Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Temporomandibular joint disorders (TMDs) are conditions that affect the muscles of mastication and joints that connect the mandible to the base of the skull. Although TMJ disorders are associated with symptoms, the causes are not well proven. Chemokines play an important role in the pathogenesis of TMJ disease by promoting chemotaxis inflammatory cells to destroy the joint synovium, cartilage, subchondral bone, and other structures.
- chemokine
- temporomandibular joint disease
- inflammation
- β-catenin
1. Introduction
Temporomandibular joint disorders (TMDs) are conditions related to the masticatory muscles and the temporomandibular joint itself that result in pain and mandibular movement dysfunction [1]. TMDs affect patients’ quality of life by causing chronic muscle pain and limited mouth opening [2]. The prevalence of TMDs, as the second most common musculoskeletal pain, was approximately 31% in adults and 11% in children [3,4]. According to the diagnostic criteria of the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) and the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD), TMD can be categorized into the three groups: arthrogenous TMD (including disc disfunction and joint pathology), myogenous TMD (masticatory muscle disorders), and headache attributed to TMD [5,6,7]. Temporomandibular joint disorders (TMDs), including disc displacement (DD), and/or osteoarthritis (OA), often lead to joint pain and restrictions in mandibular movement [8,9]. Joint noise or crepitance during jaw movement, as well as less specific signs including ear pain and stuffiness, tinnitus, dizziness, neck pain and headache can also occur [10]. Moreover, oral disease is one of the most common public health issues worldwide, with significant socio-economic impacts [11]. Temporomandibular disorders (TMD) are prevalent and debilitating, and are reported to affect 5–12% of Americans [2], with affliction odds higher for females than males [12], and estimated annual costs of $4 billion [13]. Osseous changes are late-stage characteristics of degenerative joint disease [14,15,16] that are found in the TMJs of 20–30 year olds. This is over two decades earlier than in other human joints [17], and is evidence of the distinct susceptibility of the TMJ. Why TMJ tissues prematurely fail compared to post-cranial joints is not understood. TMJ internal disc derangement (ID) and/or OA, are accompanied by synovitis characterized by chronic inflammatory changes like synovial lining hyperplasia. In these cases, the number of new capillaries and small vessels increases, followed by inflammatory cells infiltrating around these vessels [18,19]. With a frequency of 25%, TMJ OA is one of the most common end-stage TMD pathologies, and the molecular mechanisms associated with TMD remain undiscovered [20]. Chemokines are a family of small cytokines, which play a key role in the exudation of inflammatory cells from the vascular system to tissues at the inflammatory site [21]. More specifically, chemokines can stimulate the chemotaxis of neutrophils, macrophages, and T lymphocytes. In addition, inflammatory cells produce inflammatory cytokines such as IL-1β, which plays a role in OA pathology by promoting the expression of matrix-degrading enzymes and oxidative metabolites, leading to extracellular matrix degradation. Furthermore, inflammatory cytokines can stimulate the synovium and other tissues to produce more chemokines [22,23].
2. Inflammation and TMJ
TMJ OA is described as low-inflammatory arthritis, while rheumatoid arthritis (RA) is described as a high-inflammatory disease. Inflammation during TMJ OA plays a key role in the onset and progression of the disease and the pain intensity [18]. Although some inflammatory mediators are common in the local TMJ OA and systemic inflammatory diseases, local levels and compositions of these mediators may change. In fact, different cytokines, chemokines, chemokine receptors, enzymes, and bone-stimulating resorption factors that are not reported in RA are considered markers of active TMJ OA [24,25]. Increased hyperemia of capillaries and infiltration of inflammatory cells, such as T cells, monocytes, or macrophages, have been observed in the TMJ synovial lining of patients with TMJ ID and OA. Several cytokines and chemokines were detected in the synovial fluid of patients with ID or OA, which include IL-1β and TNFα [26]. These cytokines have been shown to have a role in the expression of eicosanoid acid, chemokines, and proteins in TMJ synovial cells [27].
3. Chemokine and TMJ
Chemokines are pro-inflammatory peptides (8–14 kDa) that are also known as chemotactic cytokines or chemical hormones [28]. Their main biological function is to recruit leukocytes to local inflammatory sites [29]. All chemokines share major structural similarities, which include a conserved 4-cysteine motif. Some chemokines are proinflammatory cytokines, while other chemokines are thought to maintain self-regulation and control cell migration during normal tissue maintenance and development [30]. According to the arrangement of the conserved cysteine residues of mature proteins, chemokines are divided into four subfamilies: CXC(α), CC(β), C(γ) and CX3C(δ). The corresponding receptors are CXCR, CCR, CR and CX3CR [31]. Among them, α and β family members have the most extensive functions. The CXC subfamily, which has one amino acid(aa) residue separating the first two cysteine residues, includes interleukin-8 (CXCL8), growth associated oncogene GROα (CXCL1), stromal cell-derived factor 1 (SDF-1), and platelet factor 4 (PF-4 or CXCL4). Both IL-8 and GROα can send chemotactic neutrophils to inflammatory regions, while SDF-1 and PF-4 recruit lymphocytes and monocytes. The CC subfamily includes monocyte chemoattractant protein MCP-1 (CCL2), macrophage inflammatory protein MIP-1α (CCL3), MIP-1β (CCL4), exotaxin (CCL11), and RANTES (CCL5).
A chemokine receptor is a transmembrane G-protein-coupled receptor that is selectively expressed on the surface of target cells. In TMJ, the inflammatory cells in synovial tissues of RA, OA and ID are increased. Additionally, some chemokines such as IL-8, GROα, RANTES, and MIP-1 have been detected in human chondrocytes and synovial cells at significantly increased levels. The expression of CXCL8 and MCP-1 have also been reported to be increased in the synovial fluid of patients with TMJ ID and/or OA [33]. Such chemokines can promote the infiltration of inflammatory cells in TMJ joints, which causes the release of degradation enzymes, various oxidative metabolites, and inflammatory cytokines. These can lead to joint structural damage and arthritis. Furthermore, the induction of ELR CXC (CXCL1, 2, 3, 6 and 8) may lead to the recruitment of new, small vessels in inflammatory cells and synovial tissues [30,31].
3.1. CXC (α) and CX3C (δ) Subfamilies in TMJ Disease
3.1.1. IL-8
IL-8 is a member of CXC chemokines that activate leukocytes [34,35], which were formerly known as neutrophil activator protein-1 or monocyte derived neutrophil chemokine. It is a chemokine capable of inducing chemotaxis and activating neutrophils, including through direct and trans-endothelial migration, release of storage enzymes, induction of oxygen metabolites, and expression of adhesion molecules [34]. IL-8 can also attract T lymphocytes in vitro [36]. Furthermore, IL-8 is associated with many disease states, predominately angiogenic diseases such as RA. In RA, IL-8 has been shown to cause neutrophil infiltration into synovial fluid and joint inflammation. IL-8 also plays a key role in the pathogenesis of TMJ. Koch et al. reported that, compared with OA patients, the level of IL-8 in synovial fluid of RA patients increased significantly [37]. IL-8 is another type of IL-1β-targeting cytokine, one of the strongest chemokines of neutrophils and T lymphocytes. It can promote monocyte homing and activation in the synovium [36]. IL-8 can cause various pathogenic conditions, such as the release of oxidation products, apoptosis of chondrocytes, the production of MMP-13 by articular chondrocytes, and the loss of proteoglycan and subsequent cartilage degradation [38].
3.1.2. SDF-1/CXCR4
Chemokine stromal cell-derived factor-1 (SDF-1) is a small cytokine of the CXC chemokine ligand superfamily. It is mainly expressed by bone marrow stromal cells, which include osteoblasts and endothelial cells [40]. C-X-C chemokine receptor-4 (CXCR4) is a specific receptor of SDF-1. The expression of SDF-1 in synovitis or OA patients increases abnormally. In synovium and articular cartilage tissues, the activation of the SDF-1 and CXCR4 signaling pathways can regulate the expression of various inflammatory factors, which include IL-1, IL-6, TNF-α, and MMPs. IL-1 is involved in joint pathology. The SDF-1 and CXCR4 signaling pathway plays a pro-inflammatory role in the experimental TMJ OA. There may be a potential relationship between the SDF-1-CXCR4 axis and extracellular signal regulated kinase (ERK) signaling pathway. In addition, there is evidence that SDF-1α activates ERK and downstream transcription factors (c-fos and c-jun) through CXCR4, which activates adaptor protein-1 on MMP-13 and leads to cartilage damage in knee arthritis. The expression of SDF-1 was high in the synovium [41,42].
3.1.3. FKN (CXCL1)
Fractalkine (CX3CL1) is a chemokine of the CX3C family, and is both inflammatory and nociceptive. CX3CL1 has unique connectivity with CX3CR1, which is a single receptor in microglia. CX3CR1 has been associated with oral and facial inflammatory pain [46]. There is much data indicating that the activation of microglia is an effective method to treat oral and facial pain, and that glial cells play an important regulatory role in orofacial pain signaling pathway [47]. The pathogenesis of RA is a complex process. Proinflammatory cytokines such as Interleukin-1β (IL-1β) and tumor necrosis factor-A (TNF-α) are the central mediators of RA [48]. TNF-α, IL-1β, IL-6 are common cytokines. Chemokine-induced neutrophil, chemokine-1, and keratinocyte derived chemokines (KC) can trigger the release of prostaglandins and sympathetic amines, which directly act on nociceptors and cause excessive nociception [49]. The neurochemokine FKN has a pain-promoting effect in the spinal cord [50,51].
3.2. CC(β) Subfamily in TMJ Disease
3.2.1. MCP-1 Chemokines
MCP-1 is a chemokine that is part of the CC subfamily. CC chemokines mainly act on monocytes and lymphocytes [55]. In IL-1β-responsive genes, monocyte chemoattractant protein (MCP)-1 mRNA was induced by IL-1β. It was observed to be highly expressed in stimulated synovial cells. The production of MCP-1 in chondrocytes and synovial cells has been shown to play an important role in joint diseases such as RA and OA through monocyte recruitment [56,57]. It has been suggested that MCP-1 is one of the markers of RA disease activity. The protein production of MCP-1 (also known as CCL2) in TMJ has been studied in vitro and in vivo.
3.2.2. MIP3α-CCR6
Macrophage inflammatory protein-3α (MIP-3α), also known as CCL20, is a highly upregulated gene for IL-1β and TNF-α [60]. Chemokine receptor 6, also known as CCR6, is a CC chemokine receptor protein encoded in humans by the CCR6 gene. MIP-3α and CCR6 may play a role in the recruitment of monocytes and memory lymphocytes from RA peripheral blood to RA joints, which suggests that expression of the MIP-3α receptor, CCR6, may be associated with RA development [61]. RA synovial tissue contains many leukocytes expressing CCR6 [62,63], and both MIP-3α and CCR6 have been detected in the synovial fluid and synovium of RA patients [64]. The nucleotide sequence of the human MIP-3α promoter region has binding sites for Ets, AP-1, SP-1, and NFκB. This suggests that the expression of MIP-3α was regulated by several signaling molecules [65]. IL-1β, or TNF-α, induces MIP-3α production in human synovial fibroblast-like cells (SFCs) through ERK, p38 MAPK, JNK, and NFκB pathways. Increased levels of MIP-3α may trigger dendritic cells, T cells, and B cells to migrate into the synovial tissue and fluids of TMJ ID patients.
3.2.3. RANTES-CCR1
RANTES is an 8 kDa basic polypeptide in the CC chemokine superfamily. It was originally cloned from antigen-stimulated T cell line, which is also known as CCL5. RANTES can be released by chondrocytes, synovial fibroblasts, and inflammatory cells. RANTES is an effective chemical attractant for monocytes, CD4+/CD45RO+ memory helper T lymphocytes, eosinophils, basophils, and mast cells [70,71]. It has been shown that RANTES is overexpressed in normal adult tissues and increases significantly in inflammatory sites and some tumors [72]. RANTES can promote the migration of monocytes, T lymphocytes, natural killer cells, eosinophils, and macrophages. RANTES can also promote the formation of osteoclasts induced by RANKL. RANTES is also highly expressed in tissues, synovial fluid, and peripheral blood of patients with RA or OA. RANTES can trigger and aggravate the inflammatory immune response by promoting the infiltration of immunocompetent cells, and activating synovial fibroblasts to produce inflammatory mediators. MMP-1, MMP-3, MMP-13, and iNOS released by synovial fibroblasts and chondrocytes promote cartilage degradation [73]. Migration tests confirmed that RANTES was an effective chemokine of RAW264, and was responsible for attracting macrophages to inflammatory sites. C-C chemokine receptor type 1 (CCR1) is the receptor of RANTES, and acts as an inhibitor that impairs the migration of GFP BMSCs into OA cartilage and the rescue effect of GFP BMSC injection. RANTES and CCR1 signaling plays a key and synergistic role in the recruitment of GFP BMSCs into TMJ mouse OA-degraded cartilage [74].
3.3. Other Subfamilies in TMJ Disease
Chemerin-ChemR23
Chemerins are atypical chemokine ligand agonists that bind to many chemokine ligands. Chemerin acts as a chemoattractant for cells of the myeloid lineage, can signal through a potential adipokine of ChemR23, and can also bind CCRL2 in the absence of signaling [76]. Chemerin and its receptors are ubiquitous in the human body. They play a multi-functional role as chemokines, adipokines, and possible growth factors [77]. It was first isolated from human inflammatory fluid [78], which is involved in angiogenesis, osteoblast formation, and glucose homeostasis [79]. It is also a protein of interest in various medical fields, such as immunology, dermatology, metabolism, and development. Chemerin, a ligand protein of ChemR23, is a G-protein-coupled receptor that is expressed in macrophages (dentate cells), dendritic cells, and natural killer cells (NK) [80]. The interaction between Chemerin and ChemR23 is believed to be closely related to the migration of macrophages and Dendritic Cells (DC) to inflammatory sites, as well as mediating inflammatory signals to articular chondrocytes and endothelial cells (e.g., IL-1β, IL-6, IL-8 and TNF-α). It is characterized by increased secretion of MMP-1, MMP-2, MMP-3, MMP-8, and MMP-13. Wittamer et al. first reported the association between Chemerin and inflammation. They observed a high concentration of synovial fluid (SF) in patients with arthritis [81].
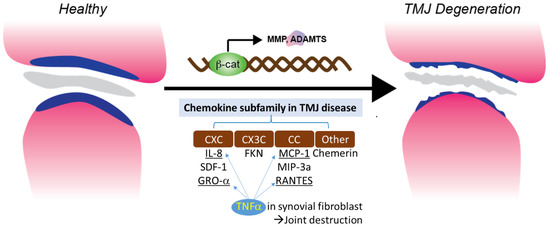
3.4. TNFα Induced Chemotaxis and Chemokine Regulation in Synovial Fibroblast
TNF-α is one of the cytokines detected in the synovial fluid of patients with intercapsular lesions with disc displacement (DD) or OA [86]. TNF-α levels are also elevated in the synovium [87]. A study evaluating the role of temporomandibular joint synovial fibroblasts in chemokine release found significant increases in the expression of IL-8, GRO-α, MCP-1, and RANTES in the conditioned medium of TNF-α-treated synovial fibroblasts. The production of IL-8 and GRO-α increased soon after exposure to TNF-α (4 and 8 hrs), suggesting that neutrophil infiltration occurs in the early stages of the inflammatory response. The production of RANTES, a potent chemoattractant for CD4+/CD45RO+ memory helper T lymphocytes, increased less than other chemokines after 4 h of exposure to TNF-α. This suggests that T lymphocytes do not migrate to sites of inflammation as early as neutrophils and monocytes. These inflammatory cells produce inflammatory cytokines, such as TNF-α, matrix-degrading enzymes, and various oxidative metabolites. These enzymes and oxidative metabolism lead to extracellular matrix degradation. At the same time, inflammatory cytokines stimulate synovial fibroblasts to produce more chemokines (Figure 1).

Figure 1. Chemokine subfamily associated with β-catenin and TNFα signaling in synovial fibroblast.
3.5. Chemokines and RA
Inflammatory cells in TMJ OA are thought to play a pathological role in the development and persistence of inflammation by their ability to release degrading enzymes and various oxidative metabolites. Infiltrating cells recruited from the blood in synovitis are mediated by chemokines released by activated synovial cells. IL-8 is a member of a chemokine superfamily of low molecular weight cytokines. These have a chemotactic effect on neutrophil and T cell subsets as well as activating neutrophil function, which includes calcium mobilization, threshing, and respiratory outbreaks [36,88]. The infiltration of neutrophils and T cells into synovial tissue are thought to play a pathological role in the development and persistence of RA through their ability to release degrading enzymes and various oxidative metabolites [88,89].
This entry is adapted from the peer-reviewed paper 10.3390/genes14020408
This entry is offline, you can click here to edit this entry!
