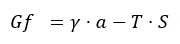Acne represents a dermatologic condition with a high prevalence, being characterized by a complex pathogenesis. Its high recurrence frequently encountered in clinical practice will affect the patient’s quality of life. Most of the treatment algorithms require at least one topical formulation, being recommended to be applied on affected areas for a long period. To treat such a versatile skin condition, smart topical vehicles capable of entrap anti-acne compounds can be considered a good option, compared with conventional systems generally used at the moment. In this direction, microemulsions are appreciated for their superior profile in matters of drug delivery, especially for challenging substances with hydrophilic or lipophilic patterns. Designed as transparent and thermodynamically stable systems, with a small number of key ingredients, microemulsion-based formulations were characterized in the present review as unique structures able to pass the skin barrier and sustain a targeted therapy in acne pathology.
- acne therapy
- microemulsions
- microstructure
- emulsifiers
- penetration enhancers
- API delivering
- evaluation methods
1. Introduction
Observed as a sophisticated mantle, the skin is designed as a first line interface that binds our body with the external environment [1]. Its coordinative character for vital functions is associated with fragility and sensitivity [2][3]. Each of these features reflect our evolution from new born existence until elderly stage, being extremely well defined in the pathological state, when the unveiled properties of the skin and its internal mechanisms will need special attention courtesy of patient and his doctor [4][5]. Dermatologic diseases are widespread over the world and have a high impact on the life quality of the patient with both physical and psychosocial repercussions [6]. The most encountered affections with a high prevalence are: eczema, acne, psoriasis, fungal or viral skin affections, autoimmune conditions, ulcerative conditions, skin burns, skin traumas, pigmentary disorders, keratinocyte carcinoma or malignant skin melanoma pathologies [7][8]. In agreement with the Global Burden of Disease Project [9], skin diseases occupied the fourth place in 2010 and 2013 as non-fatal skin injuries. These are determined by genetic factors, pre-existent systemic diseases, geographical area and socioeconomic influences such as the impairment of life quality because of the lack of access to medical care [9][10].
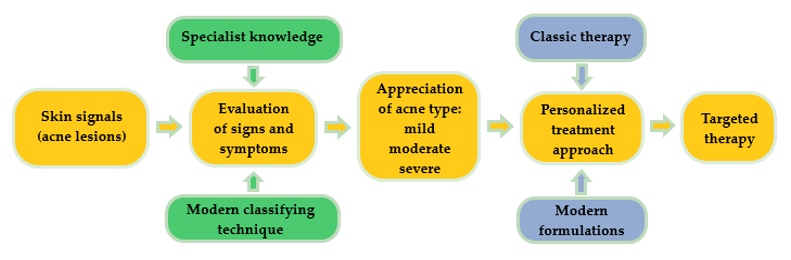
Acne is a common chronic skin condition and has been reported as one of the 10 most prevalent diseases worldwide. Its recurrent character is frequently encountered in clinical cases [11]. A proper treatment will always be suggested after a right and conclusive diagnosis, as can be observed in Figure 1. Classically, skin signal is observed in a visual inspection of the specialist. In order to increase the quality of acne diagnosis, modern classifying techniques based on convolutional neural networks are focused on the imaging and differentiation of acne lesions from healthy tissue [12]. By recognizing a skin pattern and appreciating acne evolution, personalized treatments can be created based on classical or modern pharmaceutical formulations, gently chosen to assure a targeted therapy.
Figure 1. Schematic representation concerning the impact of a right diagnosis for a personalized treatment in a dermatologic disease.
Numerous therapeutic approaches were initiated to enhance the bioavailability of dermatologic drugs at skin site in the hope to overcome the barrier effect of stratum corneum. The avoidance of oral administration of systemic drugs is also pursued, as well as attempts to integrate them in topical formulations [13].
Here, nanotechnology can put its signature, defining new ways to design proper formulations with superior therapeutic outcomes [14]. In the field of nanocolloids, microemulsions are appreciated as systems that can satisfy a high number of exigencies in matter of drug delivery—ease of preparation using a small number of ingredients like oil, water, a surfactant and a cosurfactant [15]; integration on this way of hydrophilic or lipophilic actives that can be solubilized in one of the phases [16]; generation of clear and stable dispersions with nanometric particles that can pass through biological membranes [15]—are considered a suitable platform for nanoparticle synthesis [17]. Furthermore, the use of biocompatible sources of oils like vegetable oils [18], natural surfactants [19] or biopolymers [20] can place microemulsions in the category of green alternatives in topical or systemic use of drugs with targeted action.
Early contributions in 1990s were focused on the projection of oral microemulsionate systems as vehicles based on medium chain fatty acids and their salts, able to promote calcein absorption [21]. For testosterone propionate, ample solubilization screenings were performed to find the appropriate oil phase. It was concluded that oil-based solubilizer agents are preparation key factors that can affect the internal behaviour of microemulsions and must be judiciously studied and selected for preparation [22].
Multiple applications in biomedical field were hypothesized and studied considering as effective the delivery of microemulsions in the human body. Several discoveries with positive results can be mentioned: in ophthalmology [23], for ocular delivery of retinol [24] or sacha inchi oil for dry eye treatment [25]; intranasal delivery for zidovudine [26]; sublingual delivery of insulin [27]; application of fusidic acid in wound healing [28]; vaginal delivery for fluconazole [29]; transdermal drug delivery [30]; cosmetics [31]; self-microemulsifyied systems with in situ generation of microemulsion in biological fluids for oral delivery [32]. In topical therapy of dermatologic conditions, an increased solubility and bioavailability was obtained for molecules like: cyclosporine in psoriasis [33]; ceramides for skin restructuration [34]; imiquimod in actinic keratosis or basal cell carcinoma [35]; penciclovir [36], acyclovir for herpes virus simplex cutaneous infection [37]; and tenoxicam for arthritis alleviation [38].
2. Microemulsions in a New Vision for an Optimized Acne Therapy
New modern formulations that contain actual anti-acne agents for potential dermatologic therapy represent better alternatives that must be studied for integration in clinical practice. Here can be distinguished a class of vesicular formulations which are defined as lipid-based nanosystems that include liposomes, transfersomes, niosomes, invasomes, ethosomes, cubosomes, sphingosomes, aquasomes, ufasomes or Leci Plex systems [39][40]. Besides these, superior effects were discovered by formulating systems like hydrogels which can incorporate nanoparticles [41].
Liposomal formulations are spherical vesicles with a double lipophilic membrane based on phospholipids and cholesterol. The lipidic envelope will surround a hydrophilic core, resulting in spherical vesicles [42]. For the rest of vesicular systems above mentioned, structural differences are specific considering the presence of non-ionic surfactants [43], cathionic surfactants [44], alcohol [45][46], terpenes [46] or amphiphilic lipids [47] beside phospholipids and/or cholesterol found in liposomes. The possibility to entrap hydrophilic or lipophilic substances is considered an advantage that led to multiple designs of anti-acne formulations. Favorable results considering API solubilization, protection and release were observed for liposomal formulations with tretinoin [48] or adapalene [49]. For salicylic acid [50] or rhodomyrtone [51] positive results were observed on bacterial inhibition. Other vesicular architectures were proposed as alternatives: transferosomes with clindamycin [52], niosomes with dapsone [43], invasomes with dapsone [46], ethosomes with azelaic acid [45], cubosomes with erythromycin [47] or LeciPlex with spironolactone [44].
Even if vesicular formulations are popular in this area of medical applications, several drawbacks may exist in their projection and let us to orient our interest on a different class of vehicles: a dimensional range of 50 nm until 500 nm, predisposing vesicles to hydrolytic and oxidative reactions; the presence of aggregative phenomena that can lead to unexpected dimensional changes; and the methods of preparation are laborious and expensive [53][54].
The scientific literature studied revealed multiple examples based on the formulation, preparation, and evaluation of microemulsions as ideal vehicles with multiple advantages for targeting anti-acne compounds, with positive results after the evaluation process.
With an abundant pattern of studies and multiple applications in pharmaceutical domain, microemulsions and nanoemulsions are modern colloidal dispersions with several advantages. The increase of bioavailability of active substances can be obtained in this way. Both systems have common characteristics with classical emulsions, but the addition of a co-solvent named also cosurfactant will generate unique vehicles that are capable of resolving the limitations of API integration in topical systems [55]. With referrals to particle dimension for micro- and nanoemulsions, particle dimension of 10–100 nm is specific for microemulsions, while in the case of nanoemuslions, particles dimension can reach 200 nm [55][56]. Other differences between them can be observed regarding the thermodynamic stability and the preparation methods. If microemulsions are thermodynamically and kinetically stable having the property of spontaneous generation, nanoemulsions are kinetically stable, but thermodynamically unstable systems. In the last case, the method of preparation requires the use of high-pressure homogenizers or proper sonication methods [57][58][59].
Considering these statements in the matter of nanosystem differentiation, it can be appreciated that microemulsions are fine systems with appropriate characteristics which will be further explained in what follows. Their introduction in dermatologic therapy can open new horizons toward specialized delivery, influencing in a positive manner the evolution of a skin condition like acne.
2.1. General Concepts
Microemulsions were empirically discovered in the past century, in 1943, when T. Hoar and J. H. Schulman have generated a monophasic and clear system using emulsion titration method with n-hexanol [60][61]. This was the first step for future development of smart colloidal systems named microemulsions from 1959 until today. Their initial utility at that moment was concentrated on the development of oil recovery techniques based on chemical approaches for industrial applications [62][63][64]. The evolution of the actual research methods sustains the knowledge and the discovery of unknown concepts which can help in the interpretation of physical phenomena, their physical structure and the mechanism of function for medical and pharmaceutical applications.
As a comparison with classical emulsions, microemulsions shall be differentiated, considering the following key properties: composition type, particle dimension, and thermodynamic stability. Emulsions are coarse dispersions formed by two immiscible phases which are stabilized using an emulsifier. The internal phase (discontinuous) is dispersed in the external (continuous) phase known also as a dispersion medium. The particle dimension can vary in the range 1–100 μm and is specific for opaque systems with a high interfacial energy. On the other hand, the interfacial tension has low values, assuring the stability of dispersed particles in the continuous phase [65]. The emulsion systems have a long tradition in topical application, used almost as fluid vehicles or as semi-solid forms in skin care or in dermatologic treatments [66]. A deficiency which can explain the short period of use for emulsions is the thermodynamic instability, consisting in phenomena like flocculation, coalescence, creaming, sedimentation, or Ostwald ripening. In addition, emulsion formulations are not proper for active substances with formulation challenges [65].
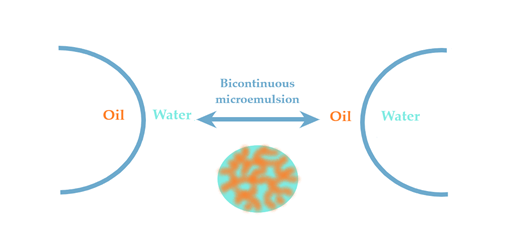
Microemulsions (MEs) are defined as microheterogeneous dispersions with a high thermodynamic stability. With a transparent appearance, the systems are observed as monophasic and isotropic structures basically formulated with an oil phase and an aqueous phase, stabilized by a mixture formed with a surfactant (S) and a cosurfactant (CoS) [63]. The clarity and homogeneity are physical markers for their dimension domain, placed in the range of 5–100 nm. The terminology of microemulsions type has a similarity with that of emulsions. Hence, the oil in water (O/W) MEs result when oil particles will be dispersed in an aqueous phase in the presence of a S/CoS mixture, while the water in oil (W/O) MEs will result in the dispersion process of water droplets in an oil phase, stabilized by a proper S/CoS mixture which will assure a superior stability [67][68]. The bicontinuous microemulsions are a particular type that requires equal amounts of oil and water in the system and are typically formed at the phase inversion temperature [69]. From a practical point of view, the transitions of ME from one type to another and their behavior are explained using the Winsor phase concept and pseudoternary phase diagrams. The study of the internal structure of microemulsions represents an important area in the research of ME thermodynamics [70][71][72].
The advantages of microemulsions are always analyzed at the beginning of a formulation process, being correlated with the actives that will be integrated and the resulting final product which can be defined as a simple system in composition, but complex in structure. In Table 1, are presented all the advantages that characterize the microemulsions as ideal vehicles for a topical delivery of active substances.
Table 1. Advantages of microemulsion (ME) formulation for topical delivery of drugs.
|
No. |
Advantages of microemulsions |
Ref. |
|
1. |
Thermodynamic stability, induced by the S/CoS mixture that provides a low interfacial tension due to a monolayer formed at the contact of oil particles with the water particles. |
[73] |
|
2. |
Spontaneous formation due to an easy preparation method, without energy consumption; economic manufacturing. |
|
|
3. |
Incorporation of both hydrophilic and lipophilic compounds, resolving the solubility drawbacks for poor soluble drugs; MEs promotes their delivery at skin site in accord with a proper diffusion process. |
|
|
4. |
The solubilization capacity for API and the superior bioavailability are proportional with a high concentration of the S/CoS mixture introduced in the system. |
[77] |
|
5. |
A high required concentration of surfactant, in association with a cosurfactant assure an easy passage through stratum corneum, acting as penetration enhancers; the barrier effect will be diminished. |
|
|
6. |
The selected API in microemulsion formulation is protected against hydrolytic and oxidative processes, being incorporated as encapsulated particles in oil or water fine droplets; MEs have a superior stability with improvement in half life. |
|
|
7. |
Incorporating APIs that are usually formulated for oral dosage forms and can be adapted in skin target. In this manner, it can be avoided the first pass liver effect and serious adverse reactions, promoting a localized action, without systemic effects. |
[79] |
2.2. Physicochemical Concepts
One of the most important characteristics of microemulsions that provokes great interest in their study is the thermodynamic stability. In this direction, were formulated five theories that can guide the research through a deeper way of understanding the behavior of MEs. Here are distinguished the thermodynamic theory, the interfacial film theory, the theory of micellar state, the solubilization theory and the theory of bicontinuous microemulsions, from basic concepts through recent discoveries [80][81]. Each of these principles can offer preliminary answers about microemulsion structure and stability which can be continued by personal discoveries rallied to a specific group of systems.
The first theory, describes thermodynamic processes that occur in microemulsion systems, using the equation of free energy, as can be seen below [82]:
The energy of the system is dependent of the interfacial tension (γ) that usually has extremely low values, being exerted at the surface of the particles (a) immersed in the dispersion medium, and is characterized by temperature (T) and an increase in the entropy (S). As the surface of the particles become larger, the stability of the system will be increased [82][83]. In addition, the interfacial film generation theory sustains the null or even negative values of interfacial tension promoted by the addition of cosurfactant in the system [82]. In this way, in three steps can be explained the formation of the interfacial monolayer as a stability promotor for microemulsions:
- In a system composed of oil and water, the surfactant will promote a low interfacial tension at the interface between oil and water, resulting thus a monomolecular film [84].
- The addition of a cosurfactant, will decrease the initial value of interfacial tension. The cosurfactant will be concentrated at the interface, among surfactant particles [84][85].
- The system will be characterized by a free energy that will assure the microemulsifying process of droplets with a size of 1–100 nm which cannot be observed at a macroscale level, but only using fine experimental techniques like transmission electron microscopy (TEM) [83][86]. In this way, will be discovered particles with a specific assembly. As an example, in a study of Reis et al. were formulated O/W microemulsion systems that can load babassu oil 12.2% as an active oil component with anti-inflammatory effects. The analysed formulation was based on a mixture of two surfactants, Span 80 and Kolliphor EL, in combination with propylene glycol as a cosurfactant with a total amount S/CoS mix of 48.8%, in a water medium of 39%. Using TEM imaging, the internal structure was visualized, confirming the particle architecture, dimensional data and the type of MEs. Thus, were observed babassu oil phase droplets covered with the monolayer stratum of tensioactive mixture and embedded in the water medium [87]. Furthermore, O/W microemulsions for dermal and transdermal delivery with a 2% flavone extract of rhizoma arisaematis with analgesic properties were formulated using a vehicle composed of a S/CoS mixture with Cremophor EL 9–27% and Transcutol 8–27% with stabilizing properties for ethyl oleate particles 4–8% in a medium of 60% water. TEM analysis revealed the presence of spherical particles of oil phase with a dimension under 100 nm, stabilized in the aqueous medium [88]. In the same direction, confocal laser microscopy can be a useful tool with application in material structure analysis, being largely accessed for microemulsion characterization [89].
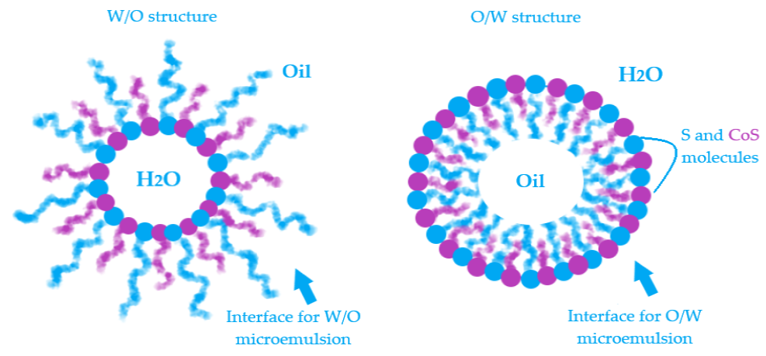
The interphases O/W and W/O are defined by a surface curvature of the monomolecular layer which is oriented as a function of oil and water content and the affinity of surfactant for hydrophilic and lipophilic groups [90][91]. In Figure 2 is exemplified a model for both W/O and O/W microemulsions and the placement of surfactant and cosurfactant at the interface.
Figure 2. Exemplification of model structure with particles as a part of water in oil microemulsions and oil in water microemulsions and the specific orientation of surfactant and cosurfactant at the interface.
The stability of microemulsions can be argued for in the same manner using the theory of elastic masses [91]. In this way, imagining microemulsion droplets as ideal spherical entities placed in a continuous phase, it can be presumed that two main characteristics are the elasticity and a proper rigidity against particle distortions [91]. The concept of elastic particles may give an input to the research of particle size using methods that can offer information about mean particle dimension, polydispersity index, interfacial tension or solubilization capacity [91][92].
Three elasticity constants are specific for microemulsion particles: firstly, the spontaneous curvature which influences the phase type and promotes stability. The solubilization capacity will be appropriate when the spontaneous curvature value will be smaller and is associated with many particles stabilized with a high amount of surfactant. Secondly, the rigidity constant, sustain the action of surfactant to resist to possible and undesirable curvature modifications. The rigidity constant increases when the third constant, the deformation (saddle-splay) constant, decreases to stabilize the system [91][93][94].
The theory of micellar state brings in front the idea of micelle generation as a common element between micelles and microemulsions. In both cases, it is selected a surface tension modulator (S). The addition of the CoS in microemulsions will make the difference among the two systems, obtaining complex structures [95]. An actual method to differentiate micellar structures and microemulsions it was proposed to be Taylor dispersion analysis which suppose the introduction of the samples in a capillary, followed by the measurement of hydrodynamic radius of the particles and their evolution in time [96]. In the case of some lipid microemulsions for oral delivery with Labrasol and Gellucire, the lipolysis process produced by pancreatic enzymes was studied in the same time with the dimensional evolution of particles, using Taylor dispersion analysis, offering clues in the matter of systems stability [97].
Apart from the classical composition of MEs, the solubilization theory suggests new insights in matter of ME spontaneous generation without surfactants, using the pre-Ouzo phenomenon [98][99]. A model system that can be easily exposed had three components: water, n-octanol as two immiscible substances one to another and ethanol as a co-solvent. Ethanol will be the hydrotropic co-solvent with solubilization power for the two immiscible compounds [98]. In a similar way were obtained ternary microemulsions based on water and eugenol in combination with ethanol [100]. As a co-solvent, ethanol will exert a hydrotropic action, transforming a turbid system into a clear one by a spontaneous emulsification as a rapid alternative to sonication methods [101].
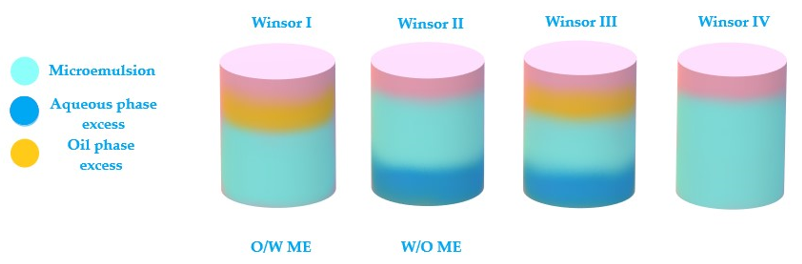
The last theory of bicontinuous microemulsions brings to the fore the four Winsor phases of microemulsions [102]. Considering this statement, a microemulsion with a continuous hydrophilic phase can pass into a system with a continuous lipophilic domain when the oil phase will be added dropwise, experiencing an intermediate state of bicontinuity where may equally coexist hydrophilic and lipophilic domains [75]. The hydrophilic and lipophilic zones are chaotically interconnected and stabilized by surfactant. This ME was observed in a study of Kogan et al. for microemulsions formulated with triacetin, D α-tocopherol acetate, ethanol and Tween 60. The system was diluted with the aqueous phase, sustaining the transition from W/O type ME (known also as inversed phase ME) to O/W ME with the normal phase [103]. In this direction, the knowledge of Winsor phases serves as a guideline in the process of formulation and preparation of ME, helping to adjust the composition of the designed systems [104].
According to Figure 3, the Winsor phases found in ME formulations can briefly be described. Winsor phase I systems are O/W microemulsions in an equilibrium with an excess of oil phase in the superior area of the vial. Winsor phase II systems are W/O microemulsions with an excess of water phase in the inferior area of the vial. Winsor phase III systems defines a ternary mixture at equilibrium composed of a microemulsion in the middle area, an excess of oil phase in the superior zone, followed by an excess of aqueous phase in the inferior area. Furthermore, the ideal Winsor IV system will contain a monophasic domain, without phase excess [72][105].
Figure 3. Schematic representation of Winsor phases I, II, III and IV, explaining the transition of phases from Winsor phase I to Winsor phase IV to obtain an ideal system.
2.3. Formulation of Microemulsions
In the formulation process of microemulsions, the attention is focused on the mixture of S/CoS and the oil phase which will be associated with a proper amount of aqueous phase, commonly selected, the distilled water [63].
A surfactant, also named emulsifier, defines a molecule with specific properties of surface tension modulation, exerted in the system where will be integrated. Structurally, the molecule is composed of hydrophilic and hydrophobic moieties, viewed as two opposite poles that assure a preferential orientation at the contact with particles of the system, in ME case, the oil and water molecules [106][107]. With respect to interfacial film theory, the surfactant will be the key molecule in the dispersion process, offering a proper flexibility for particles in the continuous phase due to the generation of the interfacial monolayer [107][108].
Chemically, surfactants are classified as ionic (anionic, cationic and zwitterionic) or non-ionic species. The ionic surfactants, at the contact with the polar phase will generate a double electric layer, while non-ionic compounds will form dipole and hydrogen bonds [109][110]. It is important to mention that anionic and cationic surfactants are not recommended in cutaneous delivery systems due to their irritative potential. Thus, non-ionic surfactants are generally selected for topical products, having a good solubilization power for APIs and a reduced toxicity [109][111].
The hydrophilic–lipophilic balance (HLB) values calculated according with the Davies’ rule, as a function of head and tail groups of a surfactant, sustain its selection for a ME system. An HLB under 8 is attributed for surfactants used in W/O systems, while surfactants with HLB over 10 are suitable for O/W MEs. Stable systems are formed also by mixing two surfactants with different HLB values, as well as in emulsion formulation case [112]. According with the Bancroft rule, which is generally accepted in ME design too, the phase in which the surfactant is most soluble represents the continuous phase [113].
Specific for microemulsions is the high amount of S/CoS mixture selected up to 70%, differing from conventional emulsions where the emulsifier is integrated up to 10–20% [72][114]. An elaborate analysis is required in the formulation of topical systems; thus, the ingredients must be non-toxic, non-irritating and biocompatible, according with GRAS (Generally Regarded as Safe) concepts [115]. Recent approaches are focused on the use of natural surfactants as a green alternative to synthetic species, associated with algorithms in order to decrease the high concentration of S/CoS mixture, maintaining in the same manner a superior stability for the systems [115].
As examples of non-ionic surfactants, sorbitan esters like Span 20, Span 40, Span 60 Span 65, Span 80 and Span 85 are usually selected to promote W/O microemulsions due to their low HLB values between 1.8 for Span 85 and 8.6 for Span 20 [109][110][116]. On the other hand, polysorbates are chosen in the formulation of O/W microemulsions due to their HLB values over 10. Here are classified Tween 20, Tween 40, Tween 60, Tween 80, Tween 85 as combinations between partial esters of sorbitol and its mono-/dianhydrides, condensed with ethylene oxide groups [109][110][116]. Tween 80 is largely selected in microemulsion design due to its biocompatibility. Its association with alcohol based cosurfactants like ethanol or 1-butanol implies some attraction phenomena that will promote the solubilization of two immiscible phases. In the study of Prieto and Calvo [117], this mixture was suitable to stabilize n-hexane/water systems. The surface activity will be modified, being correlated with an increase in dielectric constant and ionization grade. The repulsion forces of Tween 80 at the interface n-hexane/water will be diminished with the addition of the alcohol. In order to choose which alcohol is preferred for ME generation, Traube’s rule can be followed which considers that in a homologous series of surfactants, the addition of a -CH2- group will decrease the molar concentration required to promote a reduction in the surface tension [110][117][118]. In this direction, it was proposed that the solubilization power will be proportional with the chain length of alcohol, being important to assure a balance between the chain length of S and the sum of oil and CoS chain lengths. For pharmaceutical applications, ethanol can be safely selected with special consideration on structural properties of each phase [117]. This statement was observed in the same manner in the study of Chai et al., which tested the power of solubilization for Tween 20, Tween 60 and Tween 80 on a ternary system like 1-butanol/dodecane/brine. Considering that Tween 60 had a high solubilization effect on the system, it was pointed out that the sum of 1-butanol/dodecane chain lengths will be proportional with the chain length of Tween 60 [119].
Other candidates usually found in ME preparation are Labrasol (polyethylene glycol (PEG) derivative of medium chain fatty acid triglycerides C8-C10 of capric and caprylic acids) or Cremophor derivates like polyoxyl 40 hydrogenated castor oil (Cremophor RH 40) and polyoxyethyleneglycerol triricinoleate 35 (Cremophor EL, Kolliphor EL) [14][120][121][122][123].
Non-ionic surfactants derived from natural sources are sucrose- and glucose esters which are considered ideal candidates for microemulsion generation, intensively studied as biocompatible tensioactives in drug delivery. Their surface activity and biodegradability are properties that recommend them in the formulation of topical systems [124]. As an example, using a mixture of Mazol 80 (association of ethoxylated mono- and diglycerides) and sucrose laurate as a biodegradable surfactant, in association with water and peppermint oil, Fanun has suggested a potential system for solubilizing active principles [125].
Another alternative to synthetic surfactants could be phosphatidylcholine-derived products like soy lecithin or egg yolk lecithin which have HLB values in the range 4–6, being suitable for W/O microemulsions [126]. The literature confirms the use of lecithin until 10% in ME formulations [127][128]. Nevertheless, it is recommended to design mixtures of lecithin with non-ionic surfactants with an HLB over 10 for O/W microemulsions. Surabhi et al. have formulated O/W anti-acne microemulsions with tretinoin, using a surfactant mixture of lecithin 1% and Tween 80 30% with ethanol 10% as CoS, associated with isopropyl myristate (IPM) as an oil phase in aqueous medium [129].
The second component of surface-active modulator mixture is the cosurfactant (CoS), also found as co-solvent, which has the property to decrease the interfacial tension, promoting a proper flexibility for the particles [130]. Cosurfactants (CoSs) are studied as penetration enhancers, being greatly appreciated in skin delivery. In addition, the association with surfactant molecules will assure a high solubility for both hydrophilic and hydrophobic substances [75]. CoSs used in ME preparation are preferred to be medium chain alcohols C2-C10 [131]. A high stability can be obtained using CoSs with short, medium and branched chains C3-C5 [132]. Here are distinguished the most selected CoSs in the formulation of MEs: ethanol, isopropyl alcohol, n-butanol, propylene glycol (PG), glycerin, n-pentanol, polyethylene glycol 400 (PEG 400), diethylene glycol monoethyleter (Transcutol P) [133]. Propylene glycol and Transcutol P are often chosen as CoSs due to their biocompatibility, along with their additional solubilization properties for APIs which can be observed over a screening process. In a comparative study of Abd Sisak et al., the efficiency of Transcutol P and PG in the generation of a large region of ME were demonstrated, compared with PEG 400 for systems prepared with Brij 97, oleic acid and water [134].
The oil phase is considered a continuous phase for W/O MEs or a dispersed phase that can incorporate hydrophobic actives in O/W ME type. The oil phase will be selected according with the solubility of the API to obtain its delivery in an encapsulated form [135]. Isopropyl myristate, ethyl oleate and oleic acid are synthetic oils usually preferred in ME formulation [136]. On the other hand, the use of vegetable oils became an interesting approach for ME preparation, mostly preferred by those containing fatty acids with medium chains and a low molecular weight. Additional effects of vegetable oils consist in hydration promotion, skin protection and rejuvenation. These approaches were appreciated and followed in a study of Hortolomei et al., which was based on the development of MEs with avocado oil, associated with a S/CoS mixture formed with sucrose laurate and Transcutol P. An increased tolerability of the formulated systems was suggested, emphasizing their potential for skin delivery [135][137].
Recent ME systems were formulated using grape seed oil in a short study of Scomoroscenco et al., using a S/CoS mixture composed of Tween 80, Plurol diisostearique CG and ethanol. The introduction of grape seed oil as a lipophilic phase was suitable to obtain cosmeceutical microemulsions [138].
Pascoa et al., have prepared microemulsions using Pterodon emarginatus oil. It was found that microemulsions containing 5–10% oil phase exerted an anti-inflammatory effect which was superior to the oil used as a single remedy [139].
For the antioxidant and hydrating properties at skin site, olive oil was included in O/W MEs designed by Chaiyana et al. The effect of different cosurfactants like propylene glycol, ethanol, isopropanol, and PEG-400 on the generation of a larger area of microemulsion was observed, with a notable impact on their effect at skin site. Two optimal MEs with the following formulation schemes S/CoS/Oil/Water (%) were considered, namely: Tween 85 64%/Propylene glycol 16%/Olive oil 10%/Water 10% and Tween 85 64%/Ethanol 16%/Olive oil 10%/Water 10%. Propylene glycol exhibited a good influence on hydration due to its humectant properties, being comparable with a hyaluronic acid preparation, while ethanol sustained the antioxidant activity of olive oil [140].
It can be appreciated that vegetable oils can be good candidates for synthetic oil phase replacing, due to their implications in skin moisturization, skin barrier rebalancing, UV protection, which are essential for a damaged skin. Argan oil, coconut oil, jojoba oil, oat oil, pomegranate oil, almond oil, rose hip oil are vegetable oils that can be recognized as potential active species in acne alleviation [141].
The second type of oil species that are frequently selected for ME preparation, with a high impact in skin delivery, are essential oils (EO). The essential oils are products resulting from the extraction process of different parts of aromatic plants. Their active compounds can exert biological effects in the human body, offering therapeutic actions [142][143]. A main class of organic compounds which are found in the composition of essential oils are terpenes that will act on SC destabilization due to their lipophilic properties. The terpene structure and physicochemical particularities of the drug are two criteria that must be taken under consideration in the preformulation step. To assure a good penetration in skin layers, non-polar terpenes with a high grade of unsaturation are preferred for lipophilic actives, while the species with hydroxylic moieties, characterized by a minimal degree of unsaturation can be selected for hydrophilic drugs [144]. In Table 2, are presented the advantages of essential oils and their contribution in the formulation of anti-acne microemulsions.
Table 2. Advantages of essential oils (EOs) and their impact on anti-acne microemulsion formulation.
|
No. |
Advantages |
Ref. |
|
1 |
EOs can be selected as an oil phase in ME or combined with a second vegetable or synthetic oil. |
[145] |
|
2 |
EOs can be a good alternative to chemical solvents. |
[146] |
|
3 |
EOs act as penetration enhancers at skin site, increasing the localization of drugs. |
[144] |
|
4 |
EOs have low toxicity, being GRAS recognized. |
[144] |
|
5 |
EOs protect the API from degradation reactions, improving its stability; the microemulsification will protect EOs from degradation and volatilization. |
|
|
6 |
EOs can exert self-anti-acne action on microbial species like P. acnes or S. aureus. |
[148] |
Considering the antimicrobial effects of essential oils for application in acne treatment as a part of a pharmaceutical product, the potential of the following oil models can be appreciated: Acacia dealbata essential oil, Achillea millefolium essential oil, Boswellia carterii essential oil, Camellia sinensis essential oil, Citrus aurantifolia essential oil, Commiphora myrrha essential oil, Helichrysum italicum essential oil, Laurus nobilis essential oil, Lavandula angustifolia essential oil, Mentha piperita essential oil, Myrthus communis essential oil, Ocimum basilicum essential oil, Jasminum grandiflorum essential oil, Santalum album essential oil, Pogostemon patchouli essential oil, Rosmarinus officinalis essential oil, Salvia lavandulifolia essential oil, Thymus vulgaris essential oil, Vetiveria zizanioides essential oil, Viola odorata essential oil [148][149][150][151].
To exemplify the impact of essential oils in microemulsion formulation, in the study of Lv et al., ME systems were prepared with the aim to assess a high permeation of quercetin at skin level using a group of essential oils with anti-inflammatory properties and comparing their efficacy. The power of solubilization was analysed as well using peppermint oil, clove oil or rosemary oil. Hence, essential oils have improved the photostability of quercetin compared with a simple aqueous solution. From a preparation point of view, the essential oil was considered as an oil phase being mixed with an amount of S/CoS mixture formed with Cremophor EL and propylene glycol 2:1 and finally titrated with water. Over evaluation, peppermint oil MEs had a larger area than MEs prepared using clove oil or rosemary oil. On the other hand, clove oil and rosemary oil offered a protective effect, assuring quercetin stability. Quercetin was degraded in a proportion of 67% in an aqueous solution compared with only 7% degraded in the microemulsions [147]. A study by Ma et al. offered a perspective concerning the antimicrobial effects of microemulsion systems enriched with essential oils or an essential oil compound and the influence of formulation factors on their activity. Thus, the systems were formulated with cinnamon bark oil, thyme oil or eugenol in a soybean oil medium, using Tween 80 and equal amounts of PG and water. The use of a microemulsion vehicle can increase the level of minimal inhibitory concentration of cinnamon bark oil from 313 ppm (the value obtained for the use of oil alone) to 625 ppm (for microemulsion) on cultures of Listeria monocytogenes. The study draws attention to the manner in which Tween 80 and soy bean oil inclusion will decrease the antimicrobial activity of the essential oils which can be attributed to some hydrophobic interactions between Tween 80 molecules and the lipophilic moieties of essential oils, suggesting the importance of concentration control in the formulation process [152]. A superior antimicrobial activity can be sustained with a proper amount of surfactant that can assure an increase in bacterial cell permeability for the primary API [153].
2.4. Methods for Microemulsion Preparation
Microemulsions are considered adaptive systems which can be prepared without high energy consumption in an economic manner. The preparation methods at room temperature are based on two types of titration method which can be applied and adapted as a function of the selected phases, their concentrations and the type of ME that is desirable to be formulated [75]. In practice, the microemulsification technique supposes the application of two methods: the phase titration method and phase inversion method using the oil or aqueous phase [82].
According with particle concepts previously exposed at the introductive theories section, the transition from O/W type to W/O type takes place with changes in curvature orientation, experiencing the particular state of bicontinuity, with the occurring of structural modifications as can be seen in Figure 4.
Figure 4. Schematic representation of phase transition in a microemulsion system with internal structure modification, from oil in water through water in oil type and vice versa, experiencing a particular bicontinuous state.
Considering the amounts selected for the aqueous phase, the oil phase, and the S/CoS mix, the region of microemulsion generation can be deduced using a pseudoternary phase diagram design. In laborious studies, when various proportions of tensioactives and oil phases are tested, the graphical analysis based on diagrams was found to be a key step to proceed an experimental design to obtain optimal microemulsions. In a large domain of studies, pseudoternary phase diagrams offer a good way for analyzing microemulsions stability, depicting zones which are specific to O/W, W/O and biocontinuous microemulsions [154].
2.5. The mechanism of Action for Microemulsions at skin level
Considering the appropriate role of microemulsions for dermal delivery, these systems are known to be implicated in penetration activity, crossing the diffusional barrier of stratum corneum due to its main components, the S/CoS mixture along with the oil and the aqueous phase [155]. The oil in water type is almost preferred for anti-acne systems, due to its non-greasy structure, correlated with a low concentration of oil until 20%, which is the maximum required in dermatologic preparations. Here we can mention the study of Mortazavi et al., based on the preparation of microemulsions with tretinoin, where the amount of the oil phase was selected in proportion of 10% until 17% and evaluated considering its impact on particle dimension and skin delivery [156].
When a microemulsion is applied on skin, at the epidermal layer, the phenomenon of SC destabilization can be observed. The particles of the system have the capacity to intercalate among keratinocyte spaces. SC destabilization is promoted due to a high amount of tensioactive mixture which in addition will be implied in a decreasing process of interfacial tension at the skin surface. By diminishing the barrier function, accompanied by the creation of some passages of nanometric size, the passage of API particles can be promoted through the inside, with a diffusion process occurring [78][155]. Concerning the S/CoS mixture, is important to assure an equilibrium between the maximum concentration selected in the system and implied in microemulsification, solubilization and diffusion process, obtaining at the same time a high level of skin tolerability [78]. A couple of practical examples can be rendered which can emphasize the action of ethanol [157], propylene glycol [158] and Transcutol P on skin dynamics [159], considering some of the most selected excipients with penetration enhancement activity in microemulsion design. At skin site several phenomena supposed to be correlated with the mechanism of skin penetration are studied. Here we can mention: lipid extraction, alteration of protein domain, along with the increase of drug partition in skin lipids [157].
The use of natural surfactants combined or not with the non-ionic type and the use of vegetable oils can be an advantage to obtain biocompatible systems. As an example, a comparative study of Changez et al., based on the delivery of tetracaine in mice skin, proved that the addition of lecithin in ME systems can enhance the delivery at epidermal and dermal layers for tetracaine, compared with a topical solution with the same anesthetic [160].
Furthermore, the vegetable oils selected for ME systems can maintain an occlusive effect, with a similarity being discovered with mineral oils like paraffin oil. Changing the hydration gradient in the upper site of the epidermis by occlusive effect, can be a practical option for dermatologic preparations applied on dried skin [161].
2.6. Application of Microemulsions for Anti-Acne Drug Delivery
Several studies were based on the development of unique formulations that can be implied in a superior control of acne pathogenesis due to an optimal targeting of APIs at skin site, offering an alternative to conventional treatments [162]. Most of the projected systems were designed in order to resolve the solubility challenges of lipophilic actives like: vitamin E [163][164], retinoids [129][165][166], antibiotics [167][168] and other antimicrobial agents like metronidazole [169] or dapsone [170], offering in the same manner protection against undesirable internal processes by avoiding interactions and photochemical reactions. For hydrophilic substances, the easy passage through the stratum corneum can be handled for vitamin C [164], azelaic acid [171], nicotinamide [172], or hyaluronic acid [173], which are used as adjuvants in acne treatment.
Concerning the use of essential oils in the development of microemulsions with anti-acne activity, Pansang et al. used the essential basil oil 3% extracted from Ocimum basilicum as an oil phase. The ME was analyzed from a tolerability point of view on 30 patients, proving the safety profile of the vehicle at the skin site [174].
Furthermore, Jantrawut et al., designed ME systems using the antibacterial properties of orange oil. The MEs were prepared to be applied on skin as a part of a pectin film that can maintain an intimate contact with the tissue. The study revealed the importance of surfactant and cosurfactant screening in order to obtain large regions, proving that this may be one of the essences that form the basis of ME design [175].
It can be appreciated that microemulsion properties are closely influenced by the formulation parameters, where each of the selected phases will sustain the final action of the API in the targeted zone. Microemulsion systems will improve the localization of APIs in skin layers and can be optimized using Quality by Design principles. The use of combined systems like microemulsion-based gels or emulgels and the use of natural derived excipients will improve the tolerability and the biocompatibility at the application zone.
3. Conclusions
The study of nanocolloids offers a generous contribution to the evolution of topical treatments in order to sustain the healing process in dermatologic diseases. Microemulsions as a part of soft matter systems are characterized by high stability, biocompatibility, and tolerability, influencing in a positive manner the skin dynamics due to their special internal structure. Hence, the limitations of conventional systems can be overcome due to a balanced composition represented by the pseudoternary structure of oil-surfactant/cosurfactant-water. Multiple approaches were proposed to enhance skin bioavailability for both hydrophilic and lipophilic anti-acne compounds, offering a pathway through superior topical pharmaceutical options. On this way, it can be concluded that microemulsions are ideal vehicles for anti-acne drug targeting.
This entry is adapted from the peer-reviewed paper 10.3390/nano10112292
References
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin's neuroendocrine system. Anat. Embryol. Cell Biol. 2012, 212, 1‒98, doi:10.1007/978-3-642-19683-6_1.
- Stalder, J.F.; Tennstedt, D.; Deleuran, M.; Fabbrocini, G.; de Lucas, R.; Haftek, M.; Taieb, C.; Coustou, D.; Mandeau, A.; Fabre, B.; et al. Fragility of epidermis and its consequence in dermatology. Eur. Acad. Dermatol. Venereol. 2014, 28, 1‒18, doi:10.1111/jdv.12509.
- Duarte, I.; Silveira, J.E.P.S.; Hafner, M.F.S.; Toyota, R.; Pedroso, D.M.M. Sensitive skin: Review of an ascending concept. Bras. Dermatol. 2017, 92, 521‒525, doi:10.1590/abd1806-4841.201756111.
- Oranges, T.; Dini, V.; Romanelli, M. Skin Physiology of the Neonate and Infant: Clinical Implications. Wound Care 2015, 4, 587‒595, doi:10.1089/wound.2015.0642.
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.B.; Hay, R.J.; Griffiths, C.E.M. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56, 230‒242, doi:10.1093/geront/gnw003.
- Lim, H.W.; Collins, S.; Resneck, J.S.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. Am. Acad. Dermatol. 2017, 76, 958‒972, doi:10.1016/j.jaad.2016.12.043.
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global Skin Disease Morbidity and Mortality: An Update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017, 153, 406–412, doi:10.1001/jamadermatol.2016.5538.
- Seth, D.; Cheldize, K.; Brown, D.; Freeman, E.F. Global Burden of Skin Disease: Inequities and Innovations. Dermatol. Rep. 2017, 6, 204‒210, doi:10.1007/s13671-017-0192-7.
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. Investig. Dermatol. 2014, 134, 1527–1534, doi:10.1038/jid.2013.446.
- DeStefano, G.M.; Christiano, A.M. The Genetics of Human Skin Disease. Cold Spring Harb. Perspect. Med. 2014, 4, 1‒25, doi:10.1101/cshperspect.a015172.
- Truchuelo, M.T.; Vitale, M.A.; Bettoli, V.; Estebaranz, J.L. Acne Relapses and Maintenance Therapy: An Update on Definition and Prevention. J. Clin. Res. Dermatol. 2017, 2, 18‒27.
- Shen, X.; Zhang, J.; Yan, C.; Zhou, H. An Automatic Diagnosis Method of Facial Acne Vulgaris Based on Convolutional Neural Network. Rep. 2018, 8, 1‒10, doi:10.1038/s41598-018-24204-6.
- Barbieri, J.S.; Spaccarelli, N.; Margolis, D.J.; James, W.D. Approaches to limit systemic antibiotic use in acne: Systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. Am. Acad. Dermatol. 2019, 80, 538–549, doi:10.1016/j.jaad.2018.09.055.
- Nastiti, C.M.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37, doi:10.3390/pharmaceutics9040037.
- Sapra, B.; Thatai, P.; Bhandari, S.; Sood, J.; Jindal, M.; Tiwary, A.K. A critical appraisal of microemulsions for drug delivery: Part II. Deliv. 2014, 5, 83–94, doi:10.4155/tde.13.125.
- Heuschkel S, Goebel A, Neubert RH. Microemulsions-modern colloidal carrier for dermal and transdermal drug delivery. Pharm. Sci. 2008, 97, 603–631, doi:10.1002/jps.20995.
- Nam, N.H.; Luong, N.H. Nanoparticles: Synthesis and applications. In Materials for Biomedical Engineering; Grumzescu, V., Grumzescu, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–240, doi:10.1016/B978-0-08-102814-8.00008-1.
- Do, L.D.; Withayyapayanon, A.; Harwell, J.H.; Sabatini, D.A. Environmentally Friendly Vegetable Oil Microemulsions Using Extended Surfactants and Linkers. Surfact. Deterg. 2009, 12, 91–99, doi:10.1007/s11743-008-1096-0.
- Schwarz, J.C.; Klang, V.; Hoppel, M.; Mahrhauser, D.; Valenta, C. Natural microemulsions: Formulation design and skin interaction. J. Pharm. Biopharm. 2012, 81, 557–562, doi:10.1016/j.ejpb.2012.04.003.
- Alkrad, J.A.; Mrestani, Y.; Neubert, R.H. Development and characterization of microemulsions containing hyaluronic acid. J. Pharm. Sci. 2016, 86, 84–90, doi:10.1016/j.ejps.2016.02.008.
- Constantinides, P.P.; Welzel, G.; Ellens, H.; Smith, P.L.; Sturgis, S.; Yiv, S.H.; Owen, A.B. Water-in-oil microemulsions containing medium-chain fatty acids/salts: Formulation and intestinal absorption enhancement evaluation. Pharm Res. 1996, 13, 210–215, doi:10.1023/a:1016030812272.
- Malcolmson, C.; Satra, C.; Kantaria, S.; Sidhu, A.; Lawrence, M.J. Effect of oil on the level of solubilization of testosterone propionate into nonionic oil-in-water microemulsions. Pharm. Sci. 1998, 87, 109–116, doi:10.1021/js9700863.
- Radomska-Soukharev, A.; Wojciechowska, J. Microemulsions as potential ocular drug delivery systems: Phase diagrams and physical properties depending on ingredients. Pol. Pharm. 2005, 62, 465–471.
- Radomska, A.; Dobrucki, R. The use of some ingredients for microemulsion preparation containing retinol and its esters. J. Pharm. 2000, 196, 131–134, doi:10.1016/s0378-5173(99)00436-6.
- Laihia, J.; Järvinen, R.; Wylęgała, E.; Kaarniranta, K. Disease aetiology-based design of multifunctional microemulsion eye drops for moderate or severe dry eye: A randomized, quadruple-masked and active-controlled clinical trial. Acta Ophthalmol. 2020, 98, 244–254, doi:10.1111/aos.14252.
- Carvalho, F.C.; Barbi, M.S.; Sarmento, V.H.; Chiavacci, L.A.; Netto, F.M.; Gremião, M.P. Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. Pharm. Pharmacol. 2010, 62, 430–439, doi:10.1211/jpp.62.04.0004.
- Patil, N.H.; Devarajan, P.V. Enhanced insulin absorption from sublingual microemulsions: Effect of permeation enhancers. Drug Deliv. Transl. Res. 2014, 4, 429–438, doi:10.1007/s13346-014-0205-z.
- Okur, M.E.; Ayla, Ş.; Yozgatlı, V.; Aksu, N.B.; Yoltaş, A.; Orak, D.; Sipahi, H.; Üstündağ Okur, N. Evaluation of burn wound healing activity of novel fusidic acid loaded microemulsion based gel in male Wistar albino rats. Saudi Pharm. J. 2020, 28, 338–348, doi:10.1016/j.jsps.2020.01.015.
- Bachhav, Y.G.; Patravale, V.B. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. J. Pharm. 2009, 365, 175–179, doi:10.1016/j.ijpharm.2008.08.021.
- Date, A.A.; Patravale, V.B. Microemulsions: Applications in transdermal and dermal delivery. Rev. Ther. Drug Carrier Syst. 2007, 24, 547–596, doi:10.1615/critrevtherdrugcarriersyst.v24.i6.20.
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Khan, Z.I.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Emerging role of microemulsions in cosmetics. Recent Pat. Drug Deliv. Formul. 2008, 2, 275–289, doi:10.2174/187221108786241624. PMID: 19075913.
- Krstić, M.; Medarević, Đ.; Đuriš, J.; Ibrić, S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In Lipid Nanocarriers for Drug Targeting; Grumzescu, A.M., Ed.; William Andrew (Elsevier): Oxford, UK, 2018; pp. 473–508, doi:10.1016/B978-0-12-813687-4.00012-8.
- Benigni, M.; Pescina, S.; Grimaudo, M.A.; Padula, C.; Santi, P.; Nicoli, S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. J. Pharm. 2018, 545, 197–205, doi:10.1016/j.ijpharm.2018.04.049.
- Sahle, F.F.; Wohlrab, J.; Neubert, R.H. Controlled penetration of ceramides into and across the stratum corneum using various types of microemulsions and formulation associated toxicity studies. J. Pharm. Biopharm. 2014, 86, 244–250, doi:10.1016/j.ejpb.2013.07.011.
- Pescina, S.; Garrastazu, G.; Del Favero, E.; Rondelli, V.; Cantù, L.; Padula, C.; Santi, P.; Nicoli, S. Microemulsions based on TPGS and isostearic acid for imiquimod formulation and skin delivery. J. Pharm. Sci. 2018, 125, 223–231, doi:10.1016/j.ejps.2018.10.007.
- Zhu, W.; Guo, C.; Yu, A.; Gao, Y.; Cao, F.; Zhai, G. Microemulsion-based hydrogel formulation of penciclovir for topical delivery. J. Pharm. 2009, 378, 152–158, doi:10.1016/j.ijpharm.2009.05.019.
- Kaur, A.; Sharma, G.; Gupta, V.; Ratho, R.K.; Katare, O.P. Enhanced acyclovir delivery using w/o type microemulsion: Preclinical assessment of antiviral activity using murine model of zosteriform cutaneous HSV-1 infection. Cells Nanomed. Biotechnol. 2018, 46, 346–354, doi:10.1080/21691401.2017.1313262.
- Goindi, S.; Narula, M.; Kalra, A. Microemulsion-Based Topical Hydrogels of Tenoxicam for Treatment of Arthritis. AAPS PharmSciTech. 2016, 17, 597‒606, doi:10.1208/s12249-015-0383-0.
- Kotla, N.; Chandrasekar, B.; Rooney, P.; Sivaraman, G.; Larrañaga, A.; Kanala, V.K.; Pandi, A.; Rochev, Y. Biomimetic Lipid-Based Nanosystems for Enhanced Dermal Delivery of Drugs and Bioactive Agents. ACS Biomater. Sci. Eng. 2017, 3, 1262‒1272, doi:10.1021/acsbiomaterials.6b00681.t.
- Shah, S.M.; Ashtikar, M.; Jain, A.S.; Makhija, D.T.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Jagtap, A.A.; Nagarsenker, M.S.; Fahr, A. LeciPlex, invasomes, and liposomes: A skin penetration study. J. Pharm. 2015, 490, 391‒403, doi:10.1016/j.ijpharm.2015.05.042.
- Jurairattanaporn, N.; Chalermchai, T.; Ophaswongse, S.; Udompataikul, M. Comparative Trial of Silver Nanoparticle Gel and 1% Clindamycin Gel when Use in Combination with 2.5% Benzoyl Peroxide in Patients with Moderate Acne Vulgaris. Med. Assoc. Thai. 2017, 100, 78‒85.
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Pharmacol. 2015, 6, 1–13, doi:10.3389/fphar.2015.00286.
- Al Sabaa, H.; Mady, F.M.; Hussein, A.K.; Abdel-Wahab, H.M.; Ragaie, M.H. Dapsone in topical niosomes for treatment of acne vulgaris. J. Pharm. Pharmacol. 2018, 12, 221–230, doi:10.5897/AJPP2018.4925.
- Salama, A.; Badran, M.; Elmowafy, M.; Soliman, G.M. Spironolactone-Loaded LeciPlexes as Potential Topical Delivery Systems for Female Acne: In Vitro Appraisal and Ex Vivo Skin Permeability Studies. Pharmaceutics 2020, 12, 25, doi:10.3390/pharmaceutics12010025.
- Mistry, A.; Ravikumar, P. Development and Evaluation of Azelaic Acid Based Ethosomes for Topical Delivery for the Treatment of Acne. Indian J. Pharm. Educ. Res. 2016, 50, S232–S243, doi:10.5530/ijper.50.3.34.
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Dapsone-Loaded Invasomes as a Potential Treatment of Acne: Preparation, Characterization, and In Vivo Skin Deposition Assay. AAPS PharmSciTech 2018, 19, 2174–2184, doi:10.1208/s12249-018-1025-0.
- Khan, S.; Jain, P.; Jain, S.; Jain, R.; Bhargava, S.; Jain, A. Topical Delivery of Erythromycin Through Cubosomes for Acne. Nanotechnol. 2018, 6, 38–47, doi:10.2174/2211738506666180209100222.
- Rahman, S.A.; Abdelmalak, N.S.; Badawi, A.; Elbayoumy, T.; Sabry, N.; El Ramly, A. Tretinoin-loaded liposomal formulations: From lab to comparative clinical study in acne patients. Drug Deliv. 2016, 23, 1184–1193.
- Kumar, V.; Banga, A.K. Intradermal and follicular delivery of adapalene liposomes. Dev. Ind. Pharm. 2016, 42, 871–879, doi:10.3109/03639045.2015.1082580.
- Zhang, Y.; Zhao, J.L.; Zhou, H.L. Study on the Preparation Process of Salicylic Acid Liposome Gel. Mat. Res. 2012, 554–556, 828–831, doi:10.4028/www.scientific.net/amr.554-556.828.
- Chorachoo, J.; Amnuaikit, T.; Voravuthikunchai, S.P. Liposomal Encapsulated Rhodomyrtone: A Novel Antiacne Drug, Based Complement. Alternat. Med. 2013, 2013, 1–7, doi:10.1155/2013/157635.
- Thakur, N.; Jain, P.; Jain, V. Formulation Development and Evaluation of Transferosomal Gel. Drug. Deliv. Ther. 2018, 8, 168–177, doi:10.22270/jddt.v8i5.1826.
- Yadav, A.V.; Murthy, M.S.; Shete, A.S.; Sakhare, S. Stability Aspects of Liposomes. Indian J. Pharm. Educ. Res. 2011, 45, 402–413.
- Chaurasia, L.; Singh, S.; Arora, K.; Saxena, C. Transferosome: A Suitable Delivery System for Percutaneous Administration. Res. Pharm. Sci. 2019, 9, 1–11, doi:10.24092/CRPS.2019.090101.
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences and similarities. Soft Matter 2012, 8, 1719‒1729, doi:10.1039/C2SM06903B.
- de Oca-Ávalos, J.M.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and physical properties. Opin. Food Sci. 2017, 16, 1‒6, doi:10.1016/j.cofs.2017.06.003.
- Ngan, C.L.; Basri, M.; Tripathy, M.; Abedi, K.R.; Abdulmalek, E. Physicochemical Characterization and Thermodynamic Studies of Nanoemulsion-Based Transdermal Delivery System for Fullerene. World J. 2014, 2014, 1‒12, doi:10.1155/2014/219035.
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135, doi:10.3390/nano10010135.
- Chircov, C.; Grumzescu, A.M. Nanoemulsion preparation, characterization, and application in the field of biomedicine. In Nanoarchitectonics in Biomedicine; Grumzescu, A.M., Ed.; William Andrew (Elsevier): Amsterdam, The Netherlands, 2019; pp. 169‒188, doi:10.1016/C2017-0-04439-7.
- Hoar, T.P.; Schulman, J.H. Transparent water-oil dispersions, the oleophatic hydromycelle. Nature 1943, 152, 102‒103, doi:10.1038/152102a0.
- Ruckenstein, E. Thermodynamics of Microemulsions II. In Handbook of Microemulsion Science and Technology; Kumar, P., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 45‒58.
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanisms of Formation and Structure of Microemulsions by Electron Microscopy. Phys. Chem. 1959, 63, 1677‒1680, doi:10.1021/j150580a027.
- Najjar, R. Microemulsions—A Brief Introduction. In Microemulsions—An Introduction to Properties and Applications; Najjar, R., ; IntechOpen: Rijeka, Croatia, 2012; pp. 3‒30, doi:10.5772/36057.
- Dantas, T.N.C.; Santanna, V.C.; Souza, T.T.C.; Lucas, C.R.S.; Dantas Neto, A.A.; Aum, P.T.P. Microemulsions and Nanoemulsions Applied to Well Stimulation and Enhanced Oil Recovery. J. Petrol. Gas 2018, 12, 251‒265, doi:10.5419/bjpg2018-0023.
- Khan, B.A.; Akhtar, N.; Khan, H.M.S.; Waseem, K.; Mahmood, T.; Rasul, A.; Iqbal, M.; Khan, H. Basics of pharmaceutical emulsions: A review. J. Pharm. Pharmacol. 2011, 5, 2715‒2725, doi:10.5897/AJPP11.698.
- Estanqueiro, M.; Conceição, J.; Amaral, M.H.; Santos, D.; Silva, J.B.; Lobo, J.M.S. Characterization and stability studies of emulsion systems containing pumice. J. Pharm. Sci. 2014, 50, 361‒369, doi:10.1590/S1984-82502014000200016.
- Talegaonkar, S.; Azeem, A.; Ahmad, F.J.; Khar, R.K.; Pathan, S.A.; Khan, Z.I. Microemulsions: A novel approach to enhanced drug delivery. Recent Pat. Drug. Deliv. Formul. 2008, 2, 238‒257, doi:10.2174/187221108786241679.
- Karunaratne, D.N.; Pamunuwa, G.; Ranatunga, U. Introductory Chapter: Microemulsions. In Properties and Uses of Microemulsions; Karunaratne, D.N., Pamunuwa, G., Ranatunga, U., Eds.; IntechOpen: Rijeka, Croatia, 2017; pp. 3‒15, doi:10.5772/intechopen.68823.
- Muzaffar, F.; Singh, U.K.; Chauchan, L. Review on microemulsion as futuristic drug delivery. Pharm. Pharm. Sci. 2013, 5, 39‒53.
- Tlusty, T.; Safran, S.A. Microemulsion networks: The onset of bicontinuity. Phys. Condens. Matter 2000, 12, 253–262, doi:10.1088/0953-8984/12/8A/332.
- Nordiyana, M.S.W.; Khalil, M.; Jan, B.M.; Ali, B.S.; Tong, C.W. Formation and Phase Behavior of Winsor Type III Jatropha curcas‐Based Microemulsion Systems. Surfactants Deterg. 2016, 19, 701‒712, doi:10.1007/s11743-016-1814-y.
- Salager, J.-L. Emulsion Phase Inversion Phenomena. In Emulsions and Emulsion Stability, 2nd ed.; Sjoblӧm, J.,; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2005; Volume 132, pp. 185‒223, doi:10.1201/9781420028089.ch4.
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol. Physiol. 2008, 21, 246–259, doi:10.1159/000140228.
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. J. Pharm. 2017, 526, 425‒442, doi:10.1016/j.ijpharm.2017.05.005.
- Sharma, A.K.; Garg, T.; Goyal, A.K.; Rath, G. Role of microemulsions in advanced drug delivery. Cells Nanomed. Biotechnol. 2016, 44, 1177‒1185, doi:10.3109/21691401.2015.1012261.
- Ghosh, P.K.; Murthy, R.S.R. Microemulsions: A potential drug delivery system. Drug Deliv. 2006, 3, 167‒180, doi:10.2174/156720106776359168.
- Alaoui, Y.E.; Fahry, A.; Rahali, Y.; Cherkaoui, N.; Bensouda, Y.; Laatiris, A. Formulation, optimization and characterization of ibuprofen loaded microemulsion systems using D-optimal mixture design. J. App. Pharm. 2019, 11, 304‒312, doi:10.22159/ijap.2019v11i4.33076.
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52‒77, doi:10.3390/pharmaceutics6010052.
- Amarji, B.; Garg, N.K.; Singh, B.; Katare, O.P. Microemulsions mediated effective delivery of methotrexate hydrogel: More than a tour de force in psoriasis therapeutics. Drug Target. 2016, 24, 147‒160, doi:10.3109/1061186X.2015.1058804.
- Vadlamudi, H.C.; Yalavarthi, P.R.; Basaveswara Rao, M.V.; Sundaresan, C.R. Insights of Microemulsions – A Thermodynamic Comprehension. J. Pharm. Sci. 2017, 10, 23‒40.
- Lindman, B.; Shinoda, K.; Olsson, U.; Anderson, D.; Karlström, G.; Wennerström, H. On the demonstration of bicontinuous structures in microemulsions. Colloids Surf. 1989, 38, 205‒224, doi:10.1016/0166-6622(89)80154-4.
- Jha, S.K.; Dey, S.; Karki, R. Microemulsions-potential carriers for improved drug delivery. Asian J. Biomed. Pharm. Sci. 2011, 1, 5‒9.
- Mehta, S.K.; Kaur, G. Microemulsions: Thermodynamic and Dynamic Properties, In Thermodynamics; Mizutani, T., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 381‒407, doi:10.5772/12954.
- Kegel, W.K.; Overbeek, J.T.G.; Lekkerkerker, H.N.W. Thermodynamics of Microemulsions I. In Handbook of Microemulsion Science and Technology; Kumar, P., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 13‒44, doi:10.1201/9780203752739.
- De Gennes, P.G.; Taupin, C. Microemulsions and the flexibility of oil/water interfaces. Phys. Chem. 1982, 86, 2294–2304, doi:10.1021/j100210a011.
- Del-real, A.; Garcóa-Garduño, M.V.; Castaño, V.M. Transmission Electron Microscopy Observations of Micelle Structure in a Vinyl Acetate-based Microemulsion. J. Polym. Mater. Polym. Biomat. 1998, 42, 319‒326, doi:10.1080/00914039808033879.
- Reis, M.Y.F.A.; dos Santos, S.M.; Silva, D.R.; Silva, M.V.; Correia, M.T.S.; Ferraz Navarro, D.M.A.; Santos, G.K.N.; Hallwass, F.; Bianchi, O.; Silva, A.G.; et al. Anti-Inflammatory Activity of Babassu Oil and Development of a Microemulsion System for Topical Delivery. Based Complentary Altern. Med. 2017, 2017, 1‒14, doi:10.1155/2017/3647801.
- Shen, L.-N.; Zhang, Y.-T.; Wang, Q.; Xu, L.; Feng, N.-P. Preparation and evaluation of microemulsion-based transdermal delivery of total flavone of rhizoma arisaematis. J. Nanomed. 2014, 9, 3453–3464, doi:10.2147/IJN.S66524.
- Teng, X.; Li, F.; Lu, C. Visualization of materials using the confocal laser scanning microscopy technique. Soc. Rev. 2020, 49, 2408–2425, doi:10.1039/c8cs00061a.
- Robbins, M.L.; Bock, J.; Shuang, J. Model for microemulsions: III. Interfacial tension and droplet size correlation with phase behavior of mixed surfactants. Colloids Interface Sci. 1988, 126, 114‒133, doi:10.1016/0021-9797(88)90106-3.
- Bergström, L.M. Thermodynamics and Bending Energetics of Microemulsions. In Properties and Uses of Microemulsions; Karunaratne, D.N., Pamunuwa, G., Ranatunga, U., Eds.; IntechOpen: Rijeka, Croatia, 2017; pp. 117‒138, doi:10.5772/67369.
- Binks, B.P.; Meunier, J.; Langevin, D. Characteristic sizes, film rigidity and interfacial tensions in microemulsion systems. In Trends in Colloid and Interface Science III. Progress in Colloid & Polymer Science; Bothorel, P., Dufourc, E.J., Eds.; Steinkopff: Darmstadt, Germany, 1989; Volume 79, pp. 208‒213, doi:10.1007/BFb0116210.
- Kos, Ž.; Ravnik, M. Relevance of saddle-splay elasticity in complex nematic geometries. Soft Matter 2016, 12, 1313‒1323, doi:10.1039/C5SM02417J.
- Geethu, P.M.; Yadav, I.; Mani, E.; Aswal, V.K.; Satapathy, D.K. Saddle-splay modulus of reverse microemulsions: Experimental determination using small-angle neutron scattering and dielectric relaxation spectroscopy. Rev. E 2018, 98, 052604, doi:10.1103/PhysRevE.98.052604.
- Langevin, D. Micelles and Microemulsions. Rev. Phys. Chem. 1992, 43, 341‒369, doi:10.1146/annurev.pc.43.100192.002013.
- Chamieh, J.; Davanier, F.; Jannin, V.; Demarne, F.; Cottet, H. Size characterization of commercial micelles and microemulsions by Taylor dispersion analysis. J. Pharm. 2015, 492, 46‒54, doi:10.1016/j.ijpharm.2015.06.037.
- Chamieh, J.; Merdassi, H.; Rossi, J.-C.; Jannin, V.; Demarne, F.; Cottet, H. Size characterization of lipid-based self-microemulsifying pharmaceutical excipients durig lipolysis using Taylor dispersion analysis with fluorescence detection. J. Pharm. 2018, 537, 94‒101, doi:10.1016/j.ijpharm.2017.12.032.
- Zemb, T.N.; Klossek, M.; Lopian, T.; Marcus, J.; Schöettl, S.; Horinek, D.; Prevost, S.F.; Touraud, D.; Diat, O.; Marčelja, S.; et al. How to explain microemulsions formed by solvent mixtures without conventional surfactants. Natl. Acad. Sci. USA 2016, 113, 4260‒4265, doi:10.1073/pnas.1515708113.
- Klossek, M.L.; Touraud, D.; Zemb, T.; Kunz, W. Structure and Solubility in Surfactant‐Free Microemulsions. Chemphyschem 2012, 13, 4116‒4119, doi:10.1002/cphc.201200667.
- Lucia, A.; Argudo, P.G.; Guzmán, E.; Rubio, R.G.; Ortega, F. Formation of surfactant free microemulsions in the ternary system water/eugenol/ethanol. Colloids Surf. A Physicochem. Eng. Asp. 2017, 521, 133‒140, doi:10.1016/j.colsurfa.2016.04.062.
- François, G.; Katz, J.L. Nanoparticles and nanocapsules created using the Ouzo effect: Spontaneous emulisification as an alternative to ultrasonic and high-shear devices. Chemphyschem 2005, 6, 209‒216, doi:10.1002/cphc.200400527.
- Clausse, M.; Peyrelasse, J.; Heil, J.; Boned, C.; Lagourette, B. Bicontinuous structure zones in microemulsions. Nature 1981, 293, 636‒638, doi:10.1038/293636a0.
- Kogan, A.; Shalev, D.E.; Raviv, U.; Aserin, A.; Garti, N. Formation and Characterization of Ordered Bicontinuous Microemulsions. Phys. Chem. B 2009, 113, 10669‒10678, doi:10.1021/jp901617g.
- Sanchez-Dominguez, M.; Aubery, C.; Solans, C. New Trends on the Synthesis of Inorganic Nanoparticles Using Microemulsions as Confined Reaction Media. In Smart Nanoparticles Technology; Hashim, A.A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 195‒219, doi:10.5772/33010.
- Prévost, S.; Gradzielski, M.; Zemb, T. Self-assembly, phase behaviour and structural behaviour as observed by scattering for classical and non-classical microemulsions. Colloid Interface Sci. 2017, 247, 374‒396, doi:10.1016/j.cis.2017.07.022.
- Polarz, S.; Kunkel, M.; Donner, A.; Schlötter, M. Added-Value Surfactants. Chemistry 2018, 24, 18842‒18856, doi:10.1002/chem.201802279.
- Schramm, L.L.; Stasiuk, E.N.; Marangonic, D.G. Surfactants and their applications. Rep. Prog. Chem. Sect. C Phys. Chem. 2003, 99, 3‒48, doi:10.1039/b208499f.
- Dutt, S.; Siril, P.F.; Remita, S. Swollen liquid crystals (SLCs): A versatile template for the synthesis of nano structured materials. RSC Adv. 2017, 7, 5733‒5750, doi:10.1039/C6RA26390A.
- Sekhon, B.S. Surfactants: Pharmaceutical and Medicinal Aspects. JPTRM 2013, 1, 43‒88, doi:10.15415/jptrm.2013.11004.
- Attwood, D.; Florence, A.T. Physical Pharmacy; Pharmaceutical Press: London, UK, 2008; pp. 43‒47, ISBN 978-0-85369-725-1.
- Pandey, A.; Mittal, A.; Chauhan, N.; Alam, S. Role of Surfactants as Penetration Enhancer in Transdermal Drug Delivery System. Mol. Pharm. Org. Process Res. 2014, 2, 1‒10, doi:10.4172/2329-9053.1000113.
- Mohamed, A.I.A.; Sultan, A.S.; Hussein, I.A.; Al-Muntasheri, G.A. Influence of Surfactant Structure on the Stability of Water-in-Oil Emulsions under High-Temperature High-Salinity Conditions. Chem. 2017, 2017, 1‒11, doi:10.1155/2017/5471376.
- Ruckenstein, E. Microemulsions, Macroemulsions, and the Bancroft Rule. Langmuir 1996, 12, 6351‒6353, doi:10.1021/la960849m.
- Ramadan, E.; Borg, T.; Abdelghani, G.M.; Saleh, N.M. Formulation and evaluation of acyclovir microemulsions. Pharm. Sci. 2013, 36, 31‒47.
- Pappinen, S.; Urtti, A. Microem In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Dragicevic, N., Maibach, H.I., Eds.; Springer Verlag: Berlin/Heidelberg, Germany, 2016; pp. 253‒262, doi:10.1007/978-3-662-47862-2_16.
- Moroi, Y. Micelles: Theoretical and Applied Aspects; Plenum Press: New York, NY, USA, 1992; pp. 12‒13, ISBN 978-1-4899-0702-8.
- Prieto, C.; Calvo, L. Performance of the Biocompatible Surfactant Tween 80, for the Formation of Microemulsions Suitable for New Pharmaceutical Processing. Appl. Chem. 2013, 2013, 1‒10, doi:10.1155/2013/930356.
- Bowman, B.J.; Ofner, C.M.; Schott, H. Colloidal dispersions. In Remigton: The Science and Practice of Pharmacy, 21st ed.; Troy, D.B., Beringer, P., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 293‒319, ISBN 0-683-306472.
- Chai, J.L.; Liu, N.; Bai, T.T.; Zhang, H.M.; Liu, N.N.; Wang, D. Compositions and Physicochemical Properties of Tween Type Surfactants-Based Microemulsions. Disper. Sci. Technol. 2014, 35, 441‒447, doi:10.1080/01932691.2013.794733.
- Neghi, J.S. Nanolipid Materials for Drug Delivery Systems: A Comprehensive Review. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137‒163, doi:10.1016/B978-0-12-814031-4.00006-4.
- Ngawhirunpat, T.; Worachun, N.; Opanasopit, P.; Rojanarata, T.; Panomsuk, S. Cremophor RH40-PEG 400 microemulsions as transdermal drug delivery carrier for ketoprofen. Dev. Technol. 2013, 18, 798‒803, doi:10.3109/10837450.2011.627871.
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. J. Cancer 2001, 37, 1590‒1598, doi:10.1016/s0959-8049(01)00171-x.
- Lo, J.-T.; Lee, T.-M.; Chen, B.-H. Nonionic Microemulsions as Solubilizers of Hydrophobic Drugs: Solubilization of Paclitaxel. Materials 2016, 9, 761, doi:10.3390/ma9090761.
- Bevinakatti, H.S.; Mishra, B.K. Sugar derived surfactants. In Design and Selection of Performance Surfactants: Annual Surfactants Review; Karsa, D.R., Ed.; Sheffield Academic Press: Sheffield, UK, 1999; Volume 2, pp. 1‒40, ISBN 1-85075-993-6.
- Fanun, M. Microemulsions with Mixed Nonionic Surfactants and Flavour Oil. Surfactants Deterg. 2010, 13, 321‒328, doi:10.1007/s11743-010-1187-6.
- Weete, J.D.; Betageri, S.; Griffith, G.L. Improvement of lecithin as an emulsifier for water-in-oil emulsions by thermalization. Am. Oil. Chem. Soc. 1994, 71, 731‒737, doi:10.1007/BF02541430.
- Xuan, X.-Y.; Cheng, Y.-L.; Acosta, E. Lecithin-linker microemulsion gelatin gels for extended drug delivery. Pharmaceutics 2012, 4, 104‒129, doi:10.3390/pharmaceutics4010104.
- Zhou, H.; Yue, Y.; Liu, G.; Li, Y.; Zhang, J.; Gong, Q.; Yan, Z.; Duan, M. Preparation and characterization of a lecithin nanoemulsion as a topical delivery system. Nanoscale Res. Lett. 2009, 5, 224–230, doi:10.1007/s11671-009-9469-5.
- Surabhi, K.; Katare, O.P.; Sushma, D. Lecithinised Microemulsions for Topical Delivery of Tretinoin. J. Drug Dev. Res. 2010, 2, 711‒719.
- Golwala, P.; Rathod, S.; Patil, R.; Joshi, A.; Ray, D.; Aswal, V.K.; Bahadur, P.; Tiwari, S. Effect of cosurfactant addition on phase behavior and microstructure of a water dilutable microemulsion. Colloids Surf. B Biointerfaces 2020, 186, 110736, doi:10.1016/j.colsurfb.2019.110736.
- Zhong, F.; Xu, W.; Fu, T.; Li, Y. Preparation and Characterization of Functional Compounds Encapsulated Microemulsion with Nonionic Surfactants. Food Drug Anal. 2012, 20, 203‒207, doi:10.38212/2224-6614.2097.
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Colloid Interface Sci. 2006, 123‒126, 369‒385, doi:10.1016/j.cis.2006.05.014.
- Kumar, A.; Kushwaha, V.; Sharma, P.K. Pharmaceutical microemulsion: Formulation, characterization and drug delivery across skin. J. Drug Dev. Res. 2014, 6, 1‒21.
- Abd Sisak, M.A.; Daik, R.; Ramli, S. Study on the effect of oil phase and co-surfactant on microemulsion systems. Malaysian J. Anal. Sci. 2017, 21, 1409‒1416, doi:10.17576/mjas-2017-2106-23.
- Xavier-Junior, F.H.; Vauthier, C.; Morais, A.R.V.; Alencar, E.N.; Egito, E.S.T. Microemulsion systems containing bioactive natural oils: An overview on the state of the art. Drug Dev. Ind. Pharm. 2017, 43, 700‒714, doi:10.1080/03639045.2016.1235186.
- Djekic, L.; Primorac, M. The influence of cosurfactants and oils on the formation of pharmaceutical microemulsions based on PEG-8 caprylic/capric glycerides. J. Pharm. 2008, 352, 231‒239, doi:10.1016/j.ijpharm.2007.10.041.
- Hortolomei, M.; Popovici, I.; Ochiuz, L. Influence of formulation factors on the physico-chemical characteristics of dermal microemulsions prepared with sucrose esters. Farmacia 2012, 60, 484‒492.
- Scomoroscenco, C.; Cinteza, L.O.; Teodorescu, M.; Gifu, I.C.; Ianchis, R.; Nistor, C.L.; Petcu, C.; Ninciuleanu, C.M.; Alexandrescu, E.; Mihaescu, C.I. Preparation and Characterization of Vegetable Oil-Based Microemulsions. Proceedings 2019, 29, 74, doi:10.3390/proceedings2019029074.
- Pascoa, H.; Diniz, D.G.A.; Florentino, I.F.; Costa, E.A.; Bara, M.T.F. Microemulsion based on Pterodon emarginatus oil and its anti-inflammatory potential. J. Pharm. Sci. 2015, 51, 117‒125, doi:10.1590/S1984-82502015000100013.
- Chaiyana, W.; Leelapornpisid, P.; Phongpradist, R.; Kiattisin, K. Enhancement of antioxidant and skin moisturizing effects of olive oil by incorporation into microemulsions. Nanotechnol. 2016, 6, 1‒8, doi:10.1177/1847980416669488.
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. J. Mol. Sci. 2017, 19, 70, doi:10.3390/ijms19010070.
- Butnariu, M.; Sarac, I. Essential Oils from Plants. Biotech. Biomed. Sci. 2018, 1, 35‒43, doi:10.14302/issn.2576-6694.jbbs-18-2489.
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. Food Sci. 2014, 79, 1231‒1249, doi:10.1111/1750-3841.12492.
- Pandit, J.; Aqil, M.; Sultana, Y. Terpenes and Essential Oils as Skin Penetration Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of Stratum Corneum; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 173‒193, doi:10.1007/978-3-662-47039-8_11.
- Fernández-Peña, L.; Gutiérrez-Muro, S.; Guzmán, E.; Lucia, A.; Ortega, F.; Rubio, R.G. Oil-In-Water Microemulsions for Thymol Solubilization. Colloids Interfaces 2019, 3, 64, doi:10.3390/colloids3040064.
- Edris, A.E.; Rawlinson-Malone, C.F.R. Preferential solubilization behaviours and stability of some phenolic-bearing essential oils formulated in different microemulsion systems. J. Cosmet. Sci. 2012, 34, 441‒450, doi:10.1111/j.1468-2494.2012.00737.x.
- Lv, X.; Liu, T.; Ma, H.; Tian, Y.; Li, L.; Li, Z.; Gao, M.; Zhang, J.; Tang, Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech 2017, 18, 3097‒3104, doi:10.1208/s12249-017-0798-x.
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules 2010, 15, 3200‒3210, doi:10.3390/molecules15053200.
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Based Complement. Alternat. Med. 2017, 2017, 1‒92, doi:10.1155/2017/4517971.
- Kim, B.Y.; Shin, S. Antimicrobial and Improvement Effects of Tea Tree and Lavender Oils on Acne Lesions. Converg. Inf. Technol. 2013, 8, 339‒345.
- Kim, K.Y.; Jang, H.H.; Lee, S.N.; Kim, Y.-S.; An, S. Effects of the myrtle essential oil on the acne skin—clinical trials for Korean women. Dermatol. 2018, 2, 1‒10, doi:10.1186/s41702-018-0038-3.
- Ma, Q.; Davidson, P.M.; Zhong, Q. Antimicrobial properties of microemulsions formulated with essential oils, soybean oil, and Tween 80. J. Food Microbiol. 2016, 226, 20‒25, doi:10.1016/j.ijfoodmicro.2016.03.011.
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. J. Pharm. 2017, 529, 134‒160, doi:10.1016/j.ijpharm.2017.06.059.
- Chiappisi, L.; Noirez, L.; Gradzielski, M. A journey through the phase diagram of a pharmaceutically relevant microemulsion system. Colloid Interface Sci. 2016, 473, 52‒59, doi:10.1016/j.jcis.2016.03.064.
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Pharmacother. 2018, 108, 1477‒1494, doi:10.1016/j.biopha.2018.10.021.
- Mortazavi, S.A.; Pishrochi, S.; Jafari Azar, Z. Formulation and In-vitro Evaluation of Tretinoin Microemulsion as a Potential Carrier for Dermal Drug Delivery. J. Pharm. Res. 2013, 12, 599‒609.
- Gupta, R.; Badhe, Y.; Rai, B.; Mitragotri, S. Molecular mechanism of the skin permeation enhancing effect of ethanol: A molecular dynamics study. RSC Adv. 2020, 10, 12234‒12248, doi:10.1039/D0RA01692F.
- Carrer, V.; Alonso, C.; Pont, M.; Zanuy, M.; Córdoba, M.; Espinosa, S.; Barba, C.; Oliver, M.A.; Martí, M.; Coderch, L. Effect of propylene glycol on the skin penetration of drugs. Dermatol. Res. 2020, 312, 337–352, doi:10.1007/s00403-019-02017-5.
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®-Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533, doi:10.1208/s12249-018-1196-8.
- Changez, M.; Chander, J.; Dinda, A.K. Transdermal permeation of tetracaine hydrochloride by lecithin microemulsion: In vivo. Colloids Surf. B Biointerfaces 2006, 48, 58‒66, doi:10.1016/j.colsurfb.2006.01.007.
- Patzelt, A.; Lademann, J.; Richter, H.; Darvin, M.E.; Schanzer, S.; Thiede, G.; Sterry, W.; Vergou, T.; Hauser, M. In vivo investigations on the penetration of various oils and their influence on the skin barrier. Skin Res. Technol. 2012, 18, 364‒369, doi:10.1111/j.1600-0846.2011.00578.x.
- Agrahari, V. Novel drug delivery systems, devices, and fabrication methods. Drug Deliv. Transl. Res. 2018, 8, 303‒306, doi:10.1007/s13346-017-0459-3.
- Rangarajan, M.; Zatz, J.L. Effect of formulation on the topical delivery of alpha-tocopherol. Cosmet. Sci. 2003, 54, 161‒174. 163
- Rozman, B.; Gasperlin, M. Stability of vitamins C and E in topical microemulsions for combined antioxidant therapy. Drug Deliv. 2007, 14, 235‒245, doi:10.1080/10717540601067786.
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Improving the isotretinoin photostability by incorporating in microemulsion matrix. ISRN Pharm. 2011, 2011, 1‒6, doi:10.5402/2011/838016.
- Bhatia, G.; Zhou, Y.; Banga, A.K. Adapalene microemulsion for transfollicular drug delivery. Pharm. Sci. 2013, 102, 2622‒2631, doi:10.1002/jps.23627.
- Ochiuz, L.; Hortolomei, M. Development of Microemulsion Dermal Products Based on Avocado Oil for Topical Administration. In Properties and Uses of Microemulsions; Karunaratne, D.N., Pamunuwa, G., Ranatunga, U., Eds.; IntechOpen: Rijeka, Croatia, 2017; pp. 17‒30, doi:10.5772/66077.
- Kumar, A.; Agarwal, S.P.; Ahuja, A.; Ali, J.; Choudhry, R.; Baboota, S. Preparation, characterization, and in vitro antimicrobial assessment of nanocarrier based formulation of nadifloxacin for acne treatment. Pharmazie 2011, 66, 111‒114.
- Tirnaksiz, F.; Kayiş, A.; Çelebi, N.; Adişen, E.; Erel, A. Preparation and evaluation of topical microemulsion system containing metronidazole for remission in rosacea. Pharm. Bull. 2012, 60, 583‒592, doi:10.1248/cpb.60.583.
- Beheshti-Mall, L.; Shafaroodi, H.; Jafariazar, Z.; Afshar, M. A novel hydrogel-thickened microemulsion of dapsone for acne treatment: Development, characterization, physicochemical stability and ex vivo permeation studiesrmara Pharm. J. 2018, 22, 267‒276, doi:10.12991/mpj.2018.64.
- Salimi, A.; Zadeh, B.S.M.; Godazgari, S.; Rahdar, A. Development and Evaluation of Azelaic Acid-Loaded Microemulsion for Transfollicular Drug Delivery Through Guinea Pig Skin: A Mechanistic Study. Pharm. Bull. 2020, 10, 239‒246, doi:10.34172/apb.2020.028.
- Boonme, P.; Boonthongchuay, C.; Wongpoowarak, W.; Amnuaikit, T. Evaluation of nicotinamide microemulsion on the skin penetration enhancement. Dev. Technol. 2016, 21, 116‒120, doi:10.3109/10837450.2014.971378.
- Szumała, P.; Jungnickel, C.; Kozłowska-Tylingo, K.; Jacyna, B.; Cal, K. Transdermal transport of collagen and hyaluronic acid using water in oil microemulsion. J. Pharm. 2019, 572, doi:10.1016/j.ijpharm.2019.118738.
- Pansang, S.; Maphanta, S.; Tuntijarukorn, P.; Viyocha, J. Skin irritation test of a microemulsion containing essential oil isolated from Ocimum basilicum. Asia 2010, 36, 355‒358, doi:10.2306/scienceasia1513-1874.2010.36.355.
- Jantrawut, P.; Boonsermsukcharoen, K.; Thipnan, K.; Chaiwarit, T.; Hwang, K.-M.; Park, E.-S. Enhancement of Antibacterial Activity of Orange Oil in Pectin Thin Film by Microemulsion. Nanomaterials 2018, 8, 545, doi:10.3390/nano8070545.