΚB activation. Chem. Cent. J. 2018, 12, 1.
- Zhou, D.; Chang, W.; Liu, B.; Chen, G.; Yang, Y.; Hao, Y.; Hou, Y.; Li, N. Stilbenes from the tubers of Bletilla striata with potential anti-neuroinflammatory activity. Bioorg. Chem. 2020, 97, 103715.
- Petrovic-Djergovic, D.; Goonewardena, S.N.; Pinsky, D.J. Inflammatory disequilibrium in stroke. Circ. Res. 2016, 119, 142–158.
- Liu, H.; Zhao, L.; Yue, L.; Wang, B.; Li, X.; Guo, H.; Ma, Y.; Yao, C.; Gao, L.; Deng, J.; et al. Pterostilbene Attenuates Early Brain Injury Following Subarachnoid Hemorrhage via Inhibition of the NLRP3 Inflammasome and Nox2-Related Oxidative Stress. Mol. Neurobiol. 2017, 54, 5928–5940.
- Liu, H.; Wu, X.; Luo, J.; Wang, X.; Guo, H.; Feng, D.; Zhao, L.; Bai, H.; Song, M.; Liu, X.; et al. Pterostilbene attenuates astrocytic inflammation and neuronal oxidative injury after ischemia-reperfusion by inhibiting nf-κb phosphorylation. Front. Immunol. 2019, 10, 2408.
- Zhang, Z.; Deng, S.-M.; Chen, C.; He, Q.-H.; Peng, X.-W.; Liang, Q.-F.; Zhuang, G.-D.; Wang, S.-M.; Tang, D. Pterostilbene could alleviate diabetic cognitive impairment by suppressing TLR4/NF-κB pathway through microbiota-gut-brain axis. Phytother. Res. 2023, in press.
- Akar, A.; Öztopuz, R.Ö.; Büyük, B.; Ovali, M.A.; Aykora, D.; Malçok, Ü.A. Neuroprotective Effects of Piceatannol on Olfactory Bulb Injury after Subarachnoid Hemorrhage. Mol. Neurobiol. 2023.
- Mamada, N.; Tanokashira, D.; Ishii, K.; Tamaoka, A.; Araki, W. Mitochondria are devoid of amyloid β-protein (Aβ)-producing secretases: Evidence for unlikely occurrence within mitochondria of Aβ generation from amyloid precursor protein. Biochem. Biophys. Res. Commun. 2017, 486, 321–328.
- Choi, C.W.; Choi, Y.H.; Cha, M.-R.; Kim, Y.S.; Yon, G.H.; Hong, K.S.; Park, W.-K.; Kim, Y.H.; Ryu, S.Y. In vitro BACE-1 inhibitory activity of resveratrol oligomers from the seed extract of Paeonia lactiflora. Planta Med. 2011, 77, 374–376.
- Han, Y.S.; Zheng, W.H.; Bastianetto, S.; Chabot, J.G.; Quirion, R. Neuroprotective effects of resveratrol against β-amyloid-induced neurotoxicity in rat hippocampal neurons: Involvement of protein kinase C. Br. J. Pharmacol. 2004, 141, 997.
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joseph, J.A.; Casadesus, G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 2062–2071.
- Luo, H.; Yun, L.; Jiankui, G.; Zunjing, L.; Zhiqiang, Z.; Yong, W.; Zhao, L.; Xiangqun, S. Tetrahydroxy stilbene glucoside improved the behavioral disorders of APP695V717I transgenic mice by inhibiting the expression of Beclin-1 and LC3-II. J. Tradit. Chin. Med. 2015, 35, 295–300.
- Gao, Y.; Li, J.; Li, J.; Hu, C.; Zhang, L.; Yan, J.; Li, L.; Zhang, L. Tetrahydroxy stilbene glycoside alleviated inflammatory damage by mitophagy via AMPK related PINK1/Parkin signaling pathway. Biochem. Pharmacol. 2020, 177, 113997.
- Gandy, K.A.O.; Zhang, J.; Nagarkatti, P.; Nagarkatti, M. Resveratrol (3, 5, 4′-Trihydroxy-trans-Stilbene) Attenuates a Mouse Model of Multiple Sclerosis by Altering the miR-124/Sphingosine Kinase 1 Axis in Encephalitogenic T Cells in the Brain. J. Neuroimmune Pharmacol. 2019, 14, 477.
- Vion, E.; Page, G.; Bourdeaud, E.; Paccalin, M.; Guillard, J.; Bilan, A.R. Trans ε-viniferin is an amyloid-β disaggregating and anti-inflammatory drug in a mouse primary cellular model of Alzheimer’s disease. Mol. Cell Neurosci. 2018, 88, 1–6.
This entry is offline, you can click here to edit this entry!
