Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Carbon dots (CDs) are zero-dimensional carbon nanomaterials that have been polymerized. CDs have been extensively used as biosensors. CDs are a subclass of nanoparticles, defined by a quasi-spherical morphology with a single unit with a characteristic size < 10 nm. CDs have been extensively used as electrochemical sensing composites due to their interesting chemical, electronic, and mechanical properties giving rise to increased performance.
- Carbon Dots
- Graphene
- biosensor
1. Carbon Dots
Electroactive carbon dots (CDs) are zero-dimensional carbon nanomaterials. These materials possess crucial electronic properties as possessed by quantum dots. They show low toxicity, stability, and biocompatibility for their application as electrochemical biosensors. CDs can also be classified as carbon quantum dots (CQDs) and graphene quantum dots (GQDs).
2. Graphene Oxide and Reduced Graphene Oxide Transition Metal Composites
Carbon dots are mainly synthesized using top-down and bottom-up methods. Under top-down approaches, CDs are prepared by cutting larger carbon structure materials. Arc discharge, laser ablation, electrochemical synthesis, nanometer etching, hydrothermal, solvothermal, and special oxidation cleavage fall under the top-down methods. Xu et al. identified a mixture of unknown fluorescent nanoparticles when purifying single-walled carbon nanotubes (CNTs) from an arc discharge shoot [1]. Carbon dots were later synthesized by laser ablation of a suspension of carbon powders in poly(ethylene glycol) solvent, which simultaneously acted as a surface passivation agent [1][2]. The obtained CDs which have adequate polymer chains on the surface have a strong blue emission.
The electrochemical synthesis processes are applied more widely than arc discharge and laser ablation methods because of their simple operation and readily available equipment. Electrochemical reactions under the applied voltage at the electrode lead the carbon electrode materials to be corroded and exfoliated to obtain CDs. Graphite rods, multi-walled carbon nanotubes, carbon fibers, graphite powders, etc., are used as carbon electrode materials. Sodium hydroxide, t-butyl-3-methylimidazolium tetrafluoroborate, tetrabutylammonium perchlorate, phosphate buffer solution, potassium persulphate, and ultrapure water are used as the electrolytes. The research work of alkali metal-graphite intercalation compounds offers an avenue to prepare CDs. The metal-graphite intercalation method was used to exfoliate and disintegrate the multi-walled carbon nanotubes or graphite flakes to obtain CDs. The hydrothermal strategy to cut graphene sheets into surface-functionalized CDs was first introduced by Pan et al. [3]. The graphene oxide was dissolved in DMF and pyrolyzed in a poly(tetrafluoroethylene) (Teflon)-lined autoclave to obtain CDs under a one-step solvothermal route. Acid oxidation and photo-Fenton reactions were applied to form CDs by oxidation cleavage. Carbon dots can be readily synthesized using a microwave-assisted method [4][5] and hydrothermal method [6]. A study of the structure of carbon dots and their properties is very important to understand the structure-property relationship in CDs. For example, synthesized black CDs and carbon nitride dots appear to be more functionalized to a greater degree and more disordered and amorphous than yellow-CDs [7]. A direct conjugation of black CDs and gel-like CDs takes place through an amidation reaction to display many unique properties on novel drug carriers [8]. Amphiphilic CDs have been synthesized through hydrothermal carbonization using saccharides (D-glucose, D-galactose, and D-lactose) as the precursors [9]. CDs were prepared using tryptophan and two different dopants: urea and 1, 2 ethylenediamine [10]. Carbon nitride quantum dots (CNQDs) were first synthesized using four different precursor sets involving urea, thiourea, selenourea, and formamide [11]. CDs fractions have been prepared using a convenient one-step solvothermal route from gel-like materials [12]. A facile one-pot synthesis of a carbon dot gel material has been achieved by Ji et al. in 2017 [13]. Gel-like CDs (G-CDs) have been prepared using a rapid one-step solvothermal approach with citric acid and 1, 2-ethylenediamine as the precursors [14].
Nitrogen-rich carbon dots (N-CDs) have been reported to be well dispersed in aqueous solutions in large concentrations. Fiuza et al. discussed the potential of N-CDs to have tunable isoelectric points based on varying colloidal stability, solution pH, and ionic strength [15]. This possibility opens the door for its use as effective electrochemical sensing composites to better control the deposition of catalytically active NPs on these carbon supports by attaching them to the surface using Coulombic attraction.
3. Functionalization of Carbon Dots
Functionalized CDs, similar to CNTs, can have O-containing moeities serving as tethering points of electrocatalytically active additives. CD-COOHs were modified with EDA via carbodiimide activated coupling reaction to yield CD-3 for the detection of citrate-silver nanoparticles based on the inner filter effect as shown in Scheme 1 [16]. The covalent modification through amide coupling reactions, silylation, and various reactions with sulfonylation, esterification, and copolymerization; and non-covalent modifications via complexation/chelation, interactions, and electrostatic interactions are summarized in Scheme 1. Such modification is vital for polymerization and can be used as capping agents for NPs, applicable for electrochemical sensing (vide infra, Section 2.3.3).
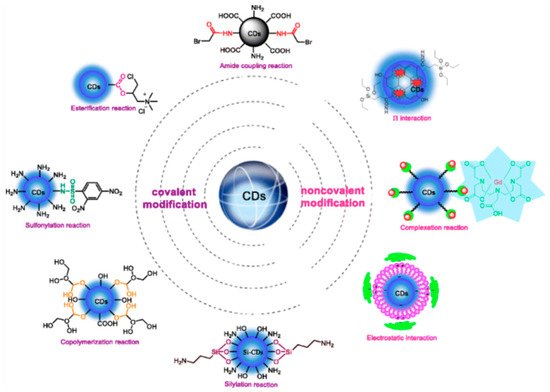
Scheme 1. Various functionalizations for modification of carbon dots. Reproduced with permission from ref. [15]. Copyright 2018, Springer Nature.
An amide coupling reaction is used to functionalize the CDs. The synthesis of CDs by treating urea, diethylenetriamine, PEI, and polyethylene glycol (PEG) as co-reaction reagents is generally rich in amino groups that affect high quantum yield and have very good biocompatibility. The presence of excessive amino groups on the surface of CDs shows low selectivity and water insolubility. Water insolubility of CDs indicates a good property for electrocatalysts. In addition, condensation of amino groups with acyl compounds using standard EDC/NHS-catalyzed reactions is applied as a surface modification to enhance the selectivity of CDs. This approach can be used for analyte detection and cell imaging [16][17][18][19]. The good stability and excellent electrochemical properties are shown by ferrocene (Fc). It has been applied to design PET-based organic molecular probes as an efficient electron donor.
Carbon quantum dots have been fabricated by microwave irradiation and were electropolymerized on GCE to sense ascorbic acid selectively. This analyte was studied by CV and DPV in the ranges of 0.11–3.0 mM and 4–12 mM, respectively. The limit of detection of AA was observed as low as 10 µM. Ascorbic acid or vitamin C plays an important role as an essential nutrient and antioxidant in the human body. The abnormal concentration of AA is related to different kinds of diseases, such as scurvy, mental disorder, cancer, AIDs, and digestive disorders. Also, as AA participates in key biological processes, e.g., cell division, iron absorption, acceleration of collagen synthesis, and melanogenesis inhibition, developing a sensor for assaying it is important. Regarding selectivity, the presence of DA and UA did not show any interference with the electrochemical detection of AA on the surface of the CQDs/GCE electrode. Moreover, there were no interferences with Na+, Cl–, Mg2+, SO4–, and glucose in samples at 100-fold concentration levels above that of the analyte using CA at +0.00 V [20].
4. Biosensing Applications of Carbon Dots
Carbon Dot Transition Metal Oxide Composites
CDs with transition metals as electrocatalysts are important for enhancing the sensitivity of biological analytes with various electrochemical techniques [21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43]. Nitrogen-doped carbon quantum dots/SnO2 (N-CQD/SnO2) nanocomposite were fabricated using a sonochemical approach with N atoms serving as tethering points for SnO2 NPs. The prepared SnO2 NPs were mixed with synthesized N-CQD solution and ultrasonicated. The mixture was filtered to obtain N-CQD/SnO2 nanocomposite for quantification of riboflavin (RF) in tablets and milk powder, as shown in Scheme 2. This sensor was used to determine RF concentration using CV. This sensor shows a wide linear range (0.05–306) µM with an LOD of 8 nM RF. RF is a water-soluble vitamin (VB2). It assists in converting fats, proteins, and carbohydrates into energy in the human body. Riboflavin itself converts into coenzymes flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). They are vital for tissue respiration. When there is a deficiency of RF, one may suffer from eye irritation, itching, sore tongue, and skin damage. Therefore, the sensitive determination of RF levels using N-CQD/SnO2 composite is necessary to monitor and diagnose such diseases [43].
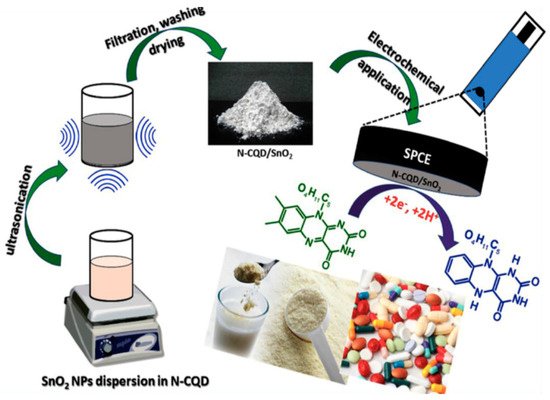
Scheme 2. Schematic illustration for the sonochemical preparation of N-CQD/SnO2 nanocomposite and its electrochemical determination of RF in milk powder and tablets. Reproduced with permission from ref. [43]. Copyright 2019, Elsevier Ltd.
Tryptophan is an essential amino acid for humans and herbivores, which is rarely available in vegetable products. It is a vital protein in the human diet and is responsible for maintaining a positive nitrogen balance. Tryptophan is also a precursor of the neurotransmitter serotonin. Tryptophan is of great importance in biochemical and pharmaceutical fields as it is a precursor molecule to hormones and neurotransmitters. It is also considered a possible cause of schizophrenia in people who cannot metabolize it properly. Under improper metabolization, it generates a waste product, a toxic analyte detected in the brain, causing hallucinations and delusions. Therefore, quantification of this amino acid is important for the detection of neuron-based diseases, such as schizophrenia, hallucinations, and delusions. A one-step electrodeposition method has been developed for the electrosynthesis of Fe3O4 magnetic nanoparticles/graphene quantum dots (Fe3O4-MNP-GQDs) on the surface of GCE. Applying this simple and effective deposition method, GQDs are effectively coated on the surface of electrodes. This sensor can quantify L-tryptophan (L-Trp) in the linear dynamic range of (0.08–150) µM with the LOD of 0.08 µM at physiological pH using DPV [24].
Cerebral Cu2+ is an essential trace element in the body, playing a vital role in various metabolic processes. Since it is a catalytic cofactor for many enzymes, including cytochrome C oxidase, tyrosinase, and superoxide dismutase, changes in cellular homeostasis of Cu2+ ions would result in cell death in addition to neurodegenerative (Alzheimer’s and Parkinson’s) diseases. Some amino compounds possess the capacity to identify copper ions selectively. With the modification of the surface of CDs, researchers can recognize Cu2+ ions selectively. In this electro-composite fabrication, N atoms served as the tethering point for the electrocatalytically active surface-attached additives. A specific recognition molecule AE-TPEA (N-(2-aminoethyl)-N, N’, N’-tris-(pyridine-2-yl-methyl) ethane-1, 2-diamine) was constructed for improving the selectivity of Cu2+ against other interfering metal ions. In this case, a functional group can coordinate with Cu2+ with high specificity to design a stable complex. Using silanization interactions, the CDs were tethered on (3-aminopropyl-) trimethoxysilane (APTMS) with a methoxy functional group. This was performed using electrochemical scanning to design a carbon-nitrogen linkage on the glassy carbon surface in advance. Finally, CDs functionalized with -OH and -COOH were conjugated via NHS and EDC as catalysts. This fabricated biosensor can selectively detect Cu2+ ions in a linear range of 1.0 to 60 µM with an LOD of 100 nM using differential pulse anodic stripping voltammetry (DPASV) due to specific recognition of the TPEA molecule [44]. Vancomycin (Van) is one of the glycopeptide antibiotics, which can be conjugated with CDs. The analyte is important for destroying harmful pathogens, such as staphylococcus aureus and methicillin-resistant staphylococcus aureus in the treatment of gram-positive bacterial infection.
UA (2, 6, 8-trihydroxy purine) is one of the fundamental waste products of nitrogenous base-like purine metabolism in humans. Various concentrations of UA levels studied have been found to relate to hyperuricemia, gout, Lesch-Nyhan disease, obesity, diabetes, high cholesterol, high blood pressure, and kidney disease. Quantifying UA in body fluids supports the diagnosis of these diseases. Hybrid nanocomposite (C-dots/Fe3O4 HCs) formation was obtained by supporting the adsorption of CDs over Fe3O4 NPs with amine-carboxyl interactions. The aggregation-free coverage of Fe3O4 NPs with a highly conductive layer of CDs as conductive centers is used to assist ultrafast electron transfer kinetics so that high signal sensitivity can be obtained. The enhancement of current signals and lower over-potential values enabled the generation of DC-amperometric (DC-AMP) sensors for uric acid (UA) with a linear working range of 0.010 to 0.014 µM and a signal sensitivity quantifiable up to 6.0 × 10−9 M. The enhancement of sensitivity results as a synergistic outcome of an active redox couple between Fe2+ and Fe3+, and a larger surface area of the Fe3O4 NPs were encapsulated with highly conductive CDs [22].
A carbon paste electrode (CPE) based on a novel nanocomposite of carbon dots/CuFe2O4 (C-dots/CuFe2O4) was fabricated by mixing pristine CuFe2O4 magnetic nanoparticles with synthesized CDs under ultrasonication for 1 h, followed by drying under vacuum at 80 °C for 12 h. This sensor is used for the simultaneous determination of rifampicin (RIF) and isoniazid (INZ) using CV and SWV, respectively. This sensor can detect RIF and NIZ in the ranges of 0.07 to 8.0 µM and 0.1 to 14.0 µM with the detection limits of 0.022 and 0.041 µM, respectively. This sensor holds great promise for fast, simple, and sensitive quantification of RIF and INZ in biological fluids and pharmaceutical samples. RIF and NIZ are classified as first-line anti-tuberculosis (anti-TB) drugs. These drugs treat mycobacterium tuberculosis (MTB) infection. Rifampicin, 3-[(4-methyl-1-piperazinyl)imino] methyl-rifamycin, also recognized as rifampin) is a semi-synthetic macrocyclic antibiotic used to treat tuberculosis (TB). Tuberculosis is a contagious bacterial infection caused by MTB, which affects the lungs. Rifampicin is also applied to fight neisseria meningitidis (a type of bacteria related to meningitis, a serious infection) infections in the nose or throat. This is also used to treat Hansen’s disease and other infections such as HIV. Isoniazid, pyridine-4-carboxylic acid hydrazide (also known as isonicotinyl hydrazine), is an antibiotic with significant bacterial activity in the initial phase of anti-TB therapy. The concentration of bacterial infection drugs is the key factor in controlling bacteria. Partial treatment of bacterial infections is common. The success or failure in therapeutic action is influenced by many factors such as the therapeutic regime, an interrupted drug supply, and/or chemical or physical interactions with other medications. However, an overdose of these drugs may lead to potential side effects, such as hepatotoxicity, allergic rashes, appetite loss, nausea, or immunological disorders. As the variations in bioavailability and pharmacokinetics of RIF and NIZ exist among people, it is necessary to fabricate therapeutic drug monitoring based on fast and reliable tools to quantify RIF and INZ concentrations [22].
Flutamide (FLU) and nitrofurantoin (NF) are anticancer and antibiotic drugs containing nitro groups, respectively. Flutamide, a non-steroidal anti-androgen drug is used to treat prostate cancer in men and to cure polycystic ovarian syndrome in women. Nitrofurantoin, an antibacterial agent is for urinary tract infections. It is used for pregnant women to treat infections in the urinary tract. However, the overdose of these drugs shows side effects, such as blood in urine, inflamed prostate, drowsiness, nausea, and liver malfunctions. In addition, the critical side effects of NF include inducing birth defects in embryos or fetuses. Moreover, due to the poor metabolism of NF and FLU, the metabolites are evacuated through urine and feces. The high electron-withdrawing nitro groups in FLU and NF resulted in poor biodegradation and kept the environment at risk. A simple microwave technique was applied to fabricate an N-CQDs/Co3O4 nanocomposite [25]. The electrocatalytic performance of the nanocomposite was enhanced with the presence of N-CQDs. Using the ultrasonication method, N-CQD/Co3O4 was hybridized with MWCNTs. The graphitic-conjugated structure of N-CQDs is responsible for tethering with MWCNTs for the formation of the hybrid N-CQD/Co3O4/MWCNTs. The synergistic effect between N-CQD, Co3O4, and MWCNT shows the high conductivity and high surface area of the hybrid nanocomposite. This sensor is used for the simultaneous determination of anticancer drugs flutamide (FLU) and nitrofurantoin (NF) using a GCE modified with N-CQDs/Co3O4/MWCNTs. This sensor shows a wide linear range of 0.05–590 µM for FLU and 0.05–1220 µM for NF with detection limits of 0.0169 µM and 0.044 µM, respectively. The selectivity of this sensor was studied using DPV in the presence of structurally relevant interfering molecules. The peaks of 100 µM FLU and NF were observed under DPV in presence of other analytes, such as 25-fold excess concentrations of nilutamide, (NLT), 4-nitroaniline (4-NA), 4-nitrophenol (4-NP), 4-nitrobenzene (4-NB), chloramphenicol (CAP), metronidazole (MTZ) and 50-fold excess concentrations of K+, Cu2+, and Br- ions. The maximum amount of interfering molecules indicates the tolerance limit, responsible for 5% relative error for the quantitation of NF and FLU. This sensor shows excellent selectivity to the recognition of NF and FLU simultaneously using DPV in an aqueous solution in presence of other analytes.
Carbon Dots Modified with Noble Metals
One member of the aminoglycoside group purified from streptomyces is streptomycin (STR) analyte used in humans and veterinary applications to treat gram-negative infections. The presence of these residues in very little amounts (200 µg/kg) can trigger potential health hazards such as nephrotoxicity and ototoxicity. Electrochemical sensing systems based on GQDs functionalized with amine (-NH) and thiol (-SH) groups (GQDs-N-S) as promising new carbon-based nanomaterial were developed for the detection of STR. In this sensing system, gold nanoparticles (AuNPs) were deposited on the GCE surface. Then GQDs-N-S was coated on the Au NPs/GCE surface. Then, the silver nanoparticles (Ag NPs) were coated on the GQDs-N-S/Au NPs/GCE. This sensor detects streptomycin (STR) using CV and DPV. This sensor has an LOD of 0.0033 pg∙mL−1 with a linear range from 0.01 pg∙mL−1 to 812.21 pg∙mL−1. Amoxicillin, ciprofloxacin, and gentamicin are the interfering analytes for the detection of STR. However, this sensor shows a higher signal of STR compared to the above-mentioned other analytes, displaying excellent selectivity [23].
Attaching noble metals to carbon dots is important for rapid selective and sensitive detection of biomolecules, such as H2O2, uric acid, riboflavin, and quercetin. Quercetin (3,3′,4′,5,7-pentahydroxy flavone), one of the abundant flavonoid molecules, is generally present in fruits and vegetables, especially in traditional Chinese herbs. It exhibits antioxidant, anti-inflammatory, anti-allergic, and antibacterial properties. It is clinically applied as a therapeutic medicine to reduce capillary permeability, lower blood pressure, and treat chronic bronchitis. On the other hand, with higher concentrations of quercetin in the biological body, adverse effects such as headache and kidney injury occur.
In an interesting development shown by Li et al., polymerized CDs can be used as a surface capping agent in the same manner as Nafion (vide supra, Section 2.3) [27]. The polymerized CD surface may be saturated with OH and -COO- moieties, which can attach to the NPs as it encapsulates the GCE surface. A drop of Au NPs of 10 µL was put onto the pretreated GCE surface to prepare the Au NPs/GCE. After drying in air, 10 µL GQDs (carbon dots in this application) was used to encapsulate Au NPs/GCE and dried under infrared lamps. The prepared GQDs/Au NP/GCE was rinsed many times to remove the residues. The nanocomposite of carbon dots and Au NP can highly accelerate the electron transfer rate and displays excellent synergistic electrochemical activity for the oxidation of quercetin. In this case (to demonstrate another application of the material), the carbon dots serve as capping agents similar to Nafion or chitosan, which is a departure from the tethering to O or N atom tethering point motif instead of serving as an NP support structure. The procedure for the fabrication of the modified electrodes and their electrochemical detection is depicted in Scheme 3. In preparing the electrode surface, Au NPs were drop-casted onto the GCE surface, followed by capping with polymerized C dots.
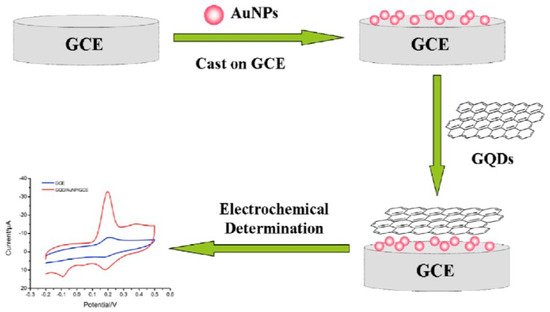
Scheme 3. Schematic illustration of the fabrication of GQD/Au NP/GCE and the electrochemical determination of quercetin. Reproduced with permission from ref. [27]. Copyright 2016, John Wiley & Sons.
The Au NP/GCE was prepared by depositing 10 µL of Au NPs on the pretreated GCE. While drying in air, 10 µL GQDs were coated on the Au NP/GCE and dried under an infrared lamp. Finally, the prepared GQD/Au NP/GCE was rinsed with DI water to avoid residues. DPV was applied for the detection of quercetin. The oxidation peak current is proportional to the range of quercetin from 0.01 to 6.0 µM with an LOD of 2 nM. This sensor can successfully quantify quercetin sensitively and selectively in human plasma samples with (95.0–98.5)% recoveries.
Various potential interfering substances for the detection of quercetin in real samples were studied in the presence of a standard solution of 1.0 µM quercetin to assess the biosensor’s selectivity. At the maximum concentration of interfering substances, the tolerance limit caused about 5% relative error in the quantification of quercetin. A 50-fold ratio (relative to quercetin) of ascorbic acid, L-tryptophan, L-phenylalanine, L-cysteine; and a 100-fold ratio of oxalic acid, glucose, Mn2+, Ca2+; and a 500-fold ratio of Ca2+, Mg2+, Fe3+, Al3+, Zn2+ did not show any impact on the accurate concentration determination of quercetin in the medium. In addition, common flavonoids, such as apigenin, naringenin, and puerarin did not interfere with the concentration determination of quercetin due to weak signal responses from these compounds [27].
H2O2 is a byproduct of many reactions by most oxidase in mitochondria and produces reactive oxygen species (ROS). The proper amount of H2O2 and ROS plays a critical role in the function and signal transduction of living cells. Many tumor cells produce more H2O2 than of normal cells because of greater ROS production and have less ROS scavenging capacity due to abnormal tumor growth. The concentration of H2O2 released from living cancer cells is used as a valuable biomarker for many kinds of cancer for early-stage recognition. The combination of H2O2′s transient nature of H2O2 and matrix effects of cancer cells makes monitoring H2O2 molecules in cancer cells a challenge. Such obstacles need to be addressed by future researchers to advance biosensing technology. A new type of functionalized hollow-structured nanospheres (HNSs) based on Pd nanoparticles (NPs) decorated double-shell structured N-doped graphene quantum dots (NGQDs)/N-doped carbon (NC) HNSs, with ultrafine Pd NPs and nanozyme NGQDs having dual signal-amplifying nanoprobes were fabricated. Due to the synergistic effect of conductive HNS supports and catalytically active Pd NPs and NGQD in facilitating electron transfer, the NGQD/NC/Pd HNS hybrid material sensor shows the quantification of H2O2 using CV and CA. This sensor is able to assay H2O2 with an LOD of 20 nM and a dynamic range of 250 nM–1.4 mM level.
H2O2 has been quantified in presence of interfering analytes, such as DA, AA, UA in PBS under CA using NGQD/NC/Pd HNS hybrid sensor. The good selectivity obtained from the favorable applied potential of 0.00 V for the amperometric study of H2O2 in which other interfering electroactive redox species do not show any response in physiological samples [28]. A large surface area, fast electron transfer rate, biocompatibility, and high electrocatalytic activity of the composite were exploited to achieve such superior sensitivity and selectivity. The detection limit of this composite is comparable to that of PB/ZnO/COOH-MWCNTs. Scheme 4 displays the ability of the NGQD/NC/Pd HNS hybrid sensor for analyzing H2O2, a cancer biomarker. The electrocatalytic H2O2 quantification in live cancer cells as low as 20 nM is the current extent of detection capability in this medium and in real-time conditions. The quantification of nanomolar levels of H2O2 excreted from various living cancer cells in conjunction with chemotherapy and radiotherapy has been achieved.
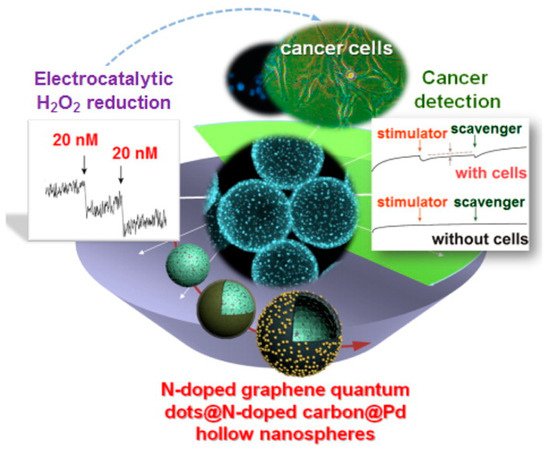
Scheme 4. Electrochemical sensing performance in cancer detection. Reproduced with permission from ref. [28]. Copyright 2016, American Chemical Society.
Hydrazine-modified graphene quantum dots (HM-GQDs) were prepared by refluxing GQDs with hydrazine hydrate. Such HM-GQDs were hybridized with gold nanoparticles (Au NPs) through the redox reaction between HM-GQDs and AuCl4−. This sensor has unique electrochemiluminescence (ECL) properties of HM-GQDs and easy self-assembly with some biomolecules of Au NPs, important for H2O2 assaying for cancer detection [28] HM-GQDs are also useful as a novel ECL immunosensor of carcinoembryonic antigen (CEA) as a model target analyte. It displays a linear response range of CEA between 0.02 and 80 ng∙mL−1 with an LOD of 0.01 ng∙mL−1 using a CV setup. Carcinoembryonic antigen is reported as a tumor marker in clinical tests [21].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26216674
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737.
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316.
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738.
- Zhou, Y.; Zahran, E.M.; Quiroga, B.A.; Perez, J.; Mintz, K.J.; Peng, Z.; Liyanage, P.Y.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Size-dependent photocatalytic activity of carbon does with surface-state determined photoluminescence. Appl. Catal. B 2019, 248, 157–166.
- Cilingir, E.K.; Seven, E.S.; Zhou, Y.; Walters, B.M.; Mintz, K.J.; Pandey, R.R.; Wikramanayake, A.H.; Chusuei, C.C.; Vanni, S.; Graham, R.M.; et al. Metformin derived carbon dots: Highly biocompatible fluorescent nanomaterials as mitochondrial targeting and blood-brain barrier penetrating biomarkers. J. Colloid Interface Sci. 2021, 592, 485–497.
- Peng, Z.; Zhou, Y.; Ji, C.; Pardo, J.; Mintz, K.J.; Pandey, R.R.; Chusuei, C.C.; Graham, R.M.; Yan, G.; Leblanc, R.M. Facile synthesis of ‘boron-doped’ carbon dots and their application in visible-light-driven photocatalytic degradation of organic dyes. Nanomaterials 2020, 10, 1560.
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A deep investigation into the structure of carbon dots. Carbon 2021, 173, 433–447.
- Zhou, Y.; Mintz, K.J.; Cheng, L.; Chen, J.; Ferreira, B.C.L.B.; Hettiarachchi, S.D.; Liyanage, P.Y.; Seven, E.S.; Miloserdov, N.; Pandey, R.R.; et al. Direct conjugation of distinct carbon dots as Lego-like building blocks for the assembly of versatile drug nanocarriers. J. Colloid Interface Sci. 2020, 576, 412–425.
- Seven, E.S.; Sharma, S.K.; Meziane, D.; Zhou, Y.; Mintz, K.J.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Close-packed Langmuir monolayers of saccharide-based carbon dots at the air-subphase interface. Langmuir 2019, 35, 6708–6718.
- Mintz, J.K.; Mercado, G.; Zhou, Y.; Ji, Y.; Hettiarachchi, D.S.; Liyanage, Y.P.; Pandey, R.R.; Chusuei, C.C.; Dallman, J.; Leblanc, M.R. Tryptophan carbon dots and their ability to cross the blood-brain barrier. Colloids Surf. B 2019, 176, 488–493.
- Liyanage, P.Y.; Geleroff, D.L.; Peng, Z.; Mintz, K.J.; Hettiarachch, S.D.; Pandey, R.R.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Carbon Nitride Dots: A Selective Bioimaging Nanomaterial. Bioconjug. Chem. 2019, 12, 111–123.
- Zhou, Y.; Liyanage, P.Y.; Geleroff, D.L.; Peng, Z.; Mintz, K.J.; Hettiarrachchi, S.D.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Photoluminescent carbon dots: A mixture of heterogeneous fractions. ChemPhysChem 2018, 19, 2589–2597.
- Ji, Y.; Zhou, Y.; Waidely, E.; Desserre, A.; Marksberry, M.H.; Chusuei, C.C.; Dar, A.A.; Chat, O.A.; Li, S.; Leblanc, R.M. Rheology of a carbon dot gel. Inorg. Chim. Acta 2017, 468, 119–124.
- Zhou, Y.; Desserre, A.; Sharma, S.K.; Li, S.; Marksberry, M.H.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Gel-like carbon dots: Characterization and their potential applications. ChemPhysChem 2017, 18, 890–897.
- Fiuza, T.; Gomide, G.; Campos, A.F.C.; Messina, F.; Depyrot, J. On the colloidal stability of nitrogen-rich carbon nanodots aqueous dispersions. C J. Carbon Res. 2019, 5, 74.
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424.
- Yan, F.; Kong, D.; Luo, Y.; Ye, Q.; He, J.; Guo, X.; Chen, L. Carbon dots serve as an effective probe for the quantitative determination and for intracellular imaging of mercury (II). Microchim. Acta 2016, 183, 1611–1618.
- Yin, J.Y.; Liu, H.J.; Jiang, S.; Chen, Y.; Yao, Y. Hyperbranched polymer functionalized carbon dots with multi stimuli-responsive property. ACS Macro Lett. 2013, 2, 1033–1037.
- Shi, W.; Li, X.; Ma, H. A tunable ratiometric pH sensor based on carbon nanodots for the quantitative measurement of the intracellular pH of whole cells. Angew. Chem. Int. Ed. Eng. 2012, 51, 6432–6435.
- Wei, Y.; Zhang, D.; Fang, Y.; Wang, H.; Liu, Y.; Xu, Z. Detection of ascorbic acid using green synthesized carbon quantum dots. J. Sens. 2019, 2019, 9869682.
- Dong, Y.; Wu, H.; Shang, P.; Zeng, X.; Chi, Y. Immobilizing water-soluble graphene quantum dots with gold nanoparticles for low potential electrochemiluminescence immunosensor. Nanoscale 2015, 7, 16366–16371.
- Abbas, M.W.; Soomro, R.A.; Kalwar, N.H.; Zahoor, M.; Avci, A.; Pehlivan, E.; Hallam, K.R.; Willander, M. Carbon quantum dot coated Fe3O4 hybrid composites for sensitive electrochemical detection of uric acid. Microchem. J. 2019, 146, 517–524.
- Shiri, S.; Pajouheshpour, N.; Khossafar, H.; Amidi, S.; Bagheri, H. An electrochemical sensor for the simulataneous determination of rifampicin and isoniazid using a 2O4 nanocomposite modified carbon paste electrode. New J. Chem. 2017, 41, 15564–15573.
- Roushani, M.; Ghanbari, K.; Hoseini, S.J. Designing an electrochemical aptasensor based on immobilization of the aptamer onto nanocomposite for detection of the streptomycin antibiotic. Microchem. J. 2018, 141, 96–103.
- Hasanzadeh, M.; Karimzadeh, A.; Shadjou, N. Magnetic graphene quantum dots as a functional nanomaterial towards voltammetric detection of L-tryptophan at physiological pH. J. Nanostruct. 2018, 8, 21–30.
- Muthusankar, G.; Devi, R.K.; Gopu, G. Nitrogen-doped carbon quantum dots embedded CO3O4 with multiwalled carbon nanotubes: An efficient probe for the simultaneous determination of anti-cancer and antibiotic drugs. Biosens. Bioelectron. 2020, 150, 111947.
- Li, J.; Qu, J.; Yang, R.; Qu, L.; Harrington, P.D.B. A sensitive and selective electrochemical sensor based on graphene quantum dot/golds nanoparticle nanocomposite modified electrode for the determination of quercertin in biological samples. Electroanalysis 2016, 28, 1322–1330.
- Xi, J.; Xie, C.; Zhang, Y.; Wang, L.; Xiao, J.; Duan, X.; Ren, J. Pd nanoparticles decorated N-doped graphene quantum carbon hollow nanospheres with high electrochemical sensing performance in cancer detection. ACS Appl. Mater. Interfaces 2016, 8, 22563–22573.
- Yola, M.L.; Atar, N. A novel detection approach for serotonin by graphene quantum dots/two dimensional (2D) hexagonal boron nitride nanosheets with molecularly imprinted polymer. Appl. Surf. Sci. 2018, 458, 648–655.
- Canevari, T.C.; Cincotto, F.H.; Gomes, D.; Landers, R.; Toma, H.E. Magnetite nanoparticles bonded carbon quantum dots magnetically confined onto screen printed carbon electrodes and their performance as electrochemical sensor for NADH. Electroanalysis 2017, 29, 1968–1975.
- Shadjou, N.; Hasanzadeh, M.; Talebi, F.; Marjani, A.P. Integration of Beta-cyclodextrin in graphene quantum dot nano-structure and its application towards detection of vitamin C at physiological pH: A new electrochemical approach. Mater. Sci. Eng. C. 2016, 67, 666–674.
- Baluta, S.; Lesiak, A.; Cabaj, J. Graphene quantum dots-based electrochemical biosensors for catecholamine neurotransmitters detection. Electroanalysis 2018, 30, 1781–1790.
- Ganganboina, A.B.; Doong, R. Functionalized N-doped graphene quantum dots for electrochemical determination of cholesterol through host-guest inclusion. Microchim. Acta. 2018, 185, 526.
- Maaoui, H.; Teodoresu, F.; Wang, Q.; Pan, C.H.; Addad, A.; Chtourou, R.; Szanerits, S.; Boukherroub, R. Non-enzymatic glucose sensing using carbon quantum dots decorated with copper oxide nanoparticles. Sensors 2016, 16, 1720.
- Shang, J.; Zhao, M.; Qu, H.; Li, H.; Chen, S. Fabrication of CQDs/MoS2/Mo foil for the improved electrochemical detection. Anal. Chim. Acta 2019, 1079, 79–85.
- Beitollah, H.; Dourandish, Z.; Ganjali, M.R.; Shakeri, S. Voltammetric determination of dopamine in the presence of tyrosine using graphite screen-printed electrode modified with graphene quantum dots. Ionics 2018, 24, 4023–4031.
- Chen, J.; He, P.; Bai, H.; He, S.; Zhang, T.; Zhang, X.; Dong, F. Poly (Beta-cyclodextrin)/carbon quantum dots modified glassy carbon electrode: Preparation, characterization, and simultaneous determination of dopamine, uric acid and tryptophan. Sens. Actuators B 2017, 252, 9–16.
- Sadhukhan, M.; Bhowmik, T.; Kundu, M.K.; Barman, S. Facile synthesis of carbon quantum dots and thin graphene sheets for non-enzymatic sensing of hydrogen peroxide. RSC Adv. 2014, 4, 4998–5005.
- Jahanbakhshi, M.; Habibi, B. A novel and facile synthesis of carbon quantum dots via salep hydrothermal treatment as the silver nanoparticles support: Application to electroanalytical determination of H2O2 in fetal bovine serum. Biosens. Bioelectron. 2016, 81, 143–150.
- Chen, W.; Weng, W.; Niu, X.; Li, X.; Men, Y.; Sun, W.; Li, G.; Dong, L. Boron-doped graphene quantum dots modified electrode for electrochemistry and electrocatalysis of hemoglobin. J. Electroanal. Chem. 2018, 823, 137–145.
- Habibi, E.; Heidari, H. Renewable surface carbon composite electrode bulk modified with GQD-RuC13 nano-composite for high sensitive detection of L-tyrosine. J. Electroanal. Chem. 2016, 28, 2559–2564.
- Wang, L.; Tricard, S.; Yue, P.; Zhao, J.; Fang, J.; Shen, W. Polypyrrole and graphene carbon Blue hybrid film on graphite felt electrodes: Application for amperometric determination of L-cysteine. Biosens. Bioelectron. 2016, 77, 1112–1118.
- Muthansankar, G.; Rajkumar, C.; Chen, S.M.; Karkuzhali, R.; Gopu, G.; Sangili, A.; Sengottuvelan, N.; Sankar, R. Sonochemical driven simple preparation of nitrogen-doped carbon quantum dots/SnO2 nanocomposite: A novel electrocatalyst for sensitive voltammetric determination of riboflavin. Sens. Actuators B Chem. 2019, 281, 602–612.
- Shao, X.; Gu, H.; Wang, Z.; Chai, X.; Tian, Y.; Shi, G. Highly selective electrochemical strategy for monitoring of cerebral Cu2+ based on a carbon dot-TPEA hybridized surface. Anal. Chem. 2013, 85, 418–425.
This entry is offline, you can click here to edit this entry!
