Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Studies have shown that during early embryogenesis, preimplantation embryos exhibit higher levels of chromosomal abnormalities in the initial stages of cleavage compared to the late morula stage or blastocysts. Thus, preimplantation embryos can acquire an aneuploidy phenotype already in early developmental stages, which points to the fact that these first mitotic cycles are more susceptible to chromosomal aberrations. The monitoring of chromatin damage, the so-called cell cycle checkpoint, is therefore an essential aspect of the cell cycle.
- : cleaving embryo
- Chk1 kinase
- cell cycle checkpoint
1. Introduction
Restoring mitotic activity is a crucial objective for the early embryo in order to maintain the subsequent course of embryonic genome expression without any disruptions. This will guarantee the accurate transfer of genetic information to the next generation of the same species. The maintenance of gene integrity is important for all types of cells, but in the case of early embryonic cells, it is absolutely essential. Disorders of the cell cycle control mechanism in somatic cells result, in the optimal case, in the elimination of such cells or tissue parts. When the control mechanisms in pre-implantation embryos fail, this may greatly influence the overall course of embryogenesis and also lead to serious consequences for the eventual offspring [1]. In this regard, early embryos are likely to use a different “strategy” to cope with DNA damage compared to somatic cells [2]. Although the complete implications of DNA lesions created during reprogramming in early embryos are not well understood, it is clear that genomic stability must be maintained during the first initial stages, when the overall dynamic epigenetic modification of the genome occurs [3]. The maintenance of gene integrity is equally important with regard to the primary differentiation of totipotent blastomeres. The damage or formation of lesions in DNA molecules is not unusual even under physiological conditions. In addition, DNA damage in cycling cells can also be induced by so-called non-physiological factors of endogenous or exogenous origin. If DNA damage occurs in germ cells (oocytes or sperm) or the fertilized oocyte and the DNA lesions are not satisfactorily repaired, this can lead to the occurrence of chromosomal aberrations during early embryogenesis and eventually to genetic instability during subsequent embryonic development. Therefore, examining the events related to DNA damage response at the sub-cellular level, particularly in germline or embryonic cells, is of utmost importance [4].
2. Cell Cycle Checkpoints
Shortly after fertilization, a significant reorganization of sperm chromatin takes place, during which, maternal histones are substituted for protamines. DNA lesions are generated during paternal DNA demethylation and repaired during the first cell cycle after fertilization to prevent chromosome fragmentation, infertility or embryo loss [5]. Subcellular abnormalities that often occur after in vitro fertilization are associated with DNA damage, which is considered to be the main reason for the decrease in the success of embryonic development [6]. In connection with the epigenetic modification of the genome in the early stages of preimplantation embryos, DNA chain breaks occur [3]. This chromatin reorganization and the simultaneous initiation of the mitotic cell cycle after fertilization followed by relatively short cycles of blastomere cleavage suggest that early embryonic cells may exhibit specific responses to different forms of DNA damage. The preimplantation embryonic period is distinguished by a series of mitotic cleavages of blastomeres from the zygote to the blastocyst stage [7]. The first embryo cleavage takes a relatively long time compared to subsequent cleavage stages. Once the first mitotic cell cycle is complete, the embryo enters into a series of rapid cell cycles, leading to an increase in the number of blastomeres, but without significant cell growth [8]. Studies have shown that during early embryogenesis, preimplantation embryos exhibit higher levels of chromosomal abnormalities in the initial stages of cleavage compared to the late morula stage or blastocysts [9]. Thus, preimplantation embryos can acquire an aneuploidy phenotype already in early developmental stages, which points to the fact that these first mitotic cycles are more susceptible to chromosomal aberrations [10]. The monitoring of chromatin damage, the so-called cell cycle checkpoint, is therefore an essential aspect of the cell cycle [11,12], because DNA damage in early embryos can lead to an extension of the cell cycle delay, leading to a reduction in the cleavage rate during blastulation [13,14,15]. A good marker of DNA repair is the well-detectable phosphorylated form of histone H2A.X (designated as γH2A.XS139) and the enzyme PARP1 (Poly [ADP-ribose] polymerase1) [16,17,18].
Oocytes generally appear to be more resistant compared with sperm, probably due to the low oxygen concentration and high levels of antioxidants in the follicular fluid [19,20,21]. However, oocytes are naturally attacked by aging processes [22]. On the other hand, spermatozoa containing damaged DNA are able to fertilize fully matured oocytes, which leads to the logical assumption that oocytes take responsibility for the possible repair and remodeling of both the maternal and paternal genomes during the very early stages of embryogenesis [23,24]. This phenomenon can probably be explained by the fact that sperm are not transcriptionally active [25]. DNA damage inherited by any germ gamete must be repaired before the first S-phase after fertilization to reduce the risk of mutagenesis and the subsequent dysregulation of primary embryonic cell differentiation. This means that the embryo must “rely” on endogenous stocks of mRNA and protein transcripts accumulated during the growth phase of the oocyte up to the stage of expression of the overall embryonic genome. However, this timing is highly specific for individual animal categories. For example, it takes place at the two-cell stage in the mouse embryo and between the four- and eight-cell stages of the early morula in the human embryo (for review see [26]). In this context, the sensory proteins of damaged DNA in the fertilized oocyte are apparently of female origin, up to the stage of the activation of the embryonic genome [27]. It was confirmed that DNA damage transmitted by sperm can thus be recognized and repaired with the help of enzymes stored in the mature oocyte [28]. If there is any deficiency or inaccuracy in the repair process by the oocyte, it has the potential to create de novo mutations in the embryo, thereby fixing paternal DNA damage. This observation could provide evidence to support the idea that assisted conception procedures have the potential to increase the mutational load passed down to the offspring [29]. Although zygotes are able to recognize DNA damage, they have the potential to protect themselves from cell death through antiapoptotic protection, which may provide an opportunity for DNA repair and continued embryogenesis [30]. The preservation of the continued cleavage of an early embryo containing damaged DNA is enabled by a certain degree of tolerance of the G1/S and G2/M checkpoints in the zygote, or by a “specific” threshold for the level of this damage, until the creation of a fully functional apoptotic mechanism during the last stages of the preimplantation embryo [31]. On the other hand, such DNA lesions followed by correct repair may promote genome diversity in response to endogenous/exogenous causes. In the worst case, a high degree of genome instability in the initial embryonic cell cycles may lead to congenital disorders caused by chromosomal abnormalities [32]. Approximately half of blastocysts are estimated to contain genomic alterations that result in a high incidence of pregnancy losses [33]. Therefore, signaling molecules induced by damaged DNA in cleavage embryos lead to activation events that control the integrity of the genome.
Studies have shown that oocytes with a moderate degree of DNA damage can complete maturation, even though there is an increased number of lagging chromosomes in anaphase I. This can lead to a cellular phenotype of chromosomal fragments at the end of oocyte maturation in metaphase II [34]. It appears that oocytes may not be thoroughly successful in such repair in order to reach the metaphase II stage and become competent for fertilization and subsequently to form the maternal pronucleus. On the other hand, the completion of meiosis fails in oocytes with a high degree of DNA damage. One recent study (focusing on the preimplantation development of mouse embryos after DNA damage induced before entry into the first S-phase) documented that even such a fertilized oocyte tolerates some degree of DNA damage, suggesting that the completion of the first cleavage stage is of utmost importance in ensuring the continuation of embryogenesis for as long as possible [35]. Several studies have addressed the cellular phenotypes of early embryos derived from DNA-damaged germ cells [36,37]. In this context, however, it will be necessary to add more detailed knowledge about the developmental consequences of these embryos, especially in the stages of primary differentiation of embryonic cells. Unlike the oocyte, the paternal contribution to the restoration of mitotic activity is limited to a highly differentiated, transcriptionally inert cell with minimal cytoplasmic content. It is evident that abnormalities in the structure of sperm chromatin, originating from spermatogenesis, can alter the chromatin configuration and result in DNA such as single-stranded or double-stranded DNA breaks [38]. After all, the resumption of mitotic activity and the initiation of embryonic genome expression take place in the maternally inherent environment of the fertilized oocyte. Nevertheless, cell cycle control checkpoints are limited in fully grown oocytes, which allows oocytes with DNA damage to resume meiosis unless the damage levels are severe [39,40]. Despite a certain degree of tolerance of maturing oocytes and very early embryos to DNA damage, the cell cycle signaling pathways are crucial for the activation of downstream effectors that control the integrity of embryonal genomes. From this aspect, early embryos overcome DNA damage using a different “strategy” compared with somatic cells [2]. In principle, the DNA damage response can result in three possible outcomes: (i) DNA damage repair; (ii) cell death mediated by the activation of the apoptotic pathway; and (iii) tolerance to the lesion, which can result in mutation or eventual carcinogenesis [41]. It was earlier documented that a few overexpressed embryonal genes are involved in DNA repair. In this sense, it seems that the repair of damaged DNA is the primary response (with certain tolerance to a low number of lesions) of the early embryo [42]. In the case of extensive or persistent DNA damage, the death of the embryo is the last resort to “protect” genomic integrity [14].
Cell cycle checkpoints play a key role in cell cycle regulation during early embryonic development, as they control the cycle sequence, genome integrity and the fidelity of major cell cycle events that determine the further course of mitotic division. This is especially important during the first cleavage stages of the early embryo, because these cell cycles are the longest during preimplantation development in mammals. The importance of this control over the course of the cycle is also confirmed by the fact that the embryonic genome is only fully activated after the S-phase of the one-cell embryo in mice and the four-to-eight-cell human embryo. Generally, the cell cycle control machinery consists of three major checkpoints that ensure the progression of the cell cycle. These include checkpoints G1/S, G2/M, and SAC (spindle assembly checkpoint). The so-called intra-S checkpoint can also be assigned to them—see Figure 1. The most sensitive checkpoint to DNA damage appears to be the G1/S checkpoint. Its activation prevents S-phase entry as well as DNA replication by the inhibition of Cdk2 or Cdk1 activation. The final control point of the cell cycle before entry into mitosis is the G2/M checkpoint. Chromosomal aberrations detected earlier can lead to the activation of this checkpoint and cell cycle arrest in the G2 stage. The SAC is the major control point in regulating the onset of cytokinesis. Its role is to prevent the premature separation of sister chromatids during metaphase–anaphase transition by delaying the anaphase onset [43]. Apparently, the most important focus of checkpoints is the control of the integrity of the DNA molecule as the central entity for the transfer of genetic information to the next generation of cells. If the DNA damage response affects cell proliferation, the cell cycle progression is reversibly inhibited to allow DNA repair. After successful DNA repair, the checkpoint is turned off and the cell cycle is restored [44]. However, precise replication of the genome during the S-phase is of fundamental importance, especially in one-cell embryos when the resumption of the mitosis cell cycle takes place. In this context, the double-stranded breakage of DNA is probably the most severe type of damage during this embryonic stage, as it can induce chromosomal instability and the failure of chromosomal remodeling [41]. If the genetic information is erroneously replicated during this process, it will lead to serious outcomes such as implantation failure, spontaneous abortion, genetic disease or embryo death [45]. It should be noted that developing human embryos are more sensitive to the consequences of DNA damage than early mouse embryos [46,47,48], which is likely related to evolutionary differences, with mouse embryos being more efficient at protecting their DNA integrity [49]. Initial experiments with exogenous DNA damage documented that the exposure of the oocyte, zygote or early embryo to γ-irradiation or laser microbeams [39,50] or certain chemical drugs, such as etoposide, bleomycin or neocarzinostatin [9,14,34,51], can cause damage that leads to delayed cleavage. As a result, cells that have been damaged may be able to complete the cell cycle, but they are more likely to experience an increase in micronuclei formation. When it comes to cleavage embryos, this damage can hinder development in the subsequent cleavage stage and frequently result in arrest prior to reaching the blastocyst stage. The application of UV irradiation or cisplatin (cis-diammineplatinum(II)dichloride) as DNA damage inducers updated the current understanding and knowledge in this field [14]. In this case, the treatment of two-cell embryos in the G2 phase caused DNA damage characterized by the increased phosphorylation of H2A.X histone. In addition, exposure to UV irradiation resulted in sustained G2/M arrest, whereas treatment with cisplatin enabled progression through mitosis and the subsequent activation of the G1/S checkpoint. Sperm-induced DNA damage caused a delay in DNA replication, leading to developmental retardation during progression into the two-cell embryonal stage. Furthermore, a significant portion of the embryos were arrested at the G2 to M phase transition [36].
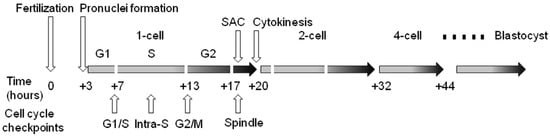
Figure 1. Cell cycle checkpoints in the temporal context of cleavage of the early mouse embryo. Time values are averages due to variability between individual lines of laboratory mice. SAC—spindle assembly checkpoint.
Recent world statistics document rising rates of infertility [52,53]. DNA damage in sperm causes the fragmentation of the paternal chromosomes. Such an event leads to the random distribution of the chromosomal fragments over the two sister cells in the subsequent first cell division. In addition, DNA damage in sperm can lead to an unforeseen secondary effect of direct unequal cleavages, including the little-understood heterogoneic cell divisions. The consequence of these various types of damage is that embryos resulting from fertilization with damaged sperm often exhibit chaotic mosaicism. Such structural variations, aneuploidies and uniparental disomies induced by sperm DNA damage may compromise fertility, cause embryonic developmental delay and lead to rare congenital disorders. Sperm-induced DNA damage can cause a delay in DNA replication, resulting in retardation during the progression into the two-cell embryonic stage. Additionally, a significant portion of the resulting embryos may become arrested in G2/M-stage transition. The cause is the high proportion of aneuploidy of mitotic origin and subsequent disorders in chromosome segregation, resulting in 20 to 30 percent of blastocysts having the so-called mosaic phenotype [54,55,56]. Considering these facts and the still incomplete knowledge about the effectiveness or activity and control points of the cell cycle in maturing oocytes as well as in very early embryos, further research in this area is important, but it must be in connection with exogenous environmental factors that have the potential to damage DNA.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24076778
This entry is offline, you can click here to edit this entry!
