Liver cancer is a malignancy that includes the structure of the liver and intrahepatic bile ducts and is classified as follows: hepatocellular carcinoma, cholangiocarcinoma, hepatic angiosarcoma (hemangiosarcoma), hepatoblastoma, and fibrolamellar carcinoma. HCC, known also as hepatoma, is the most common type of primary liver neoplasm. HCC is known today as a major malignant disease with high morbidity and mortality, which seriously threatens the health and life span of patients. Conventional cancer therapies comprise surgical procedures, radiation therapy and chemotherapy, which may be efficient but have significant adverse effects. Newer, more prominent cancer-targeted action plans, such as the phototherapy methods including photodynamic therapy (PDT), photothermal therapy (PTT), and photoimmunotherapy (PIT), have sparked excitement and hope as synergistic multimodal cancer therapies, incorporating nanomedicine to overcome the biological barriers and to help treat cancer patients.
1. Photodynamic Therapy
Light as a remedy dates back to prehistoric times, as ancient civilizations used to treat various disorders with light, including skin cancer. The implementation of “light” (visible, near-infrared (NIR) and ultraviolet (UV), as non-ionizing electromagnetic radiation) through the teamwork of photobiologists and clinicians in medical practice, which would include the elucidation of the cause of diseases, their prophylaxis and not only their diagnosis and treatment, but also for the preparation of pharmaceutical products, should be used more and more for the good of humanity, rather than just as an act or end in itself [
63,
64].
In the context of the high incidence of cancer worldwide, state-of-the-art PDT has entered as a standardized protocol and a minimally invasive procedure in the attempt to eradicate cancer. Some key moments along this long path were the accidental rediscovery at the beginning of the 20th century of the light-mediated killing effect in the presence of molecular oxygen on a unicellular protist incubated with acridine, the remarkable applications of phototherapy by Niels Finsen, for which he won the Nobel Prize in 1903, the definition of the phenomenon by Herman von Tappeiner as “photodynamic action”, and the development of photosensitizers (PSs), which started with Photofrin, as the most widely implemented PS worldwide [
64,
65].
PDT is clinically authorized for certain neoplasms and consists of the systemic or local administration of a non-toxic substance called a photosensitizer (PS) that should accumulate in the target tissue, which by illumination with a certain wavelength and appropriate energy in the presence of intracellular molecular oxygen will trigger PS photoexcitation and generate reactive oxygen species (ROS), initiating a cytotoxic effect. The intrinsic mechanism is that PS excited at certain wavelengths of the applied light source, in the presence of oxygen, releases more free radicals and various oxidation products with high cytotoxic potential, which will lead to cell death in irradiated cancer tissues. The wavelength of light must excite PS and it immediately reacts in the presence of oxygen with a nucleic acid, protein or unsaturated lipid from the underlying layer, and generates radicals that are not stable by proton or electron transfer, as a result of electron extraction or relocation to another molecule and/or ROS (type I reaction), for example the superoxide (•O
−2), the hydroxyl radical (•OH) or hydrogen peroxide (H
2O
2); or, through energy transfer (type II reaction), the PS may be generated in the presence of molecular oxygen, singlet oxygen, also named dioxygen (singlet) or dioxidene O=O (
1O
2), which is very reactive, an important cytotoxin at high oxygen concentrations in this procedure. The ratio between type I and type II photoreactions is oxygen-dependent, involves unstable species and depends on the PS concentration, the amount of irradiation energy and it can be said that the whole process of ROS generation and tumor destruction is not yet fully understood, due to the complexity and the diversity of the degradation pathways of the most significant biomolecules upon one-electron oxidation and singlet oxygen reactions. In summary, the operating phases of PDT are as follows: (a) the administration of PS in the absence of radiation by a systemic or by a topical route; (b) when a sufficient amount of PS has already penetrated the cancer cells, the PS is photoactivated in a distinct time period, during which it reaches higher excited states, but without destroying the adjacent healthy tissue; (c) the next phase of relaxation includes generated ROS, which will kill the targeted unhealthy cells. The hit of the PDT depends on various factors, such as the deepness of light piercing into the tissue, the best assimilation of PS by the unhealthy cells, as well as the photophysical and photochemical properties of PS. Concerning the depth of light into the tissues, the absorption and scattering take place at once and are directly dependent on the wavelength applied, so PS picking up photons on the “windows of optical transparency” of those tissues will trigger deeper penetration and less attenuation during the illumination step. Conventional PS is excited usually by a higher energy (UV–Vis), but for deeper tumors, an active and safe upconverter PS that absorbs light in NIR, and releases photons in the visible and NIR ranges is required. For ideal PS, the demand for nanomaterials with powerful photoluminescence emissions and relatively long luminescence decay lifetimes (µs to ms), high sensitiveness and easy preparation is a continuous research effort [
65,
66,
67]. Along the evolution of research and experiments on the use of light in photomedicine and the development of PDT, three generations of PSs can be distinguished, among which the last, that is, the third generation, includes compounds coupled to tumor-selective monoclonal antibodies for better pharmacokinetics and specificity with selective targeting and delayed delivery. In the latest trends, natural compounds (e.g., curcumin) along with nanotechnologies have increased clinical efficacy in tumor therapy [
65].
PDT is a procedure controlled by several factors and normally involves the death of cancer cells, damage to tumor blood vessels, initiation of an antitumor immune response, and a host of inflammatory reactions in the tissue exposed to photodynamic action. Many chemicals with photosensitizing properties have been researched and there is a current trend for the study and introduction of natural products, which in relation to synthetic chemicals are accounted as “green” [
14,
68].
PDT can induce cancer cell death via an interplay between apoptosis, necrosis and autophagy, which can concurrently take place depending on the genetic constitution of the cells, the class or concentration of PS, its intracellular location, the PDT dose and the tissue’s partial pressure of oxygen. In recent years, several other categories of cell death have been reported as atypical pathways such as paraptosis, parthanatos, mitotic catastrophe, pyroptosis, necroptosis, and ferroptosis, in addition to immunogenic cell death (ICD) as the most promising way to eradicate tumor cells by making T-cells and the adaptive immune response operative, as well as by inducing the long-term immunological memory [
69,
70].
PDT can not only interrupt tumor progression, but also trigger a local acute inflammatory reaction to stimulate antitumor immunity processes by the discharge of specific subsidiary inflammatory responses and abscopal effect, as a highly satisfactory anti-cancer strategy should be able to eliminate not only the initial tumors, but also prevent metastases and recurrences [
71].
In this ongoing search, researchers have undertaken and succeeded through new strategies to synthesize novel nanoparticles with good biocompatibility and biosafety as well as high PDT targeting efficiency experimentally applied to HCC, as a viable and effective future treatment options [
72,
73].
Registered clinical trials on PDT applied in HCC can be found at ClinicalTrials.gov (accessed on 30 March 2023), which is a database of privately and publicly funded clinical studies conducted around the world [
74].
In spite of important advances in PDT applied to cancer management, there are still unequivocal impediments, such as PS collection or a photobleaching effect, restricted transferal of light doses, and inefficiency in cases of shortages in the oxygen reaching tumor tissues. On the one hand, the introspection into the complex interrelationship between hypoxia-inducible factor 1 (HIF-1) and mitochondria is very important in terms of its influence on mitochondrial structure, metabolism and respiratory function, and on the other hand, the mitochondrial activation of enzymes, the respiratory chain, the complex and decoupling proteins, all of which influence HIF-1 balance and functioning, are essential for designing and applying new high-tech, effective molecular tools and interventions in cancer therapy. HIF-1 arbitrates the accommodation to hypoxia by decreasing the mitochondrial function, and thus diminishing the oxygen cellular need. Hypoxia will degenerate mtDNA and influence the expression of many genes, including HIF-1α. Meanwhile, mitochondria will generate high metabolic stress for the HIF pathway. The complex interrelationship between HIF-1 in modulating mtDNA and mitochondrial
modus operandi under hypoxic conditions is still poorly understood and needs to be unraveled soon [
75].
2. Photothermal Therapy
Methods to improve patient outcomes and even cure cancer are important challenges for modern medicine. To reduce the side effects that accompany classical cancer therapy, i.e., radiotherapy and chemotherapy, novel PTT is confirmed as one of the most promising treatments for tumor structures in the modern era. PTT relies on ablation agents, such as PS or nanomaterials with a photothermal effect, to turn light into heat that could destroy cancer cells without harming the surrounding healthy tissues. This aspect may be true because normal cells possess greater resistance and need a longer time of exposure to heat than cancer cells. PTT consists of the application of electromagnetic waves in the NIR range for the management of diverse diseases as well as cancer. PTT is in fact an extension of PDT, for which the PS is brought into an excited state with an appropriate light bandwidth, where during de-excitation, PS releases heat, i.e., high vibrational energy that will destroy those respective cells. PTT is different from PDT because it does not need oxygen to produce effects in those living cells or organs, and could use longer wavelengths, so uses less energy and is not so harmful, but could contribute to patient recovery from cancer [
76,
77].
The results of recent studies prove the effectiveness of the selective destruction of tumor cells by PTT and the diversification of the means for selective killing, increasing the density of the photothermal agents (PTAs) in the tumor area, and the photothermal conversion capacity. For ideal PTT results, one of the solutions would be to increase the quantity of PTAs in the tumor area and the self-regulating photothermal conversion capability. PTA should produce more heat in the tumor and achieve low photothermal conversion in normal tissues [
78,
79,
80].
Although noble metal nanomaterials provide particularly good photothermal effects for cancer therapy, they have some disadvantages. Nanomaterials produced on the basis of gold are not biodegradable and some structures have a relatively low photosensitivity power due to the “melting effect”. The use of light in the NIR range has several benefits because it can penetrate deeply into the tissues, where water and biomolecules absorb small amounts without causing special damage to normal tissues. When PS is exposed to continuous NIR irradiation, some gold nanomaterials would undergo structural changes and the photothermal efficiency decreases [
81,
82].
There are a variety of types of inorganic photothermal converters (such as previously mentioned gold nanoparticles, etc.) and organic photothermal converters, such as polydopamine (PDA) particles, that are ideal in PTT administration because have good biocompatibility and do not manifest cytotoxicity [
83,
84].
PTT imaging used to visualize the exact area of cancer cells and track in real-time the influx of PTT agents into tumors has recently been put into operation. Polymeric nanocomposites based on aggregation-induced emission luminogens (AIEgens) have excellent potential for applications in PTT. AIEgens are used to detect cancerous lesions and for the image guidance of neoplastic management. These AIEgens emit a bright fluorescence in the aggregated state, can contribute to the increase in the image quality, have a high toxicity, and therefore bring great benefits for photoacoustic imaging and PTT. Their multiple imaging functions can provide relevant tumor site information and direct in real-time PTT for exceptional therapeutic results with minimal adverse effects [
85,
86,
87].
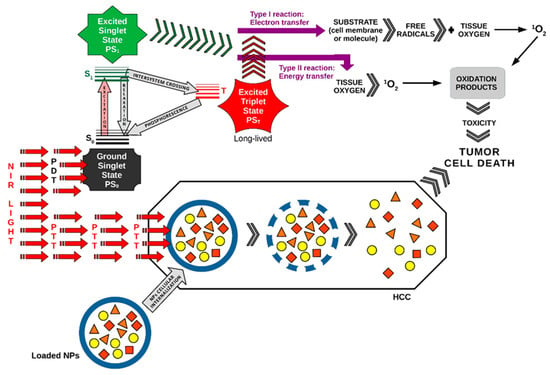
A picture of the main photomolecular processes in PDT and PTT applied synergistically in HCC as a combined duo-modal therapy is represented in Figure 1.
Figure 1. PDT and PTT applied synergistically in HCC. The pathway of action of PDT in this figure was drawn by the main author L.M.A. [
65].
3. Photoimmunotherapy
Immunotherapy is one of the most ingenious strategies for the treatment of neoplasia because it uses T-cell-activated cytokines to block the immune checkpoint blockade (ICB) or PD-1, PD-L1, and chimeric antigen receptor T cell/NK cell (CAR-T/CAR-NK). The most intensively studied are the molecules of the immune control point CTLA-4, PD-1 and PD-L1, against which inhibitory drugs have been developed. Immunotherapy for cancer includes chimeric antigen receptor (CAR)-T cells and chimeric antigen receptor (CAR) natural killer (NK) cells, i.e., CAR-NK cells, PD-1, and PD-L1 inhibitors. Through immunotherapy, the activation of the immune system is achieved to selectively control tumor growth. With all the enthusiasm and hope in these immunosuppressants, clinical trials show partial benefits, with the efficacy being limited for patients with solid tumors, and in some cases, very serious adverse reactions, such as cytokine storms, have been reported. Current concerns in the clinical therapy of solid tumors are oriented towards the discovery of new methods by which to destroy the cancer cells without affecting the patient’s immunity and with as few adverse reactions as possible [
88,
89,
90,
91,
92].
Despite all the exceptional advances in the therapy of solid cancers by ICB, complete suppression of unresectable, advanced, or metastatic HCC cannot be effectively achieved due to multiple factors, including the complex immune TME. The TME is an ecosystem that includes, in addition to the respective tumor cells, various other infiltrating immune cells, fibroblasts, non-cellular structures, stromal and neovascular elements. The vessels at the level of the tumor have a different conformation compared to those of normal tissues, being strongly contorted, with various malformities, induced by rapid growth rates through the abundance of the vascular endothelial growth factor (VEGF) secreted by the tumor cells. Local oxygen deficiency, uneven distribution of nutrients and exacerbation of aerobic glycolysis with increased lactate levels lead to decreased pH in the extracellular matrix (ECM). On the other hand, HIF-1 participates in the upregulation of glycolytic enzymes, lactate transporters and excess glucose, thus amplifying acidosis. Rapid proliferation of the tumor mass leads to the compression of local vessels with increased permeability and interstitial fluid pressure (IFP). Immunologically, the TME resembles an ineffective niche, with an abundance of innate and adaptive immune cells interspersed between endothelial cells, extracellular matrix proteins, and other tumor-supporting elements, all of which prevent T cells from contacting tumor antigens and trigger appropriate immune responses. All these elements, together with acidosis, hypoxia and IFP, are specific to the TME and constitute opposing factors to the therapeutic agents that modulate the immune process [
93,
94,
95,
96,
97].
PIT is a top-grade treatment method involving one or two completely different strategies; the first course of action uses PS in a stable form, already oxidized outside the body and then injected into the body. After administration, the PS concentrates specifically in the structure of the tumor tissue and changes the architecture of the tumor antigens, and the immune system will recognize the foreign tumor cells and will be prepared to eliminate them. A second strategy involves anchoring the PS to an antibody, creating a form of photoimmunoconjugate (PIC) so that the administered drug precisely hits the tumor tissue. This antibody specifically seeks out and targets a protein on the surface of the tumor, and by exposure to a non-thermal laser radiation source (e.g., 690 nm), the PS (dye) is activated, and the tumor cell membrane will be degraded, so the tumor will disintegrate with negligible alteration of the surrounding healthy tissue. PIC targets the most sensitive structures of cancer cells, namely the lysosomes, which it ruptures, thus releasing vesicles with lytic enzymes with the role of self-digestion [
98,
99,
100].
Sustained photoactivation therapy through PDT and PTT is a modern way of ablating many forms of cancer based on PS and photothermal conversion agents. These two techniques work in tandem, in that PDT increases the responsiveness of tumor cells to PTT by altering the TME and the heat produced by PTT increases blood supply and can improve oxygen delivery, thereby increasing the efficacy of PDT. In contrast, PIT is a more refined method than traditional PDT, as it uses the ability of antibodies to target the tumor-specific antigen with high precision, increasing the immune response, cell apoptosis, autophagy or necrosis induced by phototherapy. PIT can significantly reduce tumor size by killing many cells and by enhancing the host’s immune response. PIT is much more advantageous than monotherapy because it can also assemble chemotherapeutic agents or be combined with other cancer immunotherapy methods. Through PIT, it is possible to dissipate primary tumor formations, but also eliminate or reduce systemic metastases, thus preventing recurrences [
101,
102].
Near-infrared photoimmunotherapy (NIR-PIT) is another recently advanced method for cancer treatment based on photochemical mechanisms. An antibody-photon absorber conjugate (APC) is used, which is stimulated by laser radiation in the NIR. The core of APC is a specific monoclonal antibody (mAb) that targets the receptor or antigen on the surface of tumor cells. Following a high concentration of APC and subsequent focal projection of NIR light, the functional structures of the targeted cell membranes will be destroyed, and a prompt and characteristic photochemical ICD will occur without affecting the neighboring receptor-negative cells. Tumor cell debris generated by PIT can function as antigens, reawakening the antitumor immune responses. Meanwhile, untrained antigen-presenting dendritic cells will mature, then initiate an anti-tumor immune response directed at the host [
103,
104,
105].
The efficiency or performance of PIT depends to the greatest extent on the characteristics of the PS. There are many studies to discover and perfect the physicochemical properties of PSs. After administration, PS should selectively concentrate on cancer cells and, in a very small amount, on healthy tissues. We are looking for new PSs of natural origin, especially from plants. Ideal PS to be applied for diagnostic or therapeutic purposes for cancer pathology, in general, not only for PIT, must possess the following specific values: increased absorption coefficient to penetrate deeply into tissues in the visible spectrum, mainly in the red (R) region of the spectrum, or NIR (650–800 nm) range; high quantum capacity of triplet state formation; be easily available as a pure compound; to be able to achieve a high concentration in the fixed place; to have good biocompatibility, optimal pharmacokinetic properties and constant photoinduced release of singlet oxygen or other ROS; its chemical properties must be carefully determined in advance so that it is not harmful (low toxicity in the absence of light, and easily excreted from the body after treatment); and convenient costs. The absorption bands of PS should not overlap with the absorption bands of endogenous dyes such as melanin, hemoglobin, and water absorption bands in the NIR region. The largest variety of PSs used for PIT in cancer eradication includes porphyrins or their derivatives. Porphyrins have a light absorption spectrum structured in an intense and narrow absorption peak at a wavelength of about 400 nm (the Soret or B band), and other less intense Q bands at 500–650 nm. Other photosensitizers use chlorophyll derivatives with a chlorine backbone, in which the Q bands are at longer wavelengths (500–700 nm). Phthalocyanine dye IR700 (IR700) is the most common PS photo-immunoconjugate used in fluorescence imaging and PIT tumor therapy because it is synthetic and has a high absorption capacity in the NIR range [
106,
107,
108].
Clinical studies with the NIR-PIT technique began worldwide in 2015, and the first clinical research (NCT02422979) using the target antibody cetuximab saratolacan (cetuximab conjugated to IR700, or anti-EGFR-IR700 dye conjugate, also known as RM-1929) to target the epidermal growth factor receptor (EGFR) in patients with recurrent head and neck squamous cell carcinoma (rHNSCC) was successfully concluded in 2019, demonstrating the effectiveness and safety of the method and of the product [
109].
Currently, there are already ongoing phase 3 clinical trials with NIR-PIT for targeting EGFR using RM1929/ASP1929, i.e., the anti-EGFR antibody conjugate (cetuximab) plus the photo-absorber IRDye700DX (IR700). NIR-PIT has been given fast-track recognition by regulators in the USA and Japan. A diversity of imaging methods, including direct IR700 fluorescence imaging, could be applied to keep track of NIR-PIT [
99].
A new clinical trial was initiated in December 2020 for the therapy of carcinoma with metastatic squamous cells that express EGFR on their surface. In this study, the antibody-dye conjugate ASP-1929 is administered in association with anti-PD1 checkpoint therapy [
110].
Cancer therapy by ICB has been developed as a new technique that has revolutionized medicine by removing tumor cells that hinder the antitumor immune response. However, it has been found that only a limited number of cancer patients can benefit from this modern therapy. PDT and PTT are already known to have local beneficial effects by using PSs that can generate heat and ROS upon laser irradiation, inducing the death of immunogenic cells and opening the way for antigen-presenting cells to activate and promote a normal immune process by facilitating the infiltration of T cells into the tumor. Although ICB has emerged as a promising type of immunotherapy in advanced HCC, this ICB-only therapy is unsatisfactory with a remission rate of less than 20%. As a result, combination therapies are needed to improve therapeutic efficacy. For example, researchers have experimentally tested a targeted and ROS-sensitive micellar nanoplatform to achieve two ICD-inducing modalities by synergistic chemo-photodynamic therapy (chemo-PDT) combined with anti-PD-L1. The micellar nanoplatform was co-loaded with an aggregation-induced emission photosensitizer and paclitaxel (PTX) and was prepared for light-triggered disassembly and on-command drug release for synergistic chemo-PDT combined with anti-PD-L1, assessed both in vitro and in vivo. Chemo-PDT outstandingly increased the expression of PD-L1 on the surface of the tumor cells, which synergized with anti-PD-L1 monoclonal antibodies and set in motion an abscopal effect, generating a long-term immunological memory to hold back the tumor recurrence and metastasis. The combination of micelle-mediated chemo-PDT together with anti-PD-L1 monoclonal antibodies can synergistically enhance systemic antitumor effects and opens new avenues for novel nanomedicines with precise controlled release available for use in successful multimodal therapy for HCC [
111]. NIR-PIT has become a promising therapy method for several types of cancer because it targets cancer cells, immunoregulatory cells, or both. This technique is particularly advantageous compared to the classic methods because it removes cancer cells and at the same time promotes the immune response of the host with the protection of healthy tissues in the vicinity. Photomedicine, especially through PIT, introduces the perspective of using the immunogenic action of ICD together with various other conventional treatment methods, thus offering an optimistic future for the direct destruction of primary neoplastic cells, but also the relaunch of the suppressed immune system with the aim of increasing the survival rate and even healing cancer patients. So, combining ICB therapy with PTT-PDT could increase the capacity of the antitumor immune response and stop tumor metastasis and recurrence [
112,
113].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24098308