Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Chitosan nanoparticles (CSNPs) have prospects as a revolutionary delivery system capable of enhancing anticancer drug activity and reducing negative impacts on normal cells. The use of smart drug delivery systems (SDDs) as delivering materials to improve the bioactivity of NPs and to understand the intricacies of breast cancer has garnered significant interest.
- targeting
- chitosan
- breast cancer
- nanosystem
- nanovehicle
1. Introduction
The hallmark properties of polymeric nanoparticles as cancer nanovehicles have been proven in various research including targeted drug delivery, controlled drug release, enhanced therapeutic efficacy and safety [17,18,19,20,21]. Efforts in the development of drug delivery systems for chemotherapy have been focused on delivering chemotherapy drugs directly to cancer cells while minimizing toxic effects on healthy tissue. This strategy has the potential to increase treatment efficacy and reduce side effects [22,23,24]. Polymeric NPs (PNPs) are submicron colloidal particles broadly used in drug delivery systems, owing to the relatively easy attachment of targeting ligands to the surface of NPs. Surface modification of these PNPs with targeting ligands enables recognition by specific receptors or ligand-binding sites that are overexpressed on cancer cells or at target sites, for the controlled release of loaded drugs [25,26,27,28]. The rational design of targeted drug delivery systems based on PNPs involves taking into consideration their composition, solubility, crystallinity, molecular weight, backbone stability, hydrophobicity and polydispersity. The polymeric shell of PNPs protects the drugs from enzymatic degradation. Furthermore, hydrophilic chains can be attached to the outer surface of the PNPs to avoid the process of phagocytosis and opsonization by circulating phagocytic cells. There are several advantages of using natural polymers as nanovehicles in cancer therapy: biocompatibility, biodegradability, targeting moieties, low immunogenicity, and cost-effectiveness [29,30,31]. Polymeric nanoparticles are a promising drug delivery system for chemotherapy; however, several challenges need to be addressed to optimize their effectiveness. These challenges include achieving optimal drug loading, achieving stable drug release, and preventing the development of drug resistance in cancer cells [22,24,32,33]. Notably, several types of polymers have been employed for this purpose, including polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), chitosan, poly(ethyleneimine) (PEI), poly(methacrylate) (PMA), and poly(N-isopropylacrylamide) (PNIPAM).
Chitosan, a biodegradable and biocompatible polysaccharide derived from chitin, has been extensively studied as a promising nanovehicle for cancer therapy. Chitosan has shown potential in cancer therapy, especially as a dissolving agent, drug stabilizer, and controlled-release drug control, with a multifunctional platform for targeting, stimulus-responsive release, or use in image-guided medicine [34]. CSNPs will meet the design criteria for achieving cancer-targeting goals, which include (1) selective targeting of cancer cells, (2) efficient anticancer drug release at target sites, and (3) elimination of cytotoxicity to non-cancerous tissues [35]. Compared to other natural polymers such as alginate or gelatin, chitosan has a unique combination of properties that make it a promising option for cancer therapy. However, the optimal choice of polymer for a specific nanomedicine application will depend on the specific requirements and goals of the application, and will also consider factors such as biocompatibility, drug compatibility, and ease of production.
2. Chitosan-Based Nanosystem of Smart Drug Delivery System
Polymer nanomaterials have potential in medical treatments and therapies such as imaging, faster diagnosis, and drug distribution, and offer many attractive advantages over conventional drugs, including stability, better bioavailability, minimized toxic side effects, and enhanced drug delivery [22,45,46]. Polymeric NPs are capable of combining other functions such as controlled release, imaging agency, targeted delivery, and the loading of more than one drug for combination therapy [47,48]. Numerous studies completed over the past two decades have emphasized the importance of smart polymers in biomedicine. Smart polymers are polymers that respond to stimuli and/or environmental conditions [49,50]. CSNPs are polymers that have attracted great attention as nanocarriers with multiple multi-functionalities in forming smart DDS [6]. Bioactive compounds can interact chemically or physically with other molecules. The intelligent response of a polymer is governed by its initial state [49,51]. Smart devices boost therapeutic effects and effectiveness in cellular and animal models compared to free drug and passive nanomaterial systems. As these technologies advance and more triggering and targeting approaches emerge, smart polymer nanoparticles will enhance cancer therapy [47,48]. Smart nanosystems-based carriers possess the following characteristics (Figure 2) [50,52,53,54,55,56].
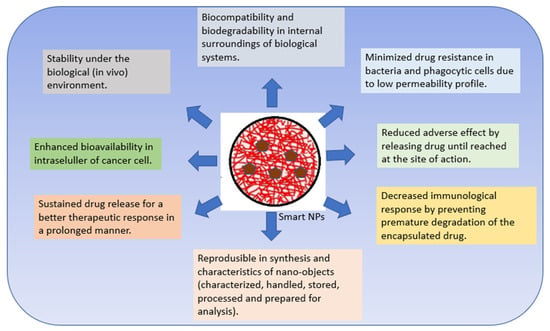
Figure 2. Characteristics of smart nanosystems-based carriers.
2.1. Synthesis, Functionalization and Characterization of Chitosan Nanoparticles
Chitosan is a deacetylated process derived from chitin, and consists of D-glucosamine and N-acetyl-D-glucosamine units linked to β-(1→4) [57,58]. Its cationic property, which enhances adhesion through electrostatic interactions to negatively charged mucosal surfaces, is one of the most significant characteristics of chitosan; it results in increased drug internalization in target cells [59,60,61]. A significant barrier to its implementation is that it is soluble only in acidic media. The broad amino and hydroxyl groups serve as target groups for chemical changes to increase solubility. CS has good biocompatibility, low immunogenicity and biodegradability. Under in vivo enzymes, CS will decompose into water and carbon dioxide and become an endogenous species, ensuring no harmful effects of degradation products. Due to its increased solubility at a slightly acidic pH such as that present in the tumour microenvironment, CS is commonly used to develop pH-sensitive DDS [62,63,64,65].
Biopolymeric NPs such as chitosan (CS) are mainly used for cancer therapy because of their benefits as drug delivery vectors:
-
Cost-effectiveness [66].
-
Drug protection through encapsulation in the core of NPs [69].
-
Enhancement of the therapeutic efficacy of therapy, especially in tumor therapy, through passive targeting or enhanced permeation and retention (EPR) effects [70].
-
Mechanical properties, targeting ability, and mode of drug release that can also be controlled by modifying the structure of natural materials with polyamines, small molecules, and targeting ligands [56].
-
The polymer may be made water-soluble depending on its usage [73].
-
A high degradation rate which ensures the material’s safety and its protection of the environment (eco-friendly properties) [76].
The use of native CS for in vivo applications is limited by its low effectiveness, which is attributed to its instability and insufficient cellular release. To overcome these limitations, there is a need for the development of effective delivery systems that involve chemical modifications (Figure 3). Chemical modifications can be made to NPs based on CS derivatives, which possess hydroxyl, acetamido, and amine functional groups. Additionally, CS polymer conjugates/complexes and the addition of functional polymers can help mask the inherent weaknesses of CS and enhance its transfection efficiency. Modified CS can be used to adjust the carrier’s functionality and delivery properties to suit the specific application requirements. The primary goal of modifying CS is to enhance its solubility, which may lead to a wider range of potential applications [68].
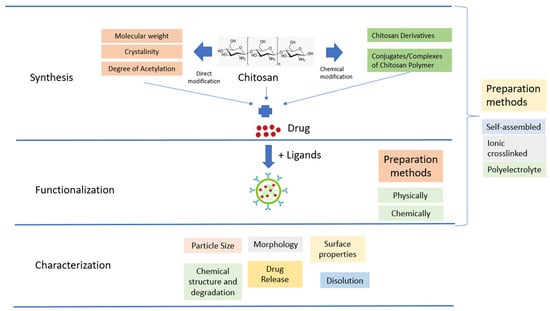
Figure 3. Schematic illustration of CSNPs.
CSNPs can be categorized into three groups depending on how they are prepared. The first group is self-assembled, wherein hydrophobic chains gather spontaneously to create reservoirs for drugs that dissolve in water. Meanwhile, hydrophilic chains form a shell around the nucleus that is affected by the aqueous phase [79]. The second group is ionic crosslinked, wherein ionic crosslinked NPs are created using electrostatic interaction between the cationic property of CS and a polyanionic crosslinker such as TPP, CaCl2, or Na2SO4. The physical characteristics of these NPs can be adjusted by varying the ionic crosslinking processing parameters; this affects the encapsulation efficiency and drug release [80]. The third group is polyelectrolyte complexes; these involve mixing CS with a negatively charged polyelectrolyte to form a CSNPs polyelectrolyte complex. This procedure is simple, and does not require toxic reagents. When mixed in solution, the polymer chains interact to create a strong but reversible electrostatic network without using crosslinking [68].
2.2. Mechanism of CSNPs for Drug Delivering
Chitosan nanoparticles are formed by the interaction of positively charged chitosan with negatively charged molecules such as DNA, RNA, and drugs, via electrostatic interactions [1,59,84,85,86,87]. These nanoparticles can be administered to the body via various routes such as oral, topical, or injection [78,88,89]. Upon administration, chitosan nanoparticles protect the encapsulated drugs from degradation and enhance their absorption and distribution [75,77].
To target specific tissues or cells, chitosan nanoparticles can be functionalized with ligands or antibodies that recognize and bind to specific receptors on the target cells [56,90]. This strategy improves drug delivery to the target site and reduces off-target effects.
Chitosan nanoparticles can also be designed to achieve controlled drug release by using different encapsulation strategies. For example, drugs can be encapsulated within the chitosan matrix, loaded onto the surface of the nanoparticles, or attached to the surface of the nanoparticles via chemical linkers. The release rate of the drugs can be controlled by varying the size and charge of the nanoparticles or modifying the chitosan matrix with different chemical groups [68,91,92].
2.3. Modification Strategies of CSNPs for BC Therapy
Given the enormous cost of drug development, exacerbated by high failure rates, anticancer drugs are usually expensive. The search for a new delivery system is expected to find a solution that can increase the effectiveness and efficiency of the use of existing cancer drugs [98,99]. In building this delivery system, there are several strategies offered by chitosan, from various studies that have been conducted:
-
CSNPs may encapsulate or conjugate chemotherapeutic medicines, therapeutic gene nucleic acids, photosensitizers, and cytokines for more reliable cancer target treatment [1].
-
Designing CSNPs according to delivery system factors, such as size and size distribution, drug loading capacity, and stability, is possible [102].
-
Chitosan has three groups: amino, acetamido, and hydroxy groups; they can provide derivatives of increased solubility and outstanding anticancer activity, offering bioavailability in cancer cells by utilizing sustained release. They also offer increased permeation, transfection, and gelation in situ [103], and easy in vivo biodegradation [104].
-
Multi-functional CSNPs can continue to offer many new opportunities for biomedical applications because they have the ability to interact with complex cellular functions in new ways [50]. There exists a multifunctional DD that combines high specificity against cancer cells with endosomal escape ability.
-
Surface modification of CSNPs can be carried out on polymers through physical or chemical methods. Surface modification of CSNPs enhances their tumor-targeting ability through different mechanisms such as a receptor or carrier-mediated transcytosis [105]. CSNPs were more effective than PLGA NPs because they targeted MCF-7 cells [102].
-
Modification of chitosan can use variations in molecular weight and the level of acetylation, which will provide different properties according to the needs of the drug delivery system [104].
-
Chitosan can be used as a solubility-enhancing polymer backbone. Advances in polymer chemistry have led to the creation of smart polymer systems [105].
-
Polymers used for drug administration can respond to stimuli such as temperature, light, or pH. Stimulus-responsive polymers may modify cell adhesion to boost gene expression or enzyme activity [96].
Chitosan-based gene delivery systems have attracted considerable attention as a promising alternatives to viral-based gene therapy due to their biocompatibility, low toxicity, and ease of functionalization [106,107,108]. Scientists have been exploring different ways to deliver genetic material into cells for gene therapy, which are categorized into two groups: viral and non-viral methods [109]. Viral vectors have been used in the majority of gene therapy clinical trials due to their high transfection efficiency and gene expression levels. However, they also have significant drawbacks such as immune responses, toxicity, immunogenicity, low loading capacity, and inflammation. Non-viral methods, on the other hand, are gaining increased attention because they can avoid many of these drawbacks. One promising non-viral method is the use cationic polymers such as chitosan, which have strong gene complexation and high transfection efficiency, for gene delivery. However, chitosan-based gene delivery carriers still face challenges such as poor water solubility, charge reduction at physiological pH, and poor targeting capability, which hinder their clinical translation [106,110,111,112]. Low-molecular-weight chitosan and a low N/P ratio (the ratio of the amine groups of chitosan to the phosphate groups of DNA) are more suitable for designing chitosan-based nonviral vectors for gene therapy. This is because the physicochemical and biological properties, as well as the stability of nanoparticles formulated with low-molecular-weight chitosan, are better than those formulated with higher-molecular-weight chitosan. [112].
2.4. Stimuli-Responsive NPs
A “smart” DDS may initiate the release of drugs near tumors or malignant cells. Systems may be regulated both internally (through the pH of the tumor microenvironment, glutathione (GSH) redox potential, and specific overexpressed enzymes) and externally (through magnetic, ultrasonic, thermal, microwave, electrical, and photochemical signals) [49,105]. Exploiting physiological variations between tumor and healthy tissue facilitates the development of stimuli-responsive DDS [38]. This reduces the systemic toxicity of chemotherapeutic drugs and restricts healthy tissue exposure to cytotoxic drugs. Quantitative mechanical description of active components plays a crucial role in their development and application, allowing the design of complex devices and the engineering of the microstructures of materials according to their intended functionality [113].
2.5. Multifunctional Delivery Systems
The chemical synthesis of nanomaterials has been a powerful force behind advances in nanoscience and nanotechnology [116]. Multifunctional delivery systems include diagnostic imaging, targeted medicine delivery, and controlled drug release. These particles allow active cancer monitoring throughout chemotherapy, which improves treatment control. Multifunctional delivery systems can provide and monitor combined drug therapy to improve cancer treatment [35] and measure pharmacokinetics and biodistribution in real time [51].
3. CSNPs and Mechanism Anticancer Action
CS has several active functional groups, offering more diversity and modification opportunities. NPs operate as nanocarriers in cancer therapy, containing anticancer drugs. The potential of nano-therapeutics relies on the proper unloading of drug cargo to the target region, which is mediated by six pathways: targeting, cellular uptake, drug release, MDR, cytotoxicity, and cell death. Methods such as emulsification, solvent evaporation/extraction, nanoprecipitation (solvent-displacement), salting-out and the supercritical anti-solvent method have been used for the preparation of PNPs.
Chitosan and its derivatives can inhibit the growth of various cancer cells induce apoptosis through increasing the concentration of calcium ions, the level of ROS and the mitochondrial membrane potential [1], and inhibit cancer cell migration and invasion. Chitosan can also modulate various signaling pathways involved in cancer progression, including the PI3K/Akt/mTOR pathway [117], the MAPK/ERK pathway [118]., and the NF-κB pathway. Chitosan and its derivatives can induce cell cycle arrest and apoptosis in cancer cells by regulating these pathways. Chitosan oligosaccharide may improve the efficacy of existing chemotherapies by inhibiting programmed cell death ligand 1 (PD-L1) expression and promoting T cell-mediated immune killing in tumors [1].
3.1. Targeting
The role of CSNPs in targeting systems has been discussed in [56]. They can be involved in both passive (based on the enhanced permeability and retention effect of cancer targeting) and active (receptor-mediated or stimuli-responsive cancer targeting) drug delivery systems for potential cancer therapy [119]. Passive and active targeting techniques for systemic medication administration target solid tumors without harming healthy cells/tissues [4].
EPR-based passive targeting may improve nano-treatment tumor aggregation and persistence by leaking tumor arteries which generate the EPR effect, thereby enabling the NPs to enter and reside in the tumor core. The holes in the leaking tumor vasculature range in size from 380 to 780 nm. NPs below this threshold may target cancer cells. Small particles less than 80 nm may readily permeate the tumor by passive diffusion and are pushed back into the circulation by the high interstitial fluid pressure inside the tumor. NPs must first pass through the tumor vasculature (usually a leaky microvasculature) and then extravasate into the tumor interstitial space, where tumor heterogeneity and hypoxia might limit real effect [4].
NPs often combine passive and active targeting; they concentrate more on cancer tissue than on normal cells and tissues, reducing side effects and therapeutic doses [35,105,120].
Multiple types of ligands are conjugated to the particle’s surface as part of the multiple-ligand targeting strategy. Due to the coupling of two stimulus-based systems in one matrix, it is believed that multiple stimulus-responsive materials should be more efficient than single stimulus-responsive materials [121].
This explains TNBC’s dismal 5-year survival rate. CD44+/CD24 BCSCs are responsible for TNBC resistance and relapse. Innovative therapeutics targeting TNBC stem cells and vulnerable cells are required. BCSCs are epithelial- or mesenchymal-like. HA binds BCSCs’ CD44 receptors [96].
3.2. Cellular Uptake
The efficiency of cellular absorption is essential for the effectiveness of nanotherapy [122,123]. The cellular absorption of NPs is determined by some variables, including particle size, charge, hydrophilicity, and the presence of ligands. CSNP provides advantages to these factors; the size of CSNP and its derivatives can reach 100 nm or less. The existence of a positive charge from CSNPs and their derivatives causes interactions with negatively charged cell membranes [77,124]. Zeta potential plays an important role. Positively charged NPs prefer to be internalized quickly and intensely by negatively charged cancer cells due to electrostatic interactions. To further enhance penetration into cells, ligands can be attached to CSNPs. NPs are predicted to be absorbed by cancer cells before releasing their therapeutic payload.
3.3. Drug Release
The starting point for the course of the therapeutic action of an NP-based drug delivery system is drug release. Drug release from CSNPs, unmodified chitosan polymers, and chitosan with various modifications has been discussed in [68]. CSNPs can controllably transfer active substances, enhancing therapeutic efficacy. CSNPs have a small size and substantial surface area for targeted delivery. CS’s hydrogen bonding and cationic charge in acidic conditions make it a good DDSs candidate.
The release of medicines from CSNPs followed a typical bi-phasic pattern, with a rapid initial release followed by a delayed, more pH-dependent release [68]. Microenvironmental acidification and other metabolic crosstalk are interesting possibilities. Increased glucose metabolism lowers the pH in the microenvironment due to lactate secretion. The acid-mediated invasion hypothesis suggests that H+ ions secreted from cancer cells diffuse into the surrounding environment and alter the tumor–stromal interface, allowing enhanced invasion [127]. The pH is lowered to 6.0–7.0 as a result of glycolysis, which creates a slightly acidic environment in a low-oxygen environment. Additionally, the pH of lysosomes within cancer cells is significantly lower, only reaching 5 [128,129]. The Warburg effect can provide advantages for cell growth in a multicellular environment. Chitosan-CD44 interactions promoted the absorption of chitosan-coated nanosystems. [130].
3.4. MDR
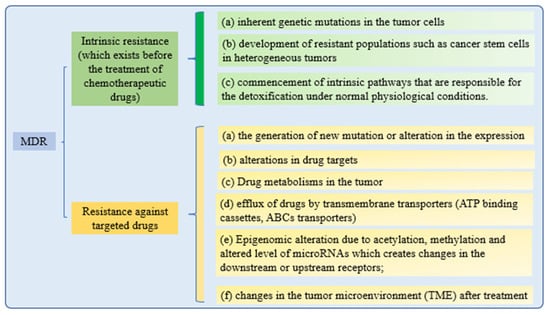
Cancer cells’ drug resistance causes the failure of anticancer therapy. Increasing drug efflux, evading drug-induced apoptosis, and activating DNA repair pathways can drive chemotherapy resistance [131]. Multiple attempts to overcome MDR in cancer have failed in recent years. Understanding MDR and cellular reprogramming may help us to overcome cancer drug resistance and improve cancer treatment (Figure 4). Cancer multidrug resistance may be addressed using novel anticancer drug candidates, molecular targets, and revolutionary DDS [9].

Figure 4. Mechanisms of drug resistance.
All of these drug resistance mechanisms may cause multidrug resistance in cancer. While retaining the effectiveness of the treatments, nanotechnological strategies are being developed to reduce the toxicity of targeted therapies to healthy tissues. These methods are based on a nanoparticle delivery system (nanocarriers) that enhances drug–site contact time and elimination time, while decreasing drug resistance [132].
3.5. Cytotoxicity
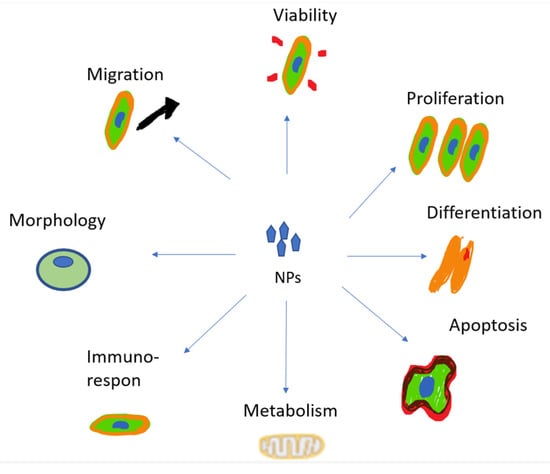
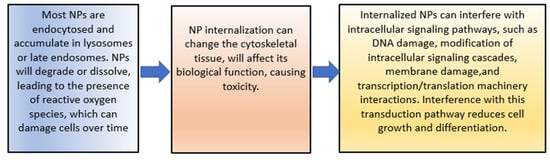
The increase in cytotoxicity is the result of the previous processes of cellular uptake, drug release, and reduction of MDR. Both can be achieved by targeting patterns from CSNPs. The toxic effects on cells induced by NPs’ uptake are mainly due to the reasons shown in Figure 5 [140].

Figure 5. The toxic effects of NPs.
The toxicity of NPs can also be caused by damage to cell membranes. The process is described in Figure 6.

Figure 6. The toxic effects of NPs.
4.6. Cell Death
There are two main apoptotic mechanisms: the death receptor and mitochondrial pathway [149]. The intrinsic mitochondria-mediated route is usually disrupted in cancer and can be triggered by lysosomal/ER stress, metabolic stress, DNA damage, ionizing radiation-induced cellular stress, hypoxia, heat, cytokine deficiencies, and chemotherapeutic drugs. The mitochondrial apoptotic pathway is the most frequently inhibited apoptotic pathway in cancer cells. Cyt c release activates the caspase activation cascade in the mitochondrial apoptotic pathway. There exists a Cyt c drug delivery device that targets the mitochondrial apoptotic pathway to promote cancer apoptosis. [40]; inducing intrinsic or extrinsic apoptosis is a promising cancer therapeutic method.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15030879
This entry is offline, you can click here to edit this entry!
