You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
In Africa, the prevalence of Antimicrobial Resistance (AMR) in wastewater is of particular concern due to the inadequate sanitation and wastewater treatment facilities, coupled with the overuse and misuse of antibiotics in healthcare and agriculture.
- low- and middle-income countries
- environmental health
- public health
1. Introduction
Antimicrobial resistance (AMR) has been recognised by countries and organisations worldwide as one of the biggest threats to public health in recent times [1][2][3]. It is estimated that without appropriate preventive or remedial measures, the world may experience approximately 10 million losses of lives and over USD 100 trillion annually in the global economy by 2050 [4].
Although microorganisms possess intrinsic resistance to naturally occurring stressors, the indiscriminate use of pharmaceuticals has been recognised as the most significant contributor to acquired resistance in these organisms, thus escalating the threat to human health [5][6]. For example, the massive and increasing demand for animal protein has engendered an unparalleled use of antibiotics in food animal production, which in 2017 was estimated at 93,309 tons per year globally, with an expected 11.5% increase by 2030 [7]. Furthermore, in humans, misdiagnosis of infections results in the inappropriate prescription of many antibiotics [8]. Therefore, to curb this ill, the World Health Organization (WHO) has identified critical factors driving AMR, including the abusive use of these pharmaceuticals, nonavailability of clean water, sanitation and hygiene (WASH) for human and animal use, inadequate measures to control and prevent infections and diseases in health and animal production settings, inaccessibility to good, and cost-effective medications, vaccines and test procedures, unawareness and lack of knowledge regarding the problem, and nonenforcement of legislation [9].
However, a considerable proportion of the antibiotics consumed by humans and animals are mostly excreted in partially or completely unmetabolised forms, usually containing active ingredients [10][11]. This results in the inevitable discharge of these pharmaceutically active compounds into the environment, especially water bodies, with the major consequence being the potential selection for the survival of resistant microorganisms. With this, wastewater treatment plants (WWTPs) have been recognised as being among the hotspots for the discharge of antibiotics, their residues and antibiotic-resistant bacteria into the environment [12][13][14][15][16][17].
Despite the perceived role of these WWTPs on the spread of AMR, studies evaluating their impact are limited, especially in low- and middle-income countries (LMICs) such as South Africa, where such facilities are usually nonfunctional or function sub-optimally. Furthermore, where such studies are available, the link between environmental and clinical isolates is not apparent, probably because of the basic analyses performed that usually have low discriminating powers to establish such associations. Moreover, the lack of proper reporting of findings influences the acquisition of such data in the public domain. Thus, the present research evaluated the existing literature on AMR in Africa between 2012 and 2022, emphasising South Africa as a case study, to identify gaps that need to be filled to inform future preventive and mitigation measures towards AMR.
2. Overview of African Studies between 2012 and 2022
In Africa, the prevalence of AMR in wastewater is of particular concern due to the inadequate sanitation and wastewater treatment facilities, coupled with the overuse and misuse of antibiotics in healthcare and agriculture. African countries, especially in the sub-Saharan region, have the highest disease burdens in the world, with infectious diseases accounting for over 227 million healthy life years and over USD 800 billion yearly productivity loss globally [18]. The ripple effect of this health situation has been identified as the primary factor driving the excessive rate of antimicrobial prescriptions within the continent [19]. For example, consumption of antibiotics in the WHO Watch list increased by 165% in LMIC (including African countries) compared to approximately 28% in their high-income counterparts between 2000 and 2015 [19].
This high antibiotic use implies that wastewater in these countries would be rich in antibiotic residues, antibiotic-resistant bacteria (ARB) and their associated antibiotic resistance genes (ARGs). For example, a study in Ghana investigated resistance genes, and mobile genetic elements (MGEs), from drainage and canalizations before and after three hospitals and an urban waste treatment plant [20]. The main idea was to establish the relationship between the hospital and the wastewater resistome. The authors used a combination of culture-dependent and independent methods, including high-throughput whole-genome sequencing on two sequencing platforms, Nanopore (long reads) and Illumina (short reads). The authors recorded higher resistance rates to carbapenems in the canalization after the hospitals, indicating that the hospital wastewater contributed significantly to the dissemination of resistant bacteria in the environment. Furthermore, the study identified several carbapenemase/β-lactamase genes, including novel variants, such as blaDIM-1, blaVIM-71, blaCARB-53, and blaCMY-172, with some of these genes associated with MGEs, meaning that these could easily be transferred within and between bacterial communities.
In Nigeria, Akpan et al. [21] isolated Gram-negative bacteria from an abattoir’s wastewater and tested them for antibiotic resistance against five antibiotics to determine the impact of the abattoir on the environmental resistome. The organisms isolated included Salmonella spp., E. coli, Klebsiella spp., Shigella spp., Pseudomonas spp. and Enterobacter spp. The authors observed that a significant proportion of the isolates (~67%) were resistant to all antibiotics tested, with a 77% multidrug resistance recorded across the samples. However, no extended-spectrum β-lactamase (ESBL)-producing traits were observed in any of the isolates. This study demonstrated that abattoirs contributed considerably to AMR in the aquatic environment.
Tesfaye et al. [22] investigated antimicrobial resistance in Enterobacteriaceae in wastewater collected from health settings, an abattoir, and a WWTP, including downstream of a river in Addis Ababa, Ethiopia. The authors obtained 54 isolates, including E. coli, Salmonella spp., Klebsiella pneumoniae, Enterobacter aerogenes, Citrobacter spp., Klebsiella oxytoca and Enterobacter cloacae. Antibiotic susceptibility testing revealed that all the isolates were multidrug resistant, while two isolates were resistant to all the 12 antibiotics tested. ESBL production was also recorded in 27.3% of the resistant isolates. Furthermore, the hospital wastewater had a higher percentage of resistance than all the other sites, again identifying hospital wastewater as a hotspot for AMR dissemination.
A major shortcoming in all the studies reviewed is that most focused on a one-off sampling, usually resulting in a very limited number of isolates or samples. Such small sample sizes would make it challenging to draw strong conclusions and would require further investigations. Furthermore, many studies used culture or sequencing and only a few used both. Using only the culture methods could underestimate the microbial load due to viable but non-culturable isolates, reducing the reported resistome. On the other hand, using only genomic approaches could overestimate the risk associated with AMR in wastewater. Nevertheless, the presence of any resistance genes and MGEs would signify the possible transmission to other related or even unrelated species. A summary of some studies on wastewater resistome in Africa is provided in Table 1.
Table 1. Summary of some studies on AMR in wastewater in Africa between 2012 and 2022.
| Country | & Wastewater Type/Source | Duration of Study | Sample Size | Targeted Resistance | Phenotypic (P)/Genotypic (G) Resistance | Method | Reference |
|---|---|---|---|---|---|---|---|
| * South Africa | WWTP | Two campaigns—actual duration not mentioned | # Not indicated | Cefotaxime-resistance | P | Culture | [23] |
| Algeria | WWTP | 3 days in 2 months | Not indicated | ESBLs and associated quinolone resistance | P, G | Culture; PCR | [24] |
| Botswana | WWTP | $ One-off sampling | one | Overall resistome | G | Shotgun metagenomics | [25] |
| Botswana | WWTP | Monthly for 1 year | 72 | General resistance—9 antibiotics tested | P | Culture | [26] |
| Burkina Faso | Urban channel | 6 months | 101 | ESBLs | P | Culture | [27] |
| Burkina Faso | WWTP | Monthly for 5 months | 15 | General resistance—19 antibiotics | P | Culture | [28] |
| Cameroon | Open-air canals | One-off | 6 (composite) samples | Overall resistome | G | Shotgun metagenomics | [29] |
| Ethiopia | Hospital wastewater | 3 months | 27 | General resistance—13 antibiotics | P | Culture | [30] |
| Ethiopia | Hospital wastewater | 4 months | 40 (composite samples) | General resistance—13 antibiotics | P | Culture | [31] |
| Ghana | WWTP | Monthly—6 months | 30 | General resistance | P | Culture | [32] |
| Kenya | University WWTP | 4 months | Not mentioned | Overall resistome | P, G | Culture; whole-genome sequencing | [33] |
| Kenya | Septic tank | 2 months | Not mentioned | General resistance | P | Culture | [34] |
| Kenya | WWTP | 6 months (covering the dry and rainy seasons) | 24 | General resistance | P | Culture | [35] |
| Nigeria | Hospital WWTP | Weekly for 4 months | Not mentioned | ESBLs | P, G | Culture; PCR | Adekanmbi |
| Senegal | Slaughterhouse wastewater and WWTP | Not mentioned | Not mentioned | General resistance—16 antibiotics | P | Culture | [36] |
| South Africa | WWTP | 7 months (Every two weeks) | 81 | Overall resistome | P, G | Culture; whole-genome sequencing | [37] |
| Tanzania | WWTP | 2013/2014 (Not specific) | 52 | General resistance—14 antibiotics | P | Microdilution | [38] |
| Tunisia | WWTP | Not mentioned | Not mentioned | intI1, ARGs blaCTX-M, blaTEM, qnrA, qnrS, sul I, ermB |
G | PCR | [39] |
| Uganda | Multiple sources | Not mentioned | Not mentioned | General resistance—15 antibiotics | P | Culture | [40] |
| Zambia | Wastewater ponds | Not mentioned | 5 samples | General resistance—8 antibiotics | P | Culture | [41] |
| Zimbabwe | Abattoir wastewater | 3 months | 600 samples | General resistance—16 antibiotics | P | Culture | [42] |
* Part of a multinational (22 countries) study in Europe, Asia, Africa, Australia, and North America. # A total of 472 samples were collected from all the countries. $ Analysed once and used to irrigate soil. Focus was not on the monitoring of the wastewater resistome but on the impact of the wastewater on the soil resistome. & Includes influent or effluent or both.
Despite the recognised role of WWTPs in AMR, studies on AMR in wastewater are not evenly distributed within the continent, with most of the studies reported in South Africa (Figure 1).
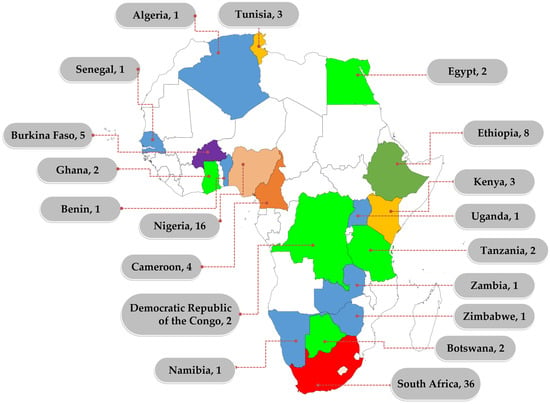
Figure 1. Distribution of African studies on AMR in wastewater between 2012 and 2022. Numbers represent the number of studies identified within the reviewed period. Only counties that reported at least one study in the review period are labelled.
However, it is evident that wastewater as a reservoir and source of AMR is gaining attention in Africa, as seen by the increasing trend of studies focusing on wastewater (Figure 2).
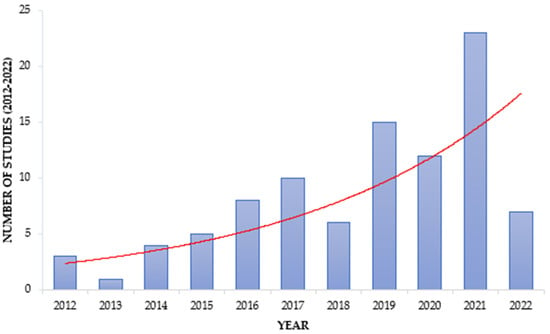
Figure 2. Trend in ARM studies focusing on wastewater. The red line shows the increasing trend within the reviewed period.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12050805
References
- UN United Nations Meeting on Antimicrobial Resistance. Bull. World Health Organ. 2016, 94, 638–639.
- World Health Organization United Nations High-Level Meeting on Antimicrobial Resistance. Available online: https://apps.who.int/mediacentre/events/2016/antimicrobial-resistance/en/index.html (accessed on 14 April 2023).
- WHO. Antimicrobial Resistance and the United Nations Sustainable Development Cooperation Framework. Guidance for United Nations Country Teams; WHO Press: Geneva, Switzerlan, 2021; pp. 1–24.
- Stanton, I.C.; Bethel, A.; Frances, A.; Leonard, C.; Gaze, W.H.; Garside, R. Existing Evidence on Antibiotic Resistance Exposure and Transmission to Humans from the Environment: A Systematic Map. Environ. Evid. 2022, 11, 8.
- Essack, S.Y.; Sartorius, B. Global Antibiotic Resistance: Of Contagion, Confounders, and the COM-B Model. Lancet Planet. Health 2018, 2, e376–e377.
- De Sosa, J.A.; Byarugaba, D.K.; Amabile-Cuevas, C.F.; Hsueh, P.R.; Kariuki, S.; Okeke, I.N. Antimicrobial Resistance in Developing Countries; Springer: New York, NY, USA, 2010; ISBN 9780387893709.
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918.
- Kubone, P.Z.; Mlisana, K.P.; Govinden, U.; Abia, A.L.K.; Essack, S.Y. Antibiotic Susceptibility and Molecular Characterization of Uropathogenic Escherichia coli Associated with Community-Acquired Urinary Tract Infections in Urban and Rural Settings in South Africa. Trop. Med. Infect. Dis. 2020, 5, 176.
- WHO Global Action Plan on Antimicrobial Resistance; WHO Document Production Services; WHO: Geneva, Switzerland, 2015.
- Chereau, F.; Opatowski, L.; Tourdjman, M.; Vong, S. Risk Assessment for Antibiotic Resistance in South East Asia. BMJ 2017, 358, j3393.
- O’Neill, J. The Review on Antimicrobial Resistance Chaired by Jim O’Neill. 2015. Available online: https://amr-review.org/sites/default/files/Report-52.15.pdf (accessed on 3 February 2023).
- Proia, L.; Anzil, A.; Borrego, C.; Farrè, M.; Llorca, M.; Sanchis, J.; Bogaerts, P.; Balcázar, J.L.; Servais, P. Occurrence and Persistence of Carbapenemases Genes in Hospital and Wastewater Treatment Plants and Propagation in the Receiving River. J. Hazard. Mater. 2018, 358, 33–43.
- Moslah, B.; Hapeshi, E.; Jrad, A.; Fatta-Kassinos, D.; Hedhili, A. Pharmaceuticals and Illicit Drugs in Wastewater Samples in North-Eastern Tunisia. Environ. Sci. Pollut. Res. 2018, 25, 18226–18241.
- Sinthuchai, D.; Boontanon, S.K.; Boontanon, N.; Polprasert, C. Evaluation of Removal Efficiency of Human Antibiotics in Wastewater Treatment Plants in Bangkok, Thailand. Water Sci. Technol. 2016, 73, 182–191.
- Li, X.; Shi, H.; Li, K.; Zhang, L.; Gan, Y. Occurrence and Fate of Antibiotics in Advanced Wastewater Treatment Facilities and Receiving Rivers in Beijing, China. Front. Environ. Sci. Eng. 2014, 8, 888–894.
- Zhang, Y.; Marrs, C.F.; Simon, C.; Xi, C. Wastewater Treatment Contributes to Selective Increase of Antibiotic Resistance among Acinetobacter spp. Sci. Total Environ. 2009, 407, 3702–3706.
- Omuferen, L.O.; Maseko, B.; Olowoyo, J.O. Occurrence of Antibiotics in Wastewater from Hospital and Convectional Wastewater Treatment Plants and Their Impact on the Effluent Receiving Rivers: Current Knowledge between 2010 and 2019. Environ. Monit. Assess. 2022, 194, 306.
- Nkengasong, J.N.; Tessema, S.K. Africa Needs a New Public Health Order to Tackle Infectious Disease Threats. Cell 2020, 183, 296–300.
- Sriram, A.; Kalanxhi, E.; Kapoor, G.; Craig, J.; Ruchita Balasubramanian, S.B.; Criscuolo, N.; Hamilton, A.; Klein, E.; Tseng, K.; Van Boeckel, T.; et al. The State of the World’s Antibiotics in 2021: A Global Analysis of Antimicrobial Resistance and Its Drivers; 2021. Available online: https://onehealthtrust.org/publications/reports/the-state-of-the-worlds-antibiotic-in-2021/ (accessed on 3 February 2023).
- Delgado-Blas, J.F.; Valenzuela Agüi, C.; Marin Rodriguez, E.; Serna, C.; Montero, N.; Saba, C.K.S.; Gonzalez-Zorn, B. Dissemination Routes of Carbapenem and Pan-Aminoglycoside Resistance Mechanisms in Hospital and Urban Wastewater Canalizations of Ghana. mSystems 2022, 7, e01019–e01021.
- Akpan, S.N.; Odeniyi, O.A.; Adebowale, O.O.; Alarape, S.A.; Adeyemo, O.K. Antibiotic Resistance Profile of Gram-Negative Bacteria Isolated from Lafenwa Abattoir Effluent and Its Receiving Water (Ogun River) in Abeokuta, Ogun State, Nigeria. Onderstepoort J. Vet. Res. 2020, 87, 1–6.
- Tesfaye, H.; Alemayehu, H.; Desta, A.F.; Eguale, T. Antimicrobial Susceptibility Profile of Selected Enterobacteriaceae in Wastewater Samples from Health Facilities, Abattoir, Downstream Rivers and a WWTP in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control 2019, 8, 134.
- Marano, R.B.M.; Fernandes, T.; Manaia, C.M.; Nunes, O.; Morrison, D.; Berendonk, T.U.; Kreuzinger, N.; Telson, T.; Corno, G.; Fatta-Kassinos, D.; et al. A Global Multinational Survey of Cefotaxime-Resistant Coliforms in Urban Wastewater Treatment Plants. Environ. Int. 2020, 144, 106035.
- Alouache, S.; Estepa, V.; Messai, Y.; Ruiz, E.; Torres, C.; Bakour, R. Characterization of ESBLs and Associated Quinolone Resistance in Escherichia coli and Klebsiella pneumoniae Isolates from an Urban Wastewater Treatment Plant in Algeria. Microb. Drug Resist. 2014, 20, 30–38.
- Onalenna, O.; Rahube, T.O. Assessing Bacterial Diversity and Antibiotic Resistance Dynamics in Wastewater Effluent-Irrigated Soil and Vegetables in a Microcosm Setting. Heliyon 2022, 8, e09089.
- Tapela, K.; Rahube, T. Isolation and Antibiotic Resistance Profiles of Bacteria from Influent, Effluent and Downstream: A Study in Botswana. Afr. J. Microbiol. Res. 2019, 13, 279–289.
- Soré, S.; Sawadogo, Y.; Bonkoungou, J.I.; Kaboré, S.P.; Béogo, S.; Sawadogo, C.; Bationo, B.G.; Ky, H.; Madingar, P.D.-M.; Ouédraogo, A.S.; et al. Detection, Identification and Characterization of Extended-Spectrum Beta-Lactamases Producing Enterobacteriaceae in Wastewater and Salads Marketed in Ouagadougou, Burkina Faso. Int. J. Biol. Chem. Sci. 2020, 14, 2746–2757.
- Abasse, O.; Boukaré, K.; Sampo, E.; Bouda, R.; CISSE, H.; Stéphane, K.; Odetokun, I.; Sawadogo, A.; Henri Nestor, B.; Savadogo, A. Spread and Antibiotic Resistance Profile of Pathogens Isolated from Human and Hospital Wastewater in Ouagadougou. Microbes Infect. Dis. 2022, 3, 318–331.
- Bougnom, B.P.; McNally, A.; Etoa, F.X.; Piddock, L.J. Antibiotic Resistance Genes Are Abundant and Diverse in Raw Sewage Used for Urban Agriculture in Africa and Associated with Urban Population Density. Environ. Pollut. 2019, 251, 146–154.
- Mekengo, B.M.; Hussein, S.; Ali, M.M. Distribution and Antimicrobial Resistance Profile of Bacteria Recovered from Sewage System of Health Institutions Found in Hawassa, Sidama Regional State, Ethiopia: A Descriptive Study. SAGE Open Med. 2021, 9, 205031212110390.
- Asfaw, T.; Negash, L.; Kahsay, A.; Weldu, Y. Antibiotic Resistant Bacteria from Treated and Untreated Hospital Wastewater at Ayder Referral Hospital, Mekelle, North Ethiopia. Adv. Microbiol. 2017, 7, 871–886.
- Adomako, L.A.B.; Yirenya-Tawiah, D.; Nukpezah, D.; Abrahamya, A.; Labi, A.K.; Grigoryan, R.; Ahmed, H.; Owusu-Danquah, J.; Annang, T.Y.; Banu, R.A.; et al. Reduced Bacterial Counts from a Sewage Treatment Plant but Increased Counts and Antibiotic Resistance in the Recipient Stream in Accra, Ghana—A Cross-Sectional Study. Trop. Med. Infect. Dis. 2021, 6, 79.
- Wawire, S.A.; Reva, O.N.; O’Brien, T.J.; Figueroa, W.; Dinda, V.; Shivoga, W.A.; Welch, M. Virulence and Antimicrobial Resistance Genes Are Enriched in the Plasmidome of Clinical Escherichia coli Isolates Compared with Wastewater Isolates from Western Kenya. Infect. Genet. Evol. 2021, 91, 104784.
- Mutuku, C. Antibiotic Resistance Profiles among Enteric Bacteria Isolated from Wastewater in Septic Tanks 2017. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 27, 99–107.
- Song’oro, E.; Nyerere, A.; Magoma, G.; Gunturu, R. Occurrence of Highly Resistant Microorganisms in Ruai Wastewater Treatment Plant and Dandora Dumpsite in Nairobi County, Kenya. Adv. Microbiol. 2019, 9, 479–494.
- Alpha, A.D.; Delphine, B.; Fatou, T.L.; Mbaye, M.; Mohamed, M.S.; Moussa, D.; Yacine, S.; Monique, K.; Rianatou, A.; Yaya, T.; et al. Prevalence of Pathogenic and Antibiotics Resistant Escherichia coli from Effluents of a Slaughterhouse and a Municipal Wastewater Treatment Plant in Dakar. African J. Microbiol. Res. 2017, 11, 1035–1042.
- Mbanga, J.; Amoako, D.G.; Abia, A.L.K.; Allam, M.; Ismail, A.; Essack, S.Y. Genomic Analysis of Enterococcus spp. Isolated from a Wastewater Treatment Plant and Its Associated Waters in Umgungundlovu District, South Africa. Front. Microbiol. 2021, 12, 648454.
- Mhongole, O.J.; Mdegela, R.H.; Kusiluka, L.J.M.; Forslund, A.; Dalsgaard, A. Characterization of Salmonella spp. from Wastewater Used for Food Production in Morogoro, Tanzania. World J. Microbiol. Biotechnol. 2017, 33, 42.
- Rafraf, I.D.; Lekunberri, I.; Sànchez-Melsió, A.; Aouni, M.; Borrego, C.M.; Balcázar, J.L. Abundance of Antibiotic Resistance Genes in Five Municipal Wastewater Treatment Plants in the Monastir Governorate, Tunisia. Environ. Pollut. 2016, 219, 353–358.
- Afema, J.A.; Byarugaba, D.K.; Shah, D.H.; Atukwase, E.; Nambi, M.; Sischo, W.M. Potential Sources and Transmission of Salmonella and Antimicrobial Resistance in Kampala, Uganda. PLoS ONE 2016, 11, e0152130.
- Mubbunu, L.; Siyumbi, S.; Katongo, C.; Mwambungu, A. Waste Water as Reservoir of Antibiotic Resistant Micro-Organisms: A Case of Luanshya Waste Water Ponds. Int. J. Res. Med. Health Sci. 2014, 4, 9.
- Gufe, C.; Ndlovu, M.N.; Sibanda, Z.; Makuvara, Z.; Marumure, J. Prevalence and Antimicrobial Profile of Potentially Pathogenic Bacteria Isolated from Abattoir Effluents in Bulawayo, Zimbabwe. Sci. African 2021, 14, e01059.
This entry is offline, you can click here to edit this entry!
