The prefrontal cortex (PFC) is thought to be the highest association area in the mammalian cortex and is required for proper executive control. Task flexibility and planning [
1], selective attention, attentional set-shifting, rule learning, strategy switching, and goal-directed behavior [
2,
3,
4] are just some of the many PFC functions. This considered, it is not surprising that PFC alterations have been associated with a variety of psychiatric conditions. For example, several investigations reported PFC-related impaired working memory [
5,
6,
7,
8] and altered network oscillations [
9,
10] in schizophrenia. Though rodent PFC is less complex than that of primates, it exerts similar functions in the executive domain [
11]. For this reason, the rodent represents a valuable model to investigate how PFC functions are determined at the molecular, cellular, and network levels. However, investigations in rodents are complicated by the lack of a univocal and unambiguous nomenclature of PFC subdivisions. Due to its recent evolution and inter-species variability, it is challenging to identify proper structural and functional criteria to define PFC regions [
12,
13]. This has been the subject of many studies aiming at characterizing differences and similarities of mammalian PFC [
14]. Ref [
15] introduced a hodological criterium based on the assumption that the mediodorsal thalamic nucleus (MD) is the primary site of projections toward the PFC. Therefore, according to this definition, the mammalian PFC could be identified based on the connectivity with the MD. Following this perspective, the effective existence in rats of two prefrontal cortex areas receiving projections from the MD, indicated as medial and orbitofrontal, was demonstrated [
16]. Clearly, this definition bears some limitations. Indeed, other criteria were then adopted. For example, other researchers proposed a cytoarchitectural criterion, though this method was deemed valid only for closely related species [
12]. To date, the best way to define PFC parcellation is proposed to be a combination of four criteria: function, architecture, connectivity, and topography [
17,
18]. In particular, the relevance of the connectivity aspect grew over time. Recent works have described the organization of cortical interconnectivity into modules along the whole brain [
18,
19] and identified a prefrontal cortical module. The areas within the prefrontal module show dense interconnections [
20,
21] and are believed to be devoted to similar functions [
22]. The regions recognized as a component of the prefrontal module are the prelimbic area, the infralimbic area, the anterior cingulate area, the frontal pole cerebral cortex, and the orbital areas. Another widely used distinction, mainly based on connectivity mapping including thalamocortical, corticothalamic, corticostriatal, and corticocortical projections, recognizes three broad PFC subdivisions: the dorsomedial PFC (dmPFC), ventromedial PFC (vmPFC), and ventrolateral PFC (vlPFC). Considering the complex scenario of rodent PFC nomenclature and the absence of a standard reference for the different studies available in the literature, it is not surprising that many studies focusing on the PFC report vague indications of the subregion actually subjected to analysis. In particular, most investigations on the highest-level cognitive functioning in rodents target the so-called medial PFC (mPFC), comprising the infralimbic, prelimbic, and anterior cingulate areas [
2,
13]. It is worth specifying that there is no direct anatomical equivalence between human and rodent PFC. However, the rodent mPFC is anatomically located in correspondence with the anterior cingulate cortex (ACC) in humans (see [
13] for a detailed review of the comparison between rodent and human PFC). Here, we will mainly refer to rodent reports on the mPFC, which is the most commonly addressed PFC area. The cytoarchitecture and the connectivity patterns are similar in rodents and humans, with the significant difference represented by the lack of the granular layer (layer IV) in rodent PFC. In both cases, the PFC is mainly composed of pyramidal neurons (PN, 80–90%) and inhibitory interneurons (IN, 10–20%) [
23]. The main excitatory output is provided by the PNs, which are strongly interconnected to form a local network that projects to other cortical and subcortical areas. PN activity is modulated by a strong network of GABAergic INs [
24,
25], which proved to be essential for controlling PN firing and generating neuronal network oscillations [
26,
27,
28].
2. Dopamine Receptors in the PFC
Dopamine (DA) is released in the mPFC by projections originating from the midbrain nuclei of the ventral tegmental area (VTA) and substantia nigra pars compacta [
29,
30]. Once released, DA interacts with five different receptors subtypes (D1, D2, D3, D4, D5) subdivided into two families: D1-like receptors comprising D1 and D5, and D2-like receptors comprising D2, D3, and D4 [
29,
31,
32]. Receptors belonging to the D1-like family are more abundant than those of the D2-like family and are expressed in all PFC layers. On the other hand, receptors of the D2-like family are primarily expressed in deeper layers (mainly layer V) [
33], and their affinity is 10–100 times higher than that of D1-like receptors [
34]. Both DA receptor families are expressed on pyramidal and non-pyramidal neurons, thus modulating excitation and inhibition [
29,
33]. Finally, these two receptor classes differ in the intracellular signaling pathway mediating their effects. Since DA receptors are G-protein coupled receptors (GPCRs), they all activate heteromeric G-proteins, but the second messenger and the effector proteins activated are usually different for different receptors and, in most cases, mediate opposite responses.
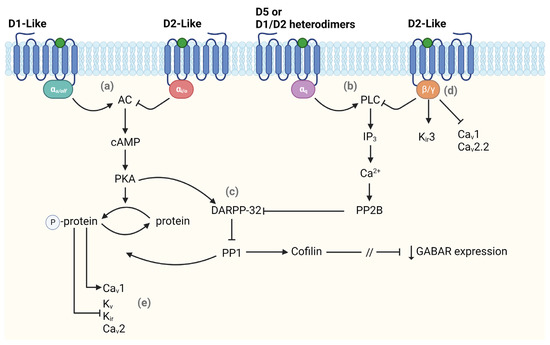
In particular, D1-like receptors activation is coupled with the G-proteins Gα
s and Gα
olf which, in turn, are associated with adenylyl cyclase (AC) that, once activated, increases the level of cyclic adenosine monophosphate (cAMP) leading to the activation of protein kinase A (PKA). PKA modulates most D1-like functions by phosphorylating many substrates including voltage-gated K
+, Na
+, and Ca
2+ channels, GABA receptors, and NMDA receptors [
32,
35]. One of the main PKA targets is the DA and cAMP-regulated phosphoprotein DARPP-32, which is crucial in regulating downstream signaling pathways. When phosphorylated, DARPP-32 inhibits the protein phosphatase 1 (PP1) that opposes PKA action, eventually amplifying PKA signaling. On the other hand, the activation of D2-like receptors leads to the opposite effect. When activated, these receptors couple with Gα
i and Gα
o that inhibit the activation of AC, thus limiting PKA signaling. Moreover, the activation of D2-like receptors determines the activation of the calmodulin-dependent protein phosphatase (PP2B), which turns DARPP-32 into a strong inhibitor of PKA signaling [
32]. Thus, DARPP-32 can bidirectionally modulate PKA activity. Besides their regulation through PKA pathways, ion channels can also be modulated directly via binding the Gβγ subunit or indirectly via activation of the phospholipase C (PLC) by both D1-like and D2-like receptors (
Figure 1). The latter is most common for modulating Ca
2+ conductance, determining a decrease in Ca
V2.2 (N-type) and Ca
V1 (L-type) currents. PLC can also be activated through coupling with Gα
q, though limited to cells expressing D5 and D1/D2 heterodimers [
36,
37]. Lastly, D1-like and D2-like receptors can modulate NMDA and GABA receptors through direct protein–protein interactions or PKA/IP3 signaling [
35]. The mechanism by which D2-like receptors, particularly D4, regulate GABA receptors involves a pathway comprising the dephosphorylation of cofilin (an actin depolymerizing factor) via PP1 activation. This leads to the loss of actin stability, with a consequent interruption of myosin motor-mediated transport of GABA receptor-containing vesicles in the membrane, resulting in a reduced GABA receptor-mediated current [
38].
Figure 1. Main intracellular pathways activated by dopamine receptors. The scheme shows different pathways in which dopamine (DA) affects the modulation of intracellular signaling. DA can regulate the activation state of (a) adenylyl cyclase (AC) or (b) phospholipase C (PLC) binding either D1-like or D2-like receptors. (c) Both pathways lead to a modulation (either positive or negative) of DARPP-32 which regulates the expression of GABA receptors. DA also affects neuronal excitability by modulating voltage-dependent ion channels via activation of (d) β/γ subunit or (e) AC pathway. The forward and stop arrows indicate activation or inhibition of the next element in the chain, respectively. This figure was created with BioRender.com.
3. Dopamine Modulation of GABAergic Inhibition
3.1. On Pyramidal Neurons (PN)
As nicely reviewed by [
29], the net effect of DA release onto the PFC also depends on cell type, synaptic properties, and interactions with other neurotransmitters. One of the critical DA roles in the PFC is the modulation of the GABAergic system. This modulation contributes to setting the proper excitation/inhibition (E/I) balance in the PFC, which requires fine-tuning to ensure correct network activity. Indeed, the E/I ratio is disrupted in a broad range of psychiatric disorders [
39,
40,
41]. Many studies focused on the role of D4 receptors in preserving the correct E/I balance. D4 receptors are enriched in the PFC and are usually expressed in dendritic processes [
42,
43,
44], while D1 receptors are most prominent at PN dendritic spines [
45]. In particular, D4 receptors are mainly expressed nearby GABA
A receptors in PFC PNs [
46]. Experimental evidence showed that D2/D4 receptor agonists decrease the inhibitory post-synaptic currents (IPSCs) of layer V PN in rodent PFC, while a D1 receptor agonist increases IPSCs amplitude in the same neurons [
46,
47,
48]. When D1- and D2-like receptors activation combines, an initial downregulation of the IPSCs mediated by D2-like receptors is followed by a D1-like receptors-dependent IPSCs increase. This suggests the biphasic nature of DA modulation of GABAergic responses in PFC PNs [
29,
47].
DA is reported to regulate inhibition through different intracellular mechanisms. In particular, high DA concentrations increase spontaneous inhibitory postsynaptic potentials (sIPSP) in PFC layer II/III [
49] and layer V-VI PNs [
50], revealing DA-mediated enhancement of GABA release. On the other hand, DA can depress evoked IPSP (eIPSP) in layer V-VI PNs [
47,
51,
52]. This evidence shows that DA can modulate spontaneous and evoked IPSPs affecting GABA release mechanisms, hence regulating the presynaptic machinery [
29]. This effect was also described in IN-PN pair recordings [
53]. A possible explanation of the different DA impact on spontaneous and evoked IPSCs is proposed by [
29]. The authors highlighted that the eIPSCs derive from activating a specific fiber through electrical stimulation, while sIPSCs derive from multiple diverse inputs. Therefore, the effect of DA on IPSCs may depend on the neuronal type generating the IPSC and the different neurons originating the GABAergic terminals impinging on that same neuron [
29]. The heterogeneity of DA modulation reported in different studies might also depend on the recording sites. Indeed, D1- and D2-like receptors have different expression patterns: while D1-like receptors mRNA are also expressed in superficial layers, D2-like receptors are restricted to deeper layers such as layer V [
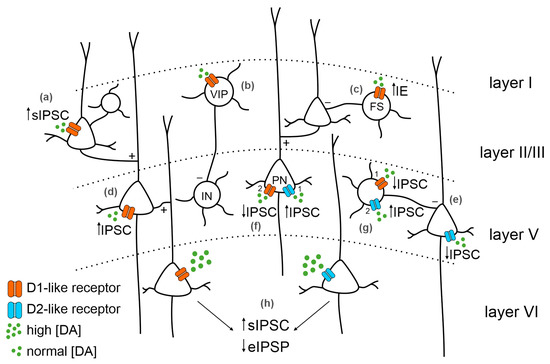
33] (
Figure 2).
Figure 2. Dopaminergic receptors distribution in the PFC and main effects on inhibition. The distribution of dopamine (DA) receptors among PFC layers and their expression on different neuronal types can variably affect inhibition. In layer II/III, DA (green dots) binding D1-like receptors (orange) on pyramidal neurons (PNs, (a)) increases spontaneous IPSC (sIPSC); (b) on vasoactive intestinal peptide (VIP) neurons, it starts internal loops inhibiting deeper layers’ inhibitory interneurons (INs); and (c) on fast-spiking interneurons (FS) increases intrinsic excitability. DA binding D1-like receptor expressed in layer V PNs (d) increases the IPSC. DA binding D2-like receptors (blue) expressed in layer V PNs (e) decreases the IPSC. Expression of both D1-like and D2-like receptors in layer V PNs (f) increases the IPSC mediated by D2-like receptor activation (1) followed by a IPSC decrease mediated by D1-like receptor activation (2). On INs (g), the decrease in the IPSC mediated by D1-like receptors (1) is followed by an increased IPSC mediated by D2-like receptors (2). (h) In layer VI PNs, the activation of DA receptors by high DA concentration leads to an increase in sIPSC and a decrease in evoked IPSC (eIPSC).
3.2. On Inhibitory Interneurons (IN)
DA receptors are expressed in a wide array of GABAergic interneurons and, therefore, DA release onto the PFC affects IN activity, too [
33,
54,
55]. DA is known to induce an increase in intrinsic excitability favoring depolarization in fast-spiking interneurons (FS) via a D1-like receptor-dependent mechanism [
56,
57]. Moreover, the effect of D1-like and D2-like receptors on PFC GABAergic INs may differ on a temporal scale. The activation of D1-like receptors induces both a depolarization and an increase in the neuronal excitability of FS. Different mechanisms mediate these two effects. The DA-induced depolarization lasts less than the increased excitability, meaning that DA can act through the same receptors to modulate different ionic currents at different time scales [
56]. Interestingly, the activation of D2-like receptors at the peak of D1-like mediated IPSC determines a decrease in the IPSC amplitude [
47,
56]. Consistent with the biphasic hypothesis of DA modulation of the GABAergic system, D2-like receptors mediate a reduction in inhibition, and D1-like receptors mediate an increase in inhibition on PFC PNs, influencing IN activity (
Figure 2). Lastly, D1-like receptors in superficial layers are often associated with vasoactive intestinal peptide (VIP) GABAergic INs and inhibit deeper INs via internal loops and interactions [
58]. This supports the D1-like receptor role in determining circuit disinhibition, which is fundamental to appropriately modulating the PFC range of activity.
3.3. Evidence In Vivo
Several studies showed that DA exerts a predominantly inhibitory effect on PFC PN in vivo, primarily suppressing spontaneous firing [59,60,61]. Importantly, microdialysis data in vivo revealed a tonic level of DA in the PFC [62,63]. Most studies reported here were performed on anesthetized animals, where little VTA activity is presumably present at rest. Nevertheless, the stimulation of fiber bundles at the medial forebrain, or direct VTA stimulation, effectively increased DA levels in the PFC. It should also be considered that the absence of not experimentally evoked DA release is an advantage in characterizing transient DA effects on PFC neurons. For these reasons, these studies are considered suitable to address the consequences of DA release on the PFC in vivo. Indeed, VTA stimulation induces a fast EPSP-IPSP sequence in PFC PNs, with the IPSP consistent with GABAA receptors activation [60]. Interestingly, the inhibitory component is eliminated not only by GABAA receptor antagonists [64] but also by D2-like receptor antagonists, which tonically inhibit neuronal excitability [65,66,67]. When the D2-like receptor tone is abolished, the entire network physiology changes: neurons increase their firing, and the inhibition produced by VTA stimulation is occluded [29]. Overall, these studies show that DA released from dopaminergic terminals in the PFC, as well as exogenous DA, modulates spontaneous firing in vivo through complex mechanisms depending on the endogenous DA tone, the amount of DA released, and the activated receptor subtype. This effect was also confirmed by a computational model in which increasing DA concentrations elicited the facilitation of FS activity, with consequent suppression of pyramidal neurons firing. Moreover, enhancing basal DA levels rescues the initial condition, through the downregulation of the GABAergic tone, with consequent hyperactivity of PN firing [68]. Interestingly, computational models primarily based on in vivo studies have proposed a dual mechanism by which D1-like receptors can modulate working memory. First, the spontaneous activity of PN is decreased by upregulating inhibitory GABA currents; then, high-activity states are induced by upregulating excitatory NMDA currents [69,70]. This effect is believed to be mediated by D1-like receptors, which might induce inhibition by amplifying IPSCs in PNs [71], or an excitatory effect by enhancing NMDA receptor-mediated responses [72,73]. The same computational model was also used to implement D2-like receptors modulation of PFC activity. It was proposed that D2-like receptors activation decreases inhibitory currents in PNs while increasing IN excitability to maintain E/I balance [74].
Taken together, these findings provide evidence for a delicate homeostatic interplay between dopaminergic and GABAergic systems necessary to maintain PFC network stability and output selectivity.