Angiogenesis-related cell-surface molecules, including integrins, aminopeptidase N, vascular endothelial growth factor, and gastrin-releasing peptide receptor (GRPR), play a crucial role in tumour formation. Radiolabelled imaging probes targeting angiogenic biomarkers serve as valuable vectors in tumour identification. Nowadays, there is a growing interest in novel radionuclides other than gallium-68 (68Ga) or copper-64 (64Cu) to establish selective radiotracers for the imaging of tumour-associated neo-angiogenesis. Given its ideal decay characteristics (Eβ+average: 632 KeV) and a half-life (T1/2 = 3.97 h) that is well matched to the pharmacokinetic profile of small molecules targeting angiogenesis, scandium-44 (44Sc) has gained meaningful attention as a promising radiometal for positron emission tomography (PET) imaging.
- positron emission tomography (PET)
- Arg-Gly-Asp (RGD)
- scandium-44 (44Sc)
1. Tumour-Related Angiogenesis
2. Scandium-44 (44Sc)
3. PET Radioisotopes Other Than Scandium-44 (44Sc)
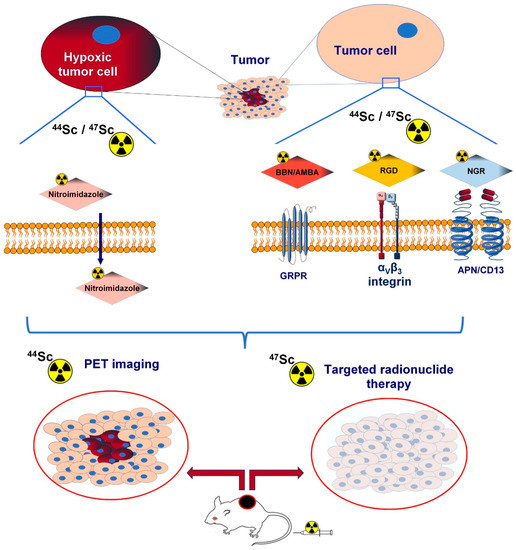
| Investigated Object | Investigated Phenomenon | Target Molecule | (Radio) Labelled Vector | Imaging Technique | Reference |
|---|---|---|---|---|---|
| PCa PC-3 and HaCaT cell lines | In vitro receptor-binding affinity utilizing in vitro blocking studies with BBN | GRPR | [44Sc]Sc-NODAGA-AMBA and [68Ga]Ga-NODAGA-AMBA, | In vitro gamma counter measurements %ID/million cells units | [75] |
| PCa PC-3 tumor-bearing CB17 SCID mice and healthy control | In vitro and in vivo biodistribution pattern, tumor-targeting capability based on blocking experiments | GRPR | [44Sc]Sc-NODAGA-AMBA and [68Ga]Ga-NODAGA-AMBA | In vivo miniPET imaging, ex vivo radioactivity determination by gamma counter (%ID/g), in vivo and ex vivo blocking studies with BBN | [75] |
| PC-3 cell line | In vitro receptor-binding affinity with blocking studies applying [125I-Tyr4]-BN | GRPR | natSc-DOTA-BN[2-14]NH2 and natGa-DOTA-BN[2-14]NH2 | Competitive displacement cell-binding assay | [33] |
| Healthy male Sprague-Dawley rats, male Copenhagen rats bearing androgen-independent Dunning R-3327-AT-1 prostate cancer tumour | In vivo and ex vivo organ distribution, GRPR-targeting ability applying blocking studies with BBN | GRPR | 44Sc-DOTA-BN[2-14]NH2 and 68Ga-DOTA-BN[2-14]NH2 | In vivo dynamic microPET imaging (in tumourous rats), ex vivo (in healthy rats) radioactivity calculations with a dose calibrator (% ID/g) | [33] |
| U87MG and AR42J tumour-bearing female athymic nude mice (CD-1 nude) | Evaluation of in vitro and in vivo behaviour, in vivo and ex vivo biodistribution | αvβ3 integrin | [44Sc]Sc-DOTA-RGD, [44Sc]Sc-NODAGA-RGD, [68Ga]Ga-DOTA-RGD, [68Ga]Ga-NODAGA-RGD | In vivo PET/CT acquisition, ex vivo gamma counting (%IA/g) | [76] |
| 4T1 tumor-bearing BALB/c mice and healthy control | applicability of chelator AAZTA, in vivo biodistribution | αvβ3 integrin | 44Sc3+, and 44Sc(AAZTA)− and 44Sc(CNAAZTA-c(RGDfK) | in vivo PET/MRI examinations | [77] |
| U87MG cells | In vitro receptor-binding affinity and specificity with blocking studies using 125I-Echistatin | αvβ3 integrin | cRGD, (cRGD)2, and DOTA-(cRGD)2 | In vitro competitive cell-binding assay, gamma counter-based detection of the tracer concentration | [17] |
| U87MG glioblastoma-bearing female athymic nude mice | Tumour-targeting competence, specificity applying in vivo and ex vivo receptor blocking with c(RGD)2, in vivo and ex vivo biodistribution | αvβ3 integrin | [44Sc]Sc-DOTA-c(RGD)2 | In vivo microPET/microCT imaging, ex vivo radioactivity determination (%ID/g) | [17] |
| CB17 SCID adult male mice bearing KB tumours and healthy control mice | Synthesis procedure, comparison of 44Sc- and 68Ga-labelled derivatives, in vivo and ex vivo biodistribution | Hypoxia | [44Sc]Sc-DO3AM-NI and [68Ga]Ga- DO3AM-NI | In vivo PET/MRI studies, ex vivo radiopharmaceutical uptake measurement %ID/g | [78] |
| Pro-angiogenic VEGF-A165/NRP-1 complex formation | Investigation of physicochemical properties and affinity for NRP-1 | NRP-1 | 44Sc-radiocompounds (44Sc-1, 44Sc-1bis, 44Sc-2, 44Sc-3):Sc-DOTA-Ahx-A7R (Sc-1), Lys(DOTA-Sc)-A7R (Sc-1bis), Lys(hArg)-Dab(Ahx-DOTA-Sc)-Pro-Arg (Sc-2) and DR7A-DLys(DOTA-Sc) (Sc-3) | Competitive ELISA test | [79] |
| 18F | 68Ga | 64Cu | 43Sc | 44Sc | 89Zr | |
|---|---|---|---|---|---|---|
| Half-life (h) | 1.83 | 1.13 | 12.7 | 3.89 | 3.97 | 78.41 |
| Decay method (%) | EC (3) ß+ (97) |
EC (11.1) ß+ (88.9) |
EC (43.9) ß+ (17.6) ß− (38.5) |
EC (12) ß+ (88) |
EC (5.7) ß+ (94.3) |
EC (77.3) ß+ (22.7) |
| β+ endpoint energy, keV (%) | 633.5 (96.7%) | 1899 (87.7) 822 (1.2) |
653 (17.6) | 1200 (70.9) 826 (17.2) |
1474 (94.3) | 902 (22.8) |
| Principal γ energies, keV (Abs.%) | 511 (194) | 511 (177.8) 1077 (3.2) 1261 (0.1) 1883 (0.1) |
511 (35.2) 1346 (0.5) |
372.8 (23) | 511 (188.5) 1157 (99.9) 1499 (0.9) |
511 (45.5) 909 (99.0) 1713 (0.7) 1745 (0.1) |
This entry is adapted from the peer-reviewed paper 10.3390/ijms24087400
References
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18.
- Jiménez, B. Mechanistic insights on the inhibition of tumor angiogenesis. J. Mol. Med. 2001, 78, 663–672.
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364.
- Folkman, J. Tumor angiogenesis. In Cancer Medicine, 5th ed.; Holland, J.F., Frei, E., III, Bast, R.C., Jr., Eds.; B.C. Decker: Hamilton, ON, Canada, 2000; pp. 132–152.
- Folkman, J. Angiogenesis. In Harrison’s Principles of Internal Medicine, 15th ed.; Braunwald, E., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Jameson, J.L., et al., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 517–530.
- Whitelock, J.M.; Murdoch, A.D.; Iozzo, R.V.; Underwood, P.A. The Degradation of Human Endothelial Cell-derived Perlecan and Release of Bound Basic Fibroblast Growth Factor by Stromelysin, Collagenase, Plasmin, and Heparanases. J. Biol. Chem. 1996, 271, 10079–10086.
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770.
- Asabella, A.N.; Altini, C.; Ferrari, C.; Rubini, G.; Di Palo, A. Multimodality Imaging in Tumor Angiogenesis: Present Status and Perspectives. Int. J. Mol. Sci. 2017, 18, 1864.
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307.
- Bergsland, E.K. Update on Clinical Trials Targeting Vascular Endothelial Growth Factor in Cancer. Am. J. Health Pharm. 2004, 61, S12–S20.
- Siemann, D.W.; Chaplin, D.J.; Horsman, M. Vascular-targeting therapies for treatment of malignant disease. Cancer 2004, 100, 2491–2499.
- Stacy, M.R.; Maxfield, M.W.; Sinusas, A.J. Targeted molecular imaging of angiogenesis in PET and SPECT: A review. Yale J. Biol. Med. 2012, 85, 75–86.
- Huclier-Markai, S.; Alliot, C.; Kerdjoudj, R.; Mougin-Degraef, M.; Chouin, N.; Haddad, F. Promising Scandium Radionuclides for Nuclear Medicine: A Review on the Production and Chemistry up to In Vivo Proofs of Concept. Cancer Biotherapy Radiopharm. 2018, 33, 316–329.
- Vaughn, B.A.; Koller, A.J.; Chen, Z.; Ahn, S.H.; Loveless, C.S.; Cingoranelli, S.J.; Yang, Y.; Cirri, A.; Johnson, C.J.; Lapi, S.E.; et al. Homologous Structural, Chemical, and Biological Behavior of Sc and Lu Complexes of the Picaga Bifunctional Chelator: Toward Development of Matched Theranostic Pairs for Radiopharmaceutical Applications. Bioconjugate Chem. 2021, 32, 1232–1241.
- Snow, M.S.; Foley, A.; Ward, J.L.; Kinlaw, M.T.; Stoner, J.; Carney, K.P. High purity 47Sc production using high-energy photons and natural vanadium targets. Appl. Radiat. Isot. 2021, 178, 109934.
- Türler, A. Matched Pair Theranostics. Chimia 2019, 73, 947–949.
- Hernandez, R.; Valdovinos, H.F.; Yang, Y.; Chakravarty, R.; Hong, H.; Barnhart, T.E.; Cai, W. 44Sc: An Attractive Isotope for Peptide-Based PET Imaging. Mol. Pharm. 2014, 11, 2954–2961.
- Severin, G.; Engle, J.W.; Valdovinos, H.; Barnhart, T.; Nickles, R. Cyclotron produced 44gSc from natural calcium. Appl. Radiat. Isot. 2012, 70, 1526–1530.
- Valdovinos, H.; Hernandez, R.; Barnhart, T.; Graves, S.; Cai, W.; Nickles, R. Separation of cyclotron-produced 44Sc from a natural calcium target using a dipentyl pentylphosphonate functionalized extraction resin. Appl. Radiat. Isot. 2015, 95, 23–29.
- van der Meulen, N.P.; Bunka, M.; Domnanich, K.A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015, 42, 745–751.
- Norman, E.B.; Browne, E.; Chan, Y.D.; Goldman, I.D.; Larimer, R.-M.; Lesko, K.T.; Nelson, M.; Wietfeldt, F.E.; Zlimen, I. Half-life of44Ti. Phys. Rev. C 1998, 57, 2010–2016.
- Roesch, F. Scandium-44: Benefits of a long-lived PET radionuclide available from the (44)Ti/(44)Sc generator system. Curr. Radiopharm. 2012, 5, 187–201.
- Welch, M.J.; McCarthy, T.J. The potential role of generator-produced radiopharmaceuticals in clinical PET. J. Nucl. Med. 2000, 41, 315–317.
- Filosofov, D.V.; Loktionova, N.S.; Rösch, F. A 44Ti/44Sc radionuclide generator for potential application of 44Sc-based PET-radiopharmaceuticals. Radiochim. Acta 2010, 98, 149–156.
- García-Toraño, E.; Peyres, V.; Roteta, M.; Sánchez-Cabezudo, A.; Romero, E.; Ortega, A.M. Standardisation and precise determination of the half-life of 44 Sc. Appl. Radiat. Isot. 2016, 109, 314–318.
- Mikolajczak, R.; Huclier-Markai, S.; Alliot, C.; Haddad, F.; Szikra, D.; Forgacs, V.; Garnuszek, P. Production of scandium radionuclides for theranostic applications: Towards standardization of quality requirements. EJNMMI. Radiopharm. Chem. 2021, 6, 19.
- Sitarz, M.; Cussonneau, J.-P.; Matulewicz, T.; Haddad, F. Radionuclide candidates for β+γ coincidence PET: An overview. Appl. Radiat. Isot. 2020, 155, 108898.
- Ferguson, S.; Jans, H.-S.; Wuest, M.; Riauka, T.; Wuest, F. Comparison of scandium-44 g with other PET radionuclides in pre-clinical PET phantom imaging. EJNMMI Phys. 2019, 6, 23.
- Martiniova, L.; De Palatis, L.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207.
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207.
- Rösch, F.; Baum, R.P. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: On the way to THERANOSTICS. Dalton Trans. 2011, 40, 6104–6111.
- Eppard, E.; de la Fuente, A.; Benešová, M.; Khawar, A.; Bundschuh, R.A.; Gärtner, F.C.; Kreppel, B.; Kopka, K.; Essler, M.; Rösch, F. Clinical Translation and First In-Human Use of Sc-PSMA-617 for PET Imaging of Metastasized Castrate-Resistant Prostate Cancer. Theranostics 2017, 7, 4359–4369.
- Koumarianou, E.; Loktionova, N.; Fellner, M.; Roesch, F.; Thews, O.; Pawlak, D.; Archimandritis, S.; Mikolajczak, R. 44Sc-DOTA-BNNH2 in comparison to 68Ga-DOTA-BNNH2 in pre-clinical investigation. Is 44Sc a potential radionuclide for PET? Appl. Radiat. Isot. 2012, 70, 2669–2676.
- Eppard, E. Pre-Therapeutic Dosimetry Employing Scandium-44 for Radiolabeling PSMA-617. In Prostatectomy; IntechOpen: London, UK, 2019.
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956.
- Khawar, A.M.; Eppard, E.; Sinnes, J.P.D.; Roesch, F.; Ahmadzadehfar, H.M.; Kürpig, S.; Meisenheimer, M.M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Sc-PSMA-617 Biodistribution and Dosimetry in Patients With Metastatic Castration-Resistant Prostate Carcinoma. Clin. Nucl. Med. 2018, 43, 323–330.
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617—Preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9.
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208.
- Deilami-Nezhad, L.; Moghaddam-Banaem, L.; Sadeghi, M. Development of bone seeker radiopharmaceuticals by Scandium-47 and estimation of human absorbed dose. Appl. Radiat. Isot. 2017, 129, 108–116.
- Majkowska-Pilip, A.; Bilewicz, A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J. Inorg. Biochem. 2011, 105, 313–320.
- Viola-Villegas, N.; Doyle, R.P. The coordination chemistry of 1,4,7,10-tetraazacyclododecane-N,N′,N″,N′″-tetraacetic acid (H4DOTA): Structural overview and analyses on structure–stability relationships. Co-ord. Chem. Rev. 2009, 253, 1906–1925.
- Chakravarty, R.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Hong, H.; Nickles, R.J.; Cai, W. Matching the Decay Half-Life with the Biological Half-Life: ImmunoPET Imaging with 44Sc-Labeled Cetuximab Fab Fragment. Bioconjugate Chem. 2014, 25, 2197–2204.
- Nagy, G.; Dénes, N.; Kis, A.; Szabó, J.P.; Berényi, E.; Garai, I.; Bai, P.; Hajdu, I.; Szikra, D.; Trencsényi, G. Preclinical evaluation of melanocortin-1 receptor (MC1-R) specific 68Ga- and 44Sc-labeled DOTA-NAPamide in melanoma imaging. Eur. J. Pharm. Sci. 2017, 106, 336–344.
- Mausner, L.F.; Joshi, V.; Kolsky, K.L. Evaluation of chelating agents for radioimmunotherapy with scandium-47. J. Nucl. Med. 1995, 36.
- Kolsky, K.; Joshi, V.; Mausner, L.; Srivastava, S. Radiochemical purification of no-carrier-added scandium-47 for radioimmunotherapy. Appl. Radiat. Isot. 1998, 49, 1541–1549.
- Lima, T.V.M.; Gnesin, S.; Strobel, K.; Pérez, M.D.S.; Roos, J.E.; Müller, C.; van der Meulen, N.P. Fifty Shades of Scandium: Comparative Study of PET Capabilities Using Sc-43 and Sc-44 with Respect to Conventional Clinical Radionuclides. Diagnostics 2021, 11, 1826.
- Singh, A.; van der Meulen, N.P.; Grubmüller, B.; Klette, I.; Kulkarni, H.R.; Türler, A.; Schibli, R.; Baum, R.P.; Dash, A.; Chakraborty, S.; et al. First-in-Human PET/CT Imaging of Metastatic Neuroendocrine Neoplasms with Cyclotron-Produced 44Sc-DOTATOC: A Proof-of-Concept Study. Cancer Biotherapy Radiopharm. 2017, 32, 124–132.
- Alauddin, M.M. Positron emission tomography (PET) imaging with (18)F-based radiotracers. Am. J. Nucl. Med. Mol. Imaging 2011, 2, 55–76.
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 1–17.
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem. 2015, 26, 1–18.
- Richter, S.; Wuest, F. 18F-Labeled Peptides: The Future Is Bright. Molecules 2014, 19, 20536–20556.
- Asti, M.; De Pietri, G.; Fraternali, A.; Grassi, E.; Sghedoni, R.; Fioroni, F.; Roesch, F.; Versari, A.; Salvo, D. Validation of 68Ge/68Ga generator processing by chemical purification for routine clinical application of 68Ga-DOTATOC. Nucl. Med. Biol. 2008, 35, 721–724.
- Fani, M.; André, J.P.; Maecke, H.R. 68Ga-PET: A powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging 2008, 3, 53–63.
- Müller, C.; Bunka, M.; Reber, J.; Fischer, C.; Zhernosekov, K.; Türler, A.; Schibli, R. Promises of Cyclotron-Produced 44Sc as a Diagnostic Match for Trivalent β−-Emitters: In Vitro and In Vivo Study of a 44Sc-DOTA-Folate Conjugate. J. Nucl. Med. 2013, 54, 2168–2174.
- Breeman, W.A.; de Blois, E.; Chan, H.S.; Konijnenberg, M.; Kwekkeboom, D.J.; Krenning, E.P. 68Ga-labeled DOTA-Peptides and 68Ga-labeled Radiopharmaceuticals for Positron Emission Tomography: Current Status of Research, Clinical Applications, and Future Perspectives. Semin. Nucl. Med. 2011, 41, 314–321.
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-Octreotide PET in Neuroendocrine Tumors: Comparison with Somatostatin Receptor Scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518.
- Roesch, F.; Riss, P.J. The Renaissance of the 68Ge/68Ga Radionuclide Generator Initiates New Developments in 68Ga Radiopharmaceutical Chemistry. Curr. Top. Med. Chem. 2010, 10, 1633–1668.
- Fichna, J.; Janecka, A. Synthesis of Target-Specific Radiolabeled Peptides for Diagnostic Imaging. Bioconjugate Chem. 2003, 14, 3–17.
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997, 24, 35–43.
- Anderson, C.J.; Ferdani, R. Copper-64 Radiopharmaceuticals for PET Imaging of Cancer: Advances in Preclinical and Clinical Research. Cancer Biotherapy Radiopharm. 2009, 24, 379–393.
- Holland, J.P.; Ferdani, R.; Anderson, C.J.; Lewis, J.S. Copper-64 Radiopharmaceuticals for Oncologic Imaging. PET Clin. 2009, 4, 49–67.
- Walczak, R.; Krajewski, S.; Szkliniarz, K.; Sitarz, M.; Abbas, K.; Choiński, J.; Jakubowski, A.; Jastrzębski, J.; Majkowska, A.; Simonelli, F.; et al. Cyclotron production of 43Sc for PET imaging. EJNMMI Phys. 2015, 2, 33.
- Zhang, Y.; Hong, H.; Cai, W. PET Tracers Based on Zirconium-89. Curr. Radiopharm. 2011, 4, 131–139.
- de Ruijter, L.K.; Hooiveld-Noeken, J.S.; Giesen, D.; Hooge, M.N.L.-D.; Kok, I.C.; Brouwers, A.H.; Elias, S.G.; Nguyen, M.T.; Lu, H.; Gietema, J.A.; et al. First-in-Human Study of the Biodistribution and Pharmacokinetics of 89Zr-CX-072, a Novel Immunopet Tracer Based on an Anti–PD-L1 Probody. Clin. Cancer Res. 2021, 27, 5325–5333.
- Mulgaonkar, A.; Elias, R.; Woolford, L.; Guan, B.; Nham, K.; Kapur, P.; Christie, A.; Tcheuyap, V.T.; Singla, N.; Bowman, I.A.; et al. ImmunoPET Imaging with 89Zr-Labeled Atezolizumab Enables In Vivo Evaluation of PD-L1 in Tumorgraft Models of Renal Cell Carcinoma. Clin. Cancer Res. 2022, 28, 4907–4916.
- Dijkers, E.C.; Kosterink, J.G.; Rademaker, A.P.; Perk, L.R.; van Dongen, G.A.; Bart, J.; de Jong, J.R.; de Vries, E.G.; Lub-de Hooge, M.N. Development and Characterization of Clinical-Grade 89Zr-Trastuzumab for HER2/neu ImmunoPET Imaging. J. Nucl. Med. 2009, 50, 974–981.
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression In Vivo. J. Nucl. Med. 2010, 51, 1293–1300.
- Nagengast, W.B.; De Vries, E.G.; Hospers, G.A.; Mulder, N.H.; De Jong, J.R.; Hollema, H.; Brouwers, A.H.; Van Dongen, G.A.; Perk, L.R.; Lub-de Hooge, M.N. In Vivo VEGF Imaging with Radiolabeled Bevacizumab in a Human Ovarian Tumor Xenograft. J. Nucl. Med. 2007, 48, 1313–1319.
- Perk, L.R.; Visser, G.W.M.; Vosjan, M.J.W.D.; Walsum, M.S.-V.; Tijink, B.M.; Leemans, C.R.; van Dongen, G.A.M.S. (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J. Nucl. Med. 2005, 46, 1898–1906.
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with 89Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2012, 40, 3–14.
- Verel, I.; Visser, G.W.M.; Boellaard, R.; Boerman, O.C.; Van Eerd, J.; Snow, G.B.; A Lammertsma, A.; van Dongen, G.A.M.S. Quantitative 89Zr immuno-PET for in vivo scouting of 90Y-labeled monoclonal antibodies in xenograft-bearing nude mice. J. Nucl. Med. 2003, 44, 1663–1670.
- Zeglis, B.M.; Houghton, J.L.; Evans, M.J.; Viola, N.; Lewis, J.S. Underscoring the Influence of Inorganic Chemistry on Nuclear Imaging with Radiometals. Inorg. Chem. 2014, 53, 1880–1899.
- Alzimami, K.S.; Ma, A.K. Effective dose to staff members in a positron emission tomography/CT facility using zirconium-89. Br. J. Radiol. 2013, 86, 20130318.
- Vugts, D.J.; van Dongen, G.A. 89Zr-labeled compounds for PET imaging guided personalized therapy. Drug Discov. Today Technol. 2011, 8, e53–e61.
- Kálmán-Szabó, I.; Szabó, J.P.; Arató, V.; Dénes, N.; Opposits, G.; Jószai, I.; Kertész, I.; Képes, Z.; Fekete, A.; Szikra, D.; et al. PET Probes for Preclinical Imaging of GRPR-Positive Prostate Cancer: Comparative Preclinical Study of Ga-NODAGA-AMBA and Sc-NODAGA-AMBA. Int. J. Mol. Sci. 2022, 23, 10061.
- Domnanich, K.A.; Müller, C.; Farkas, R.; Schmid, R.M.; Ponsard, B.; Schibli, R.; Türler, A.; van der Meulen, N.P. 44Sc for labeling of DOTA- and NODAGA-functionalized peptides: Preclinical in vitro and in vivo investigations. EJNMMI. Radiopharm. Chem. 2017, 1, 8.
- Nagy, G.; Szikra, D.; Trencsényi, G.; Fekete, A.; Garai, I.; Giani, A.M.; Negri, R.; Masciocchi, N.; Maiocchi, A.; Uggeri, F.; et al. AAZTA: An Ideal Chelating Agent for the Development of 44 Sc PET Imaging Agents. Angew. Chem. Int. Ed. 2017, 56, 2118–2122.
- Szücs, D.; Csupász, T.; Szabó, J.P.; Kis, A.; Gyuricza, B.; Arató, V.; Forgács, V.; Vágner, A.; Nagy, G.; Garai, I.; et al. Synthesis, Physicochemical, Labeling and In Vivo Characterization of 44Sc-Labeled DO3AM-NI as a Hypoxia-Sensitive PET Probe. Pharmaceuticals 2022, 15, 666.
- Masłowska, K.; Redkiewicz, P.; Halik, P.K.; Witkowska, E.; Tymecka, D.; Walczak, R.; Choiński, J.; Misicka, A.; Gniazdowska, E. Scandium-44 Radiolabeled Peptide and Peptidomimetic Conjugates Targeting Neuropilin-1 Co-Receptor as Potential Tools for Cancer Diagnosis and Anti-Angiogenic Therapy. Biomedicines 2023, 11, 564.
