Multicomponent reactions are a fascinating family of organic chemistry transformations. Traditional bimolecular reactions are outperformed by such reactions, which combine three or more reactants into one reaction product. Multicomponent reactions speed up chemical space exploration by minimizing the quantity of synthesis and refinement steps needed to create a particular target. Isocyanides (isonitriles) were the only stable organic molecules containing a formally divalent carbon atom for a long period of time. The group of isocyanides are distinguishable from other functional groups due to their reactivity.
- isocyanide
- multicomponent reactions
- antibiotic resistance
1. Introduction
2. Isocyanides and the Types of Isocyanide-Based Multicomponent Reactions
2.1. The Reactions of Passerini
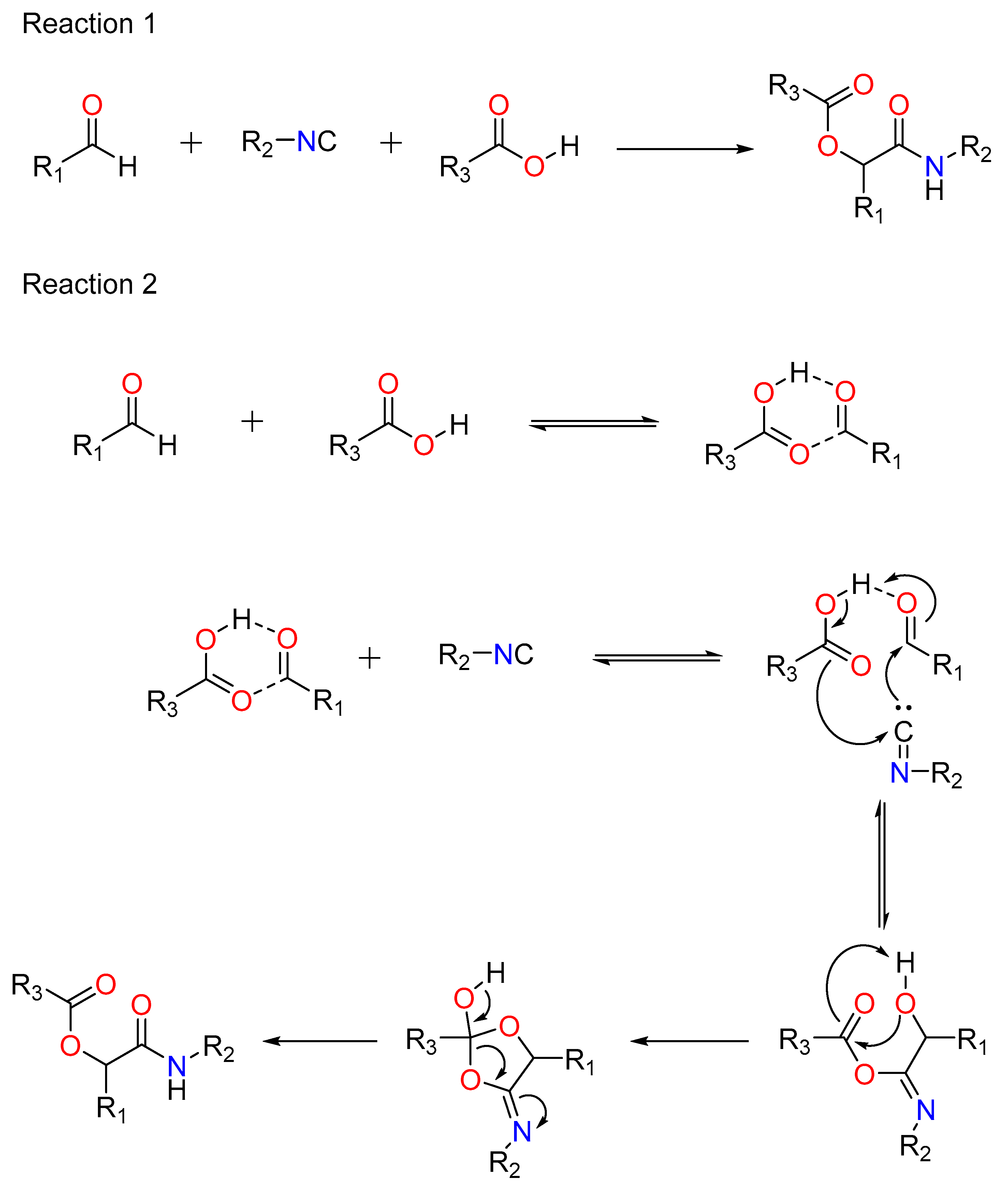
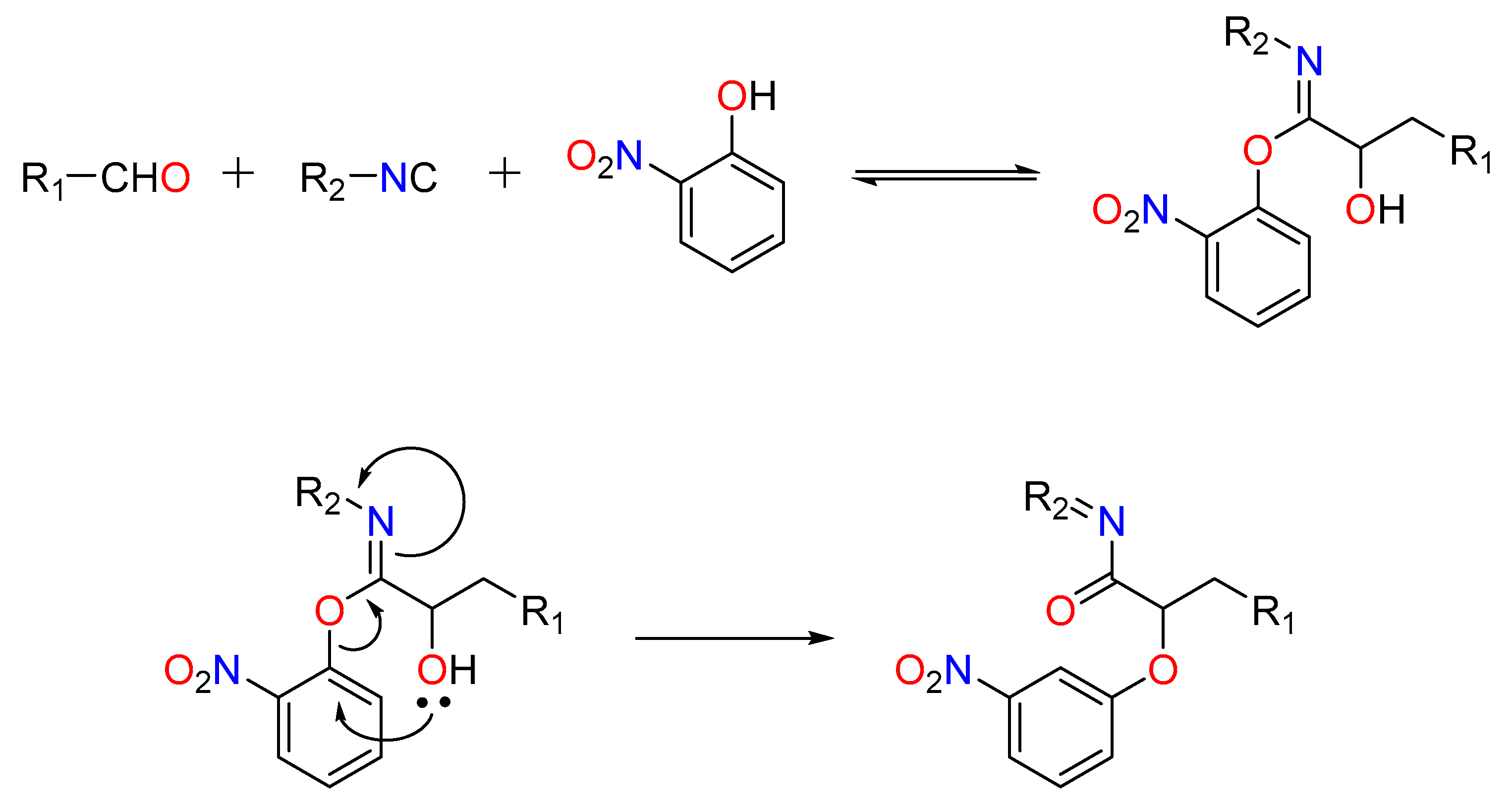
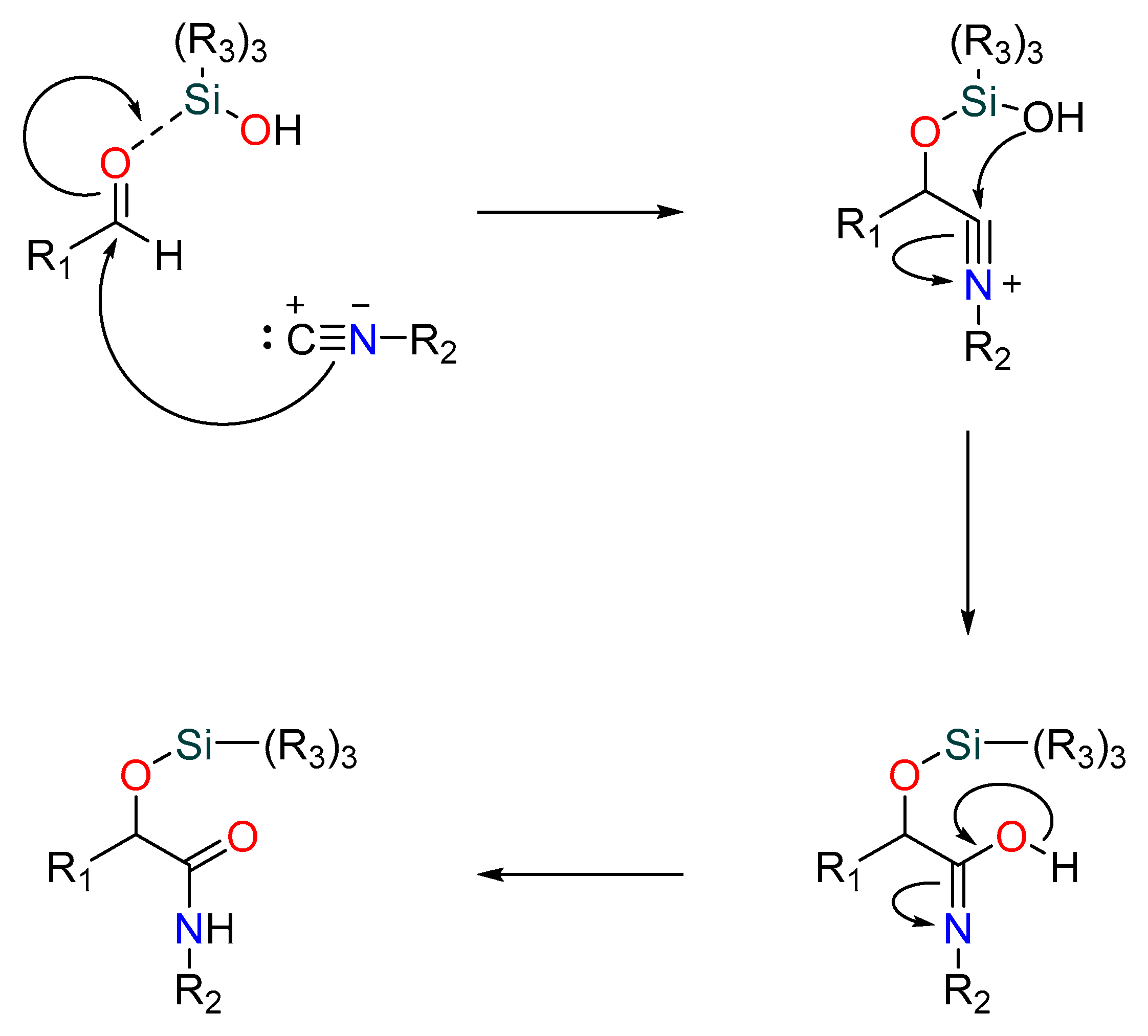
2.2. Ugi-4C Reaction
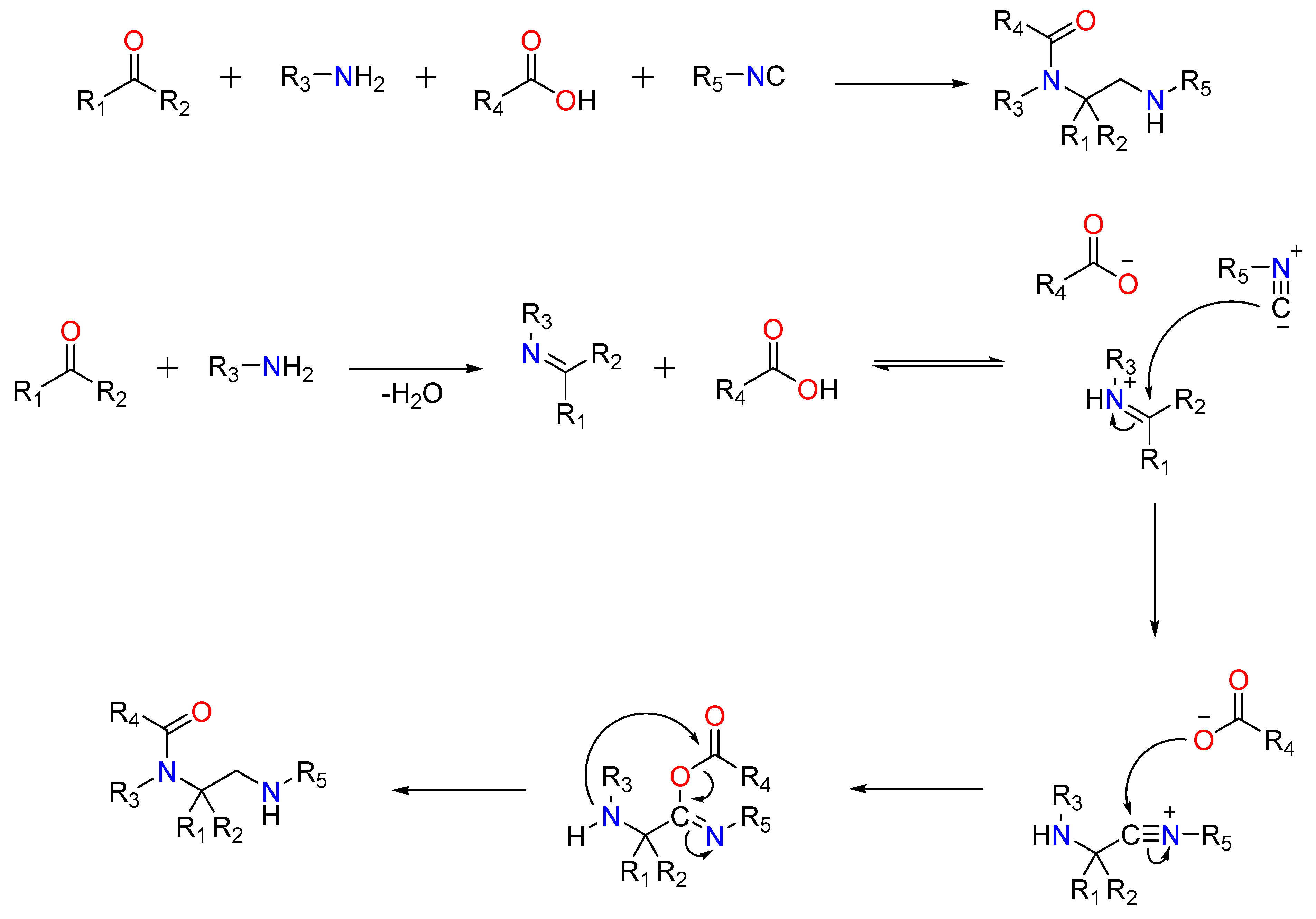
2.3. Groebke–Blackburn–Bienaymé Reaction
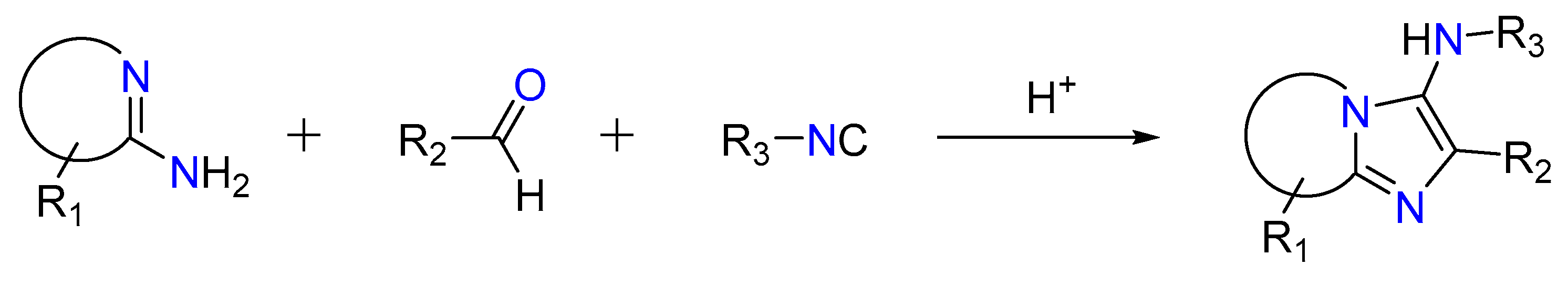
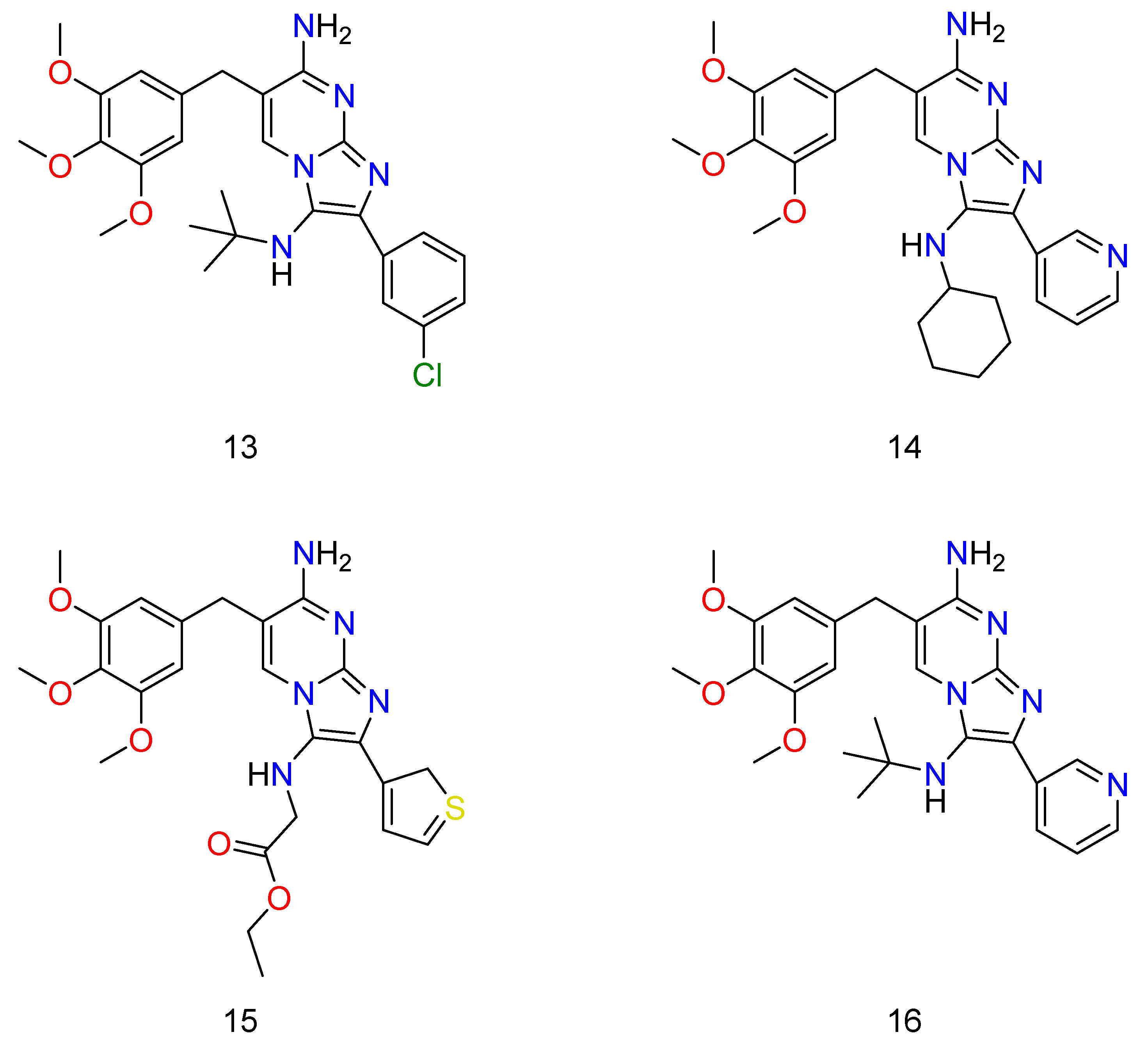
3. Groebke–Blackburn–Bienaymé Reaction in the Discovery of the Modified Antibiotic Trimethoprim
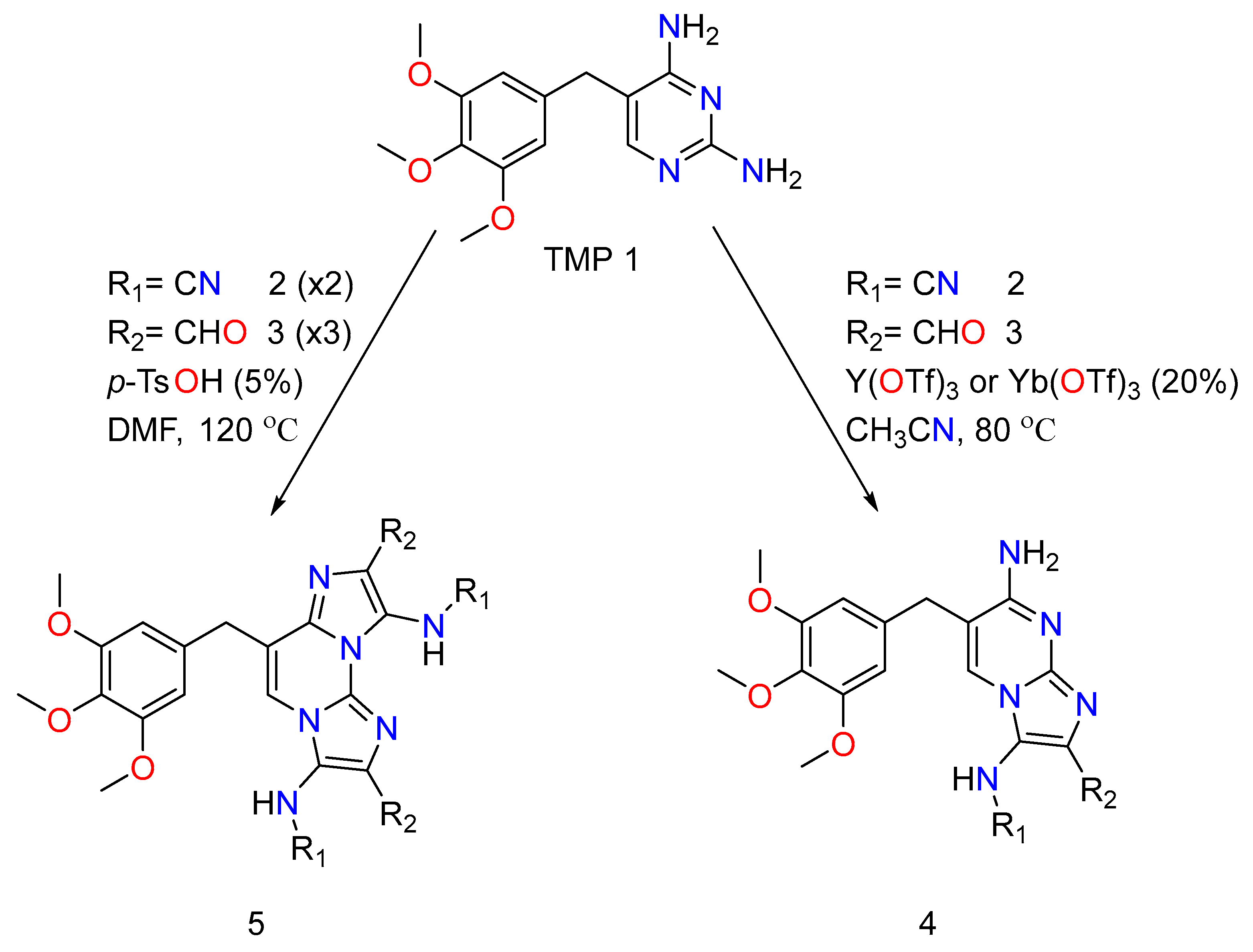
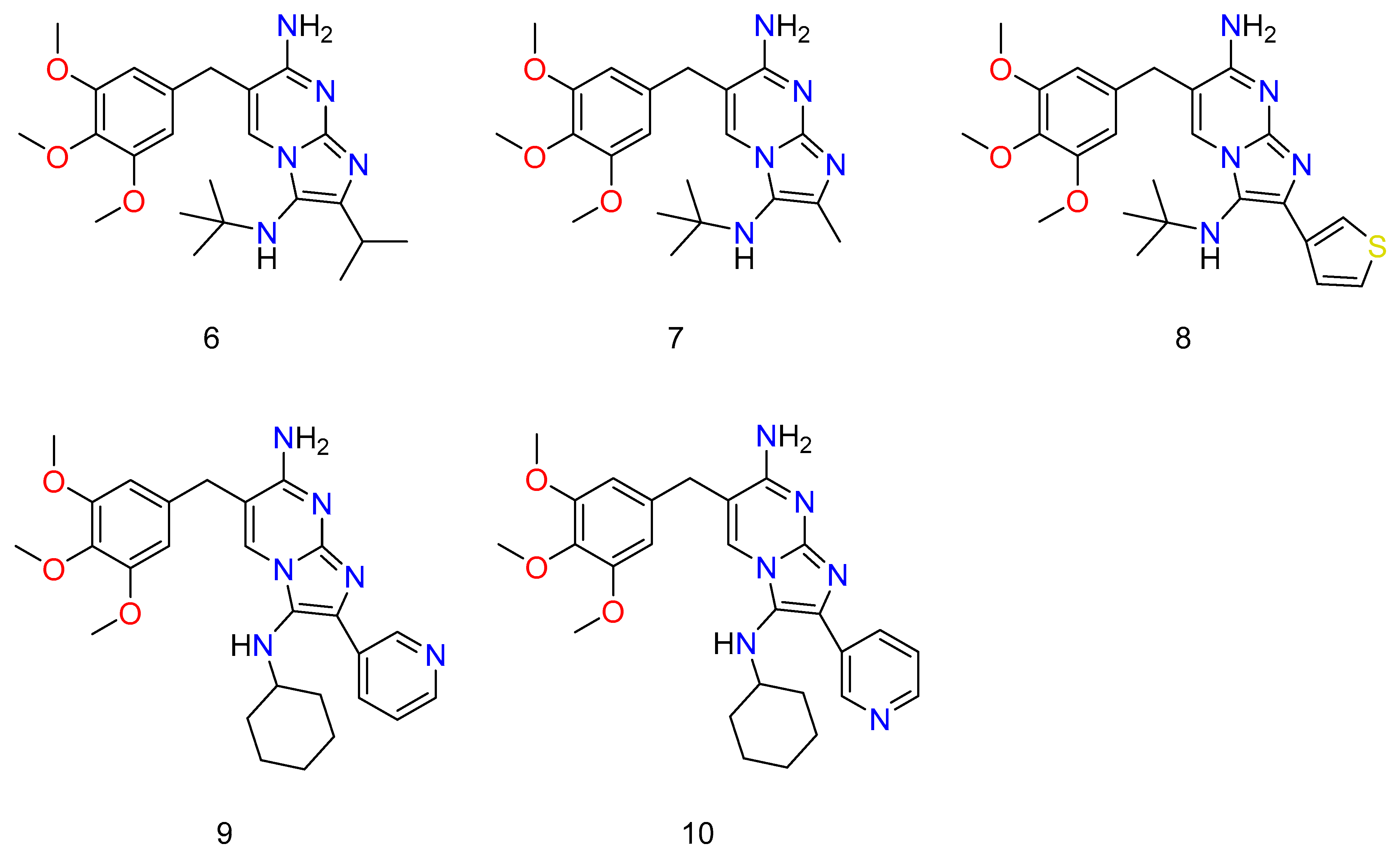
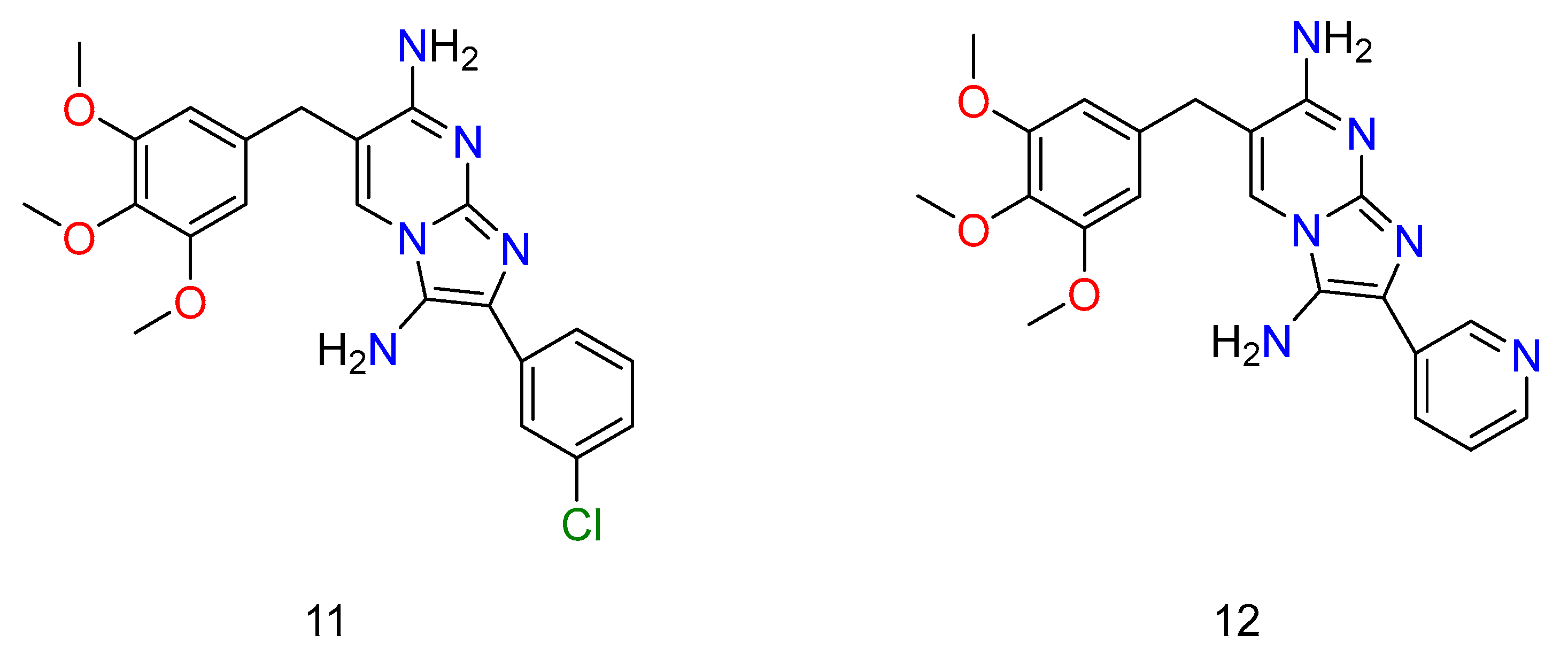
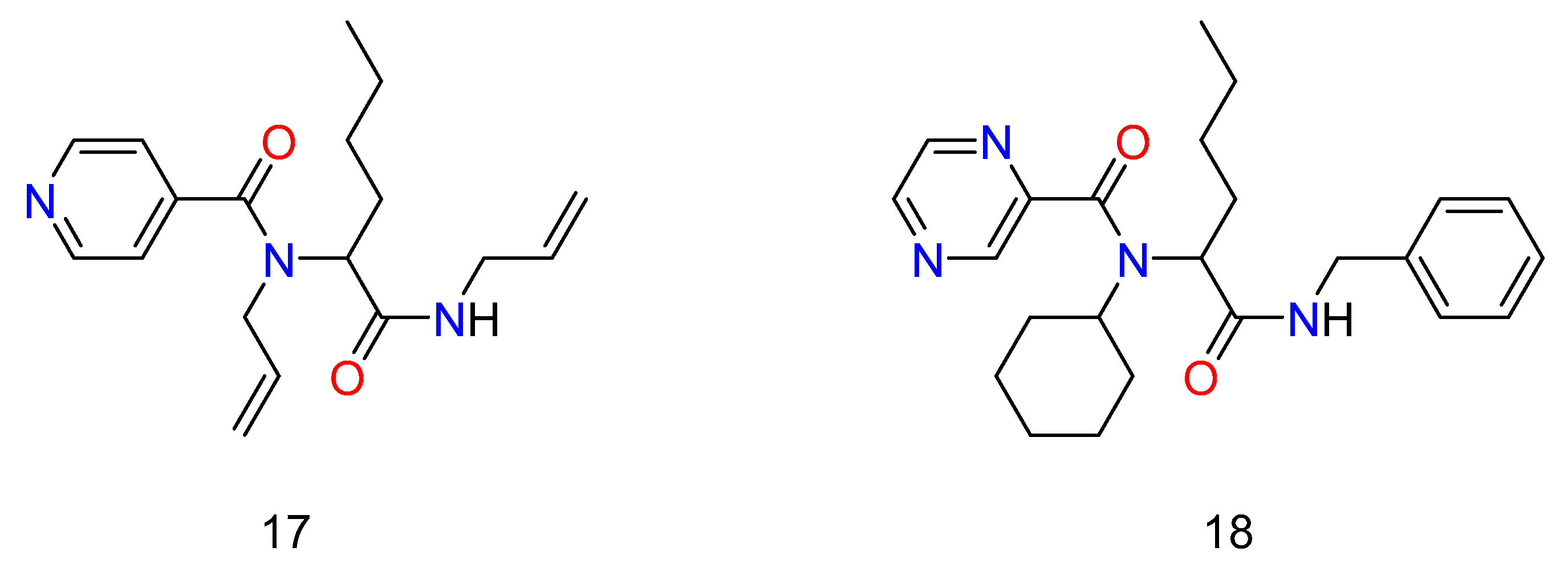
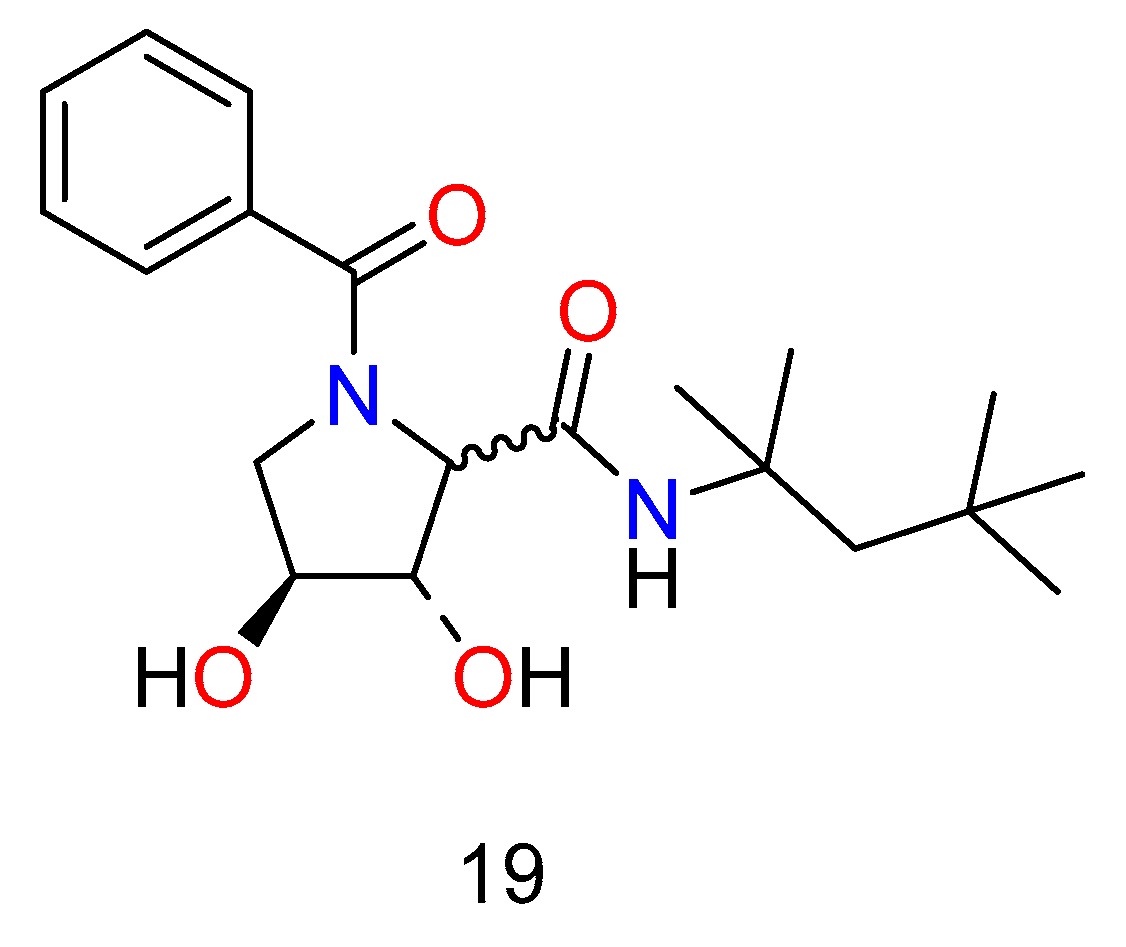
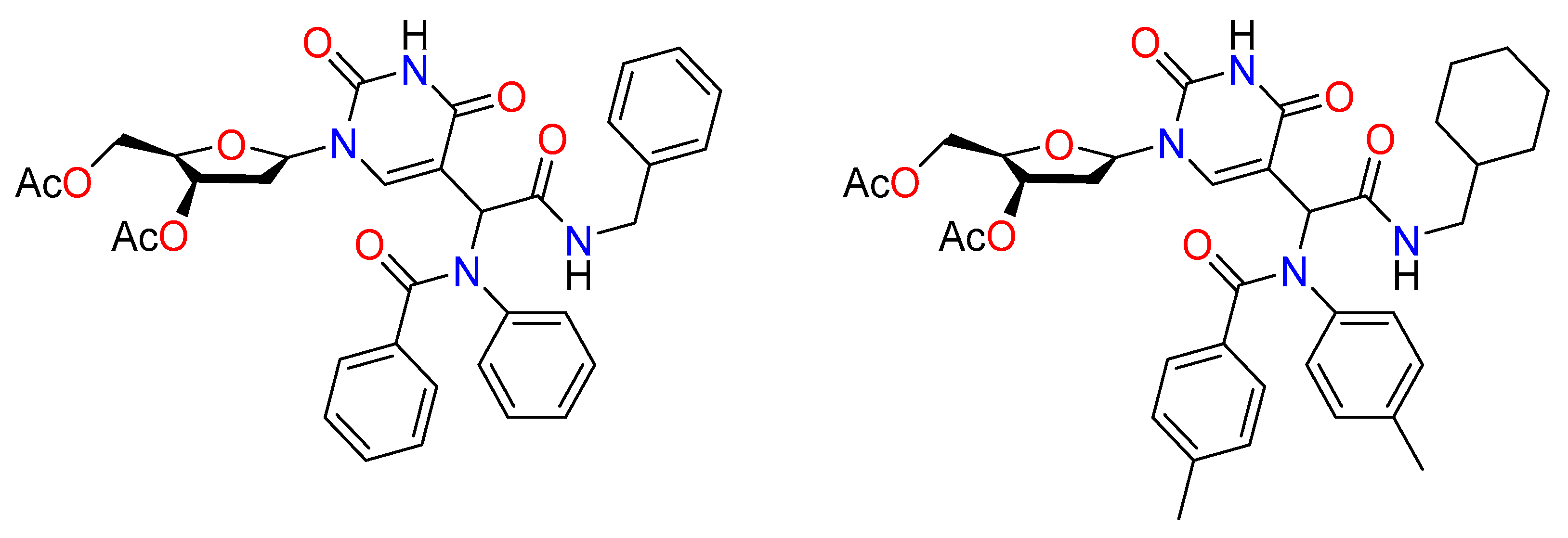
4. The Discovery of Antimicrobial Compounds against Infectious Diseases through the Application of Ugi’s Reaction
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12050849
References
- Zarganes-Tzitzikas, T.; Chandgude, A.L.; Dömling, A. Multicomponent reactions, union of MCRs and beyond. Chem. Rec. 2015, 15, 981–996.
- Afshari, R.; Shaabani, A. Materials functionalization with multicomponent reactions: State of the art. ACS Comb. Sci. 2018, 20, 499–528.
- Quazi, S.; Gavas, S.; Malik, J.A.; Suman, K.S.; Haider, Z. In-silico pharmacophore and molecular docking based drug discovery against marburg virus’s viral protein 35; A potent of MAVD. bioRxiv 2021. preprint.
- Lieke, W. Ueber das cyanallyl. Justus Liebigs Ann. Chem. 1859, 316–321.
- Gautier, A. Ueber die einwirkung des chlorwasserstoffs u. a. Auf das aethyl- und methylcyanür. Ann. Chem. Pharm. 1867, 142, 289–294.
- Ugi, I. The α-addition of immonium ions and anions to isonitriles accompanied by secondary reactions. Angew. Chem. Int. Ed. Engl. 1962, 1, 8–21.
- Ugi, I.; Dömling, A.; Gruber, B.; Almstetter, M. Multicomponent reactions and their libraries—A new approach to preparative organic chemistry. Croat. Chem. Acta 1997, 70, 631–647.
- Ugi, I. Neuere methoden der präparativen organischen chemie IV: Mit sekundär-reaktionen gekoppelte α-additionen von immonium-ionen und anionen an isonitrile. Angew. Chem. 1962, 74, 9–22.
- Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Versuche mit isonitrilen. Angew. Chem. 1959, 71, 386.
- Arshady, R.; Ugi, I. Solid phase peptide synthesis by four component condensation: Peptide formation on an isocyano polymer support. Z. Naturforsch. B J. Chem. Sci. 1981, 36, 1202–1203.
- Oertel, K.; Zech, G.; Kunz, H. Stereoselective combinatorial ugi-multicomponent synthesis on solid phase this work was supported by the deutsche forschungsgemeinschaft and by the fonds der chemischen industrie. Angew. Chem. Int. Ed. Engl. 2000, 39, 1431–1433.
- Bienaymé, H.; Bouzid, K. A new heterocyclic multicomponent reaction for the combinatorial synthesis of fused 3-aminoimidazoles. Angew. Chem. Int. Ed 1998, 37, 2234–2237.
- Blackburn, C.; Guan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Parallel synthesis of 3-aminoimidazo pyridines and pyrazines by a new three-component condensation. Tetrahedron Lett. 1998, 39, 3635–3638.
- Groebke, K.; Weber, L.; Mehlin, F. ChemInform abstract: Synthesis of imidazo annulated pyridines, pyrazines, and pyrimidines by a novel three-component condensation. ChemInform 2010, 29.
- Ugi, I.; Meyr, R.; Isonitrile, V. Erweiterter anwendungsbereich der passerini-reaktion. Chem. Ber. 1961, 94, 2229–2233.
- Wang, S.-X.; Wang, M.-X.; Wang, D.-X.; Zhu, J. Catalytic enantioselective passerini three-component reaction. Angew. Chem. Int. Ed. Engl. 2008, 47, 388–391.
- Brioche, J.; Masson, G.; Zhu, J. Passerini three-component reaction of alcohols under catalytic aerobic oxidative conditions. Org. Lett. 2010, 12, 1432–1435.
- Yanai, H.; Oguchi, T.; Taguchi, T. Direct alkylative passerini reaction of aldehydes, isocyanides, and free aliphatic alcohols catalyzed by indium (III) triflate. J. Org. Chem. 2009, 74, 3927–3929.
- El Kaim, L.; Gizolme, M.; Grimaud, L. O-arylative passerini reactions. Org. Lett. 2006, 8, 5021–5023.
- Soeta, T.; Kojima, Y.; Ukaji, Y.; Inomata, K. O-silylative passerini reaction: A new one-pot synthesis of α-siloxyamides. Org. Lett. 2010, 12, 4341–4343.
- Sunderhaus, J.D.; Martin, S.F. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chemistry 2009, 15, 1300–1308.
- Paulvannan, K. Preparation of tricyclic nitrogen heterocycles via tandem four-component condensation/intramolecular diels-alder reaction. Tetrahedron Lett. 1999, 40, 1851–1854.
- Koopmanschap, G.; Ruijter, E.; Orru, R.V.A. Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics. Beilstein J. Org. Chem. 2014, 10, 544–598.
- Bonnaterre, F.; Bois-Choussy, M.; Zhu, J. Rapid access to oxindoles by the combined use of an ugi four-component reaction and a microwave-assisted intramolecular buchwald−hartwig amidation reaction. Org. Lett. 2006, 8, 4351–4354.
- White, C.J.; Yudin, A.K. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011, 3, 509–524.
- Quazi, S.; Jangi, R. Artificial intelligence and machine learning in medicinal chemistry and validation of emerging drug targets. Adv. Control. Drug Deliv. Syst. 2021, 27–43. Available online: https://www.igi-global.com/chapter/artificial-intelligence-and-machine-learning-in-medicinal-chemistry-and-validation-of-emerging-drug-targets/300400 (accessed on 1 March 2023).
- Hulme, C.; Lee, Y.-S. Emerging approaches for the syntheses of bicyclic imidazo -heterocycles. Mol. Divers. 2008, 12, 1–15.
- Devi, N.; Rawal, R.K.; Singh, V. Diversity-oriented synthesis of fused-imidazole derivatives via groebke–blackburn–bienayme reaction: A review. Tetrahedron 2015, 71, 183–232.
- Akritopoulou-Zanze, I.; Wakefield, B.D.; Gasiecki, A.; Kalvin, D.; Johnson, E.F.; Kovar, P.; Djuric, S.W. Scaffold oriented synthesis. Part 4: Design, synthesis and biological evaluation of novel 5-substituted indazoles as potent and selective kinase inhibitors employing heterocycle forming and multicomponent reactions. Bioorg. Med. Chem. Lett. 2011, 21, 1480–1483.
- Baviskar, A.T.; Madaan, C.; Preet, R.; Mohapatra, P.; Jain, V.; Agarwal, A.; Guchhait, S.K.; Kundu, C.N.; Banerjee, U.C.; Bharatam, P.V. N-fused imidazoles as novel anticancer agents that inhibit catalytic activity of topoisomerase IIα and induce apoptosis in G1/S phase. J. Med. Chem. 2011, 54, 5013–5030.
- Shukla, N.M.; Salunke, D.B.; Yoo, E.; Mutz, C.A.; Balakrishna, R.; David, S.A. Antibacterial activities of groebke–blackburn–bienaymé-derived imidazo pyridin-3-amines. Bioorg. Med. Chem. 2012, 20, 5850–5863.
- Burchak, O.N.; Mugherli, L.; Ostuni, M.; Lacapère, J.J.; Balakirev, M.Y. Combinatorial discovery of fluorescent pharmacophores by multicomponent reactions in droplet arrays. J. Am. Chem. Soc. 2011, 133, 10058–10061.
- Elleder, D.; Baiga, T.J.; Russell, R.L.; Naughton, J.A.; Hughes, S.H.; Noel, J.P.; Young, J.A.T. Identification of a 3-aminoimidazo pyridine inhibitor of HIV-1 reverse transcriptase. Virol. J. 2012, 9, 305.
- Bode, M.L.; Gravestock, D.; Moleele, S.S.; van der Westhuyzen, C.W.; Pelly, S.C.; Steenkamp, P.A.; Hoppe, H.C.; Khan, T.; Nkabinde, L.A. Imidazo pyridin-3-amines as potential HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. 2011, 19, 4227–4237.
- Urbancic, K.F.; Ierino, F.; Phillips, E.; Mount, P.F.; Mahony, A.; Trubiano, J.A. Taking the challenge: A protocolized approach to optimize pneumocystis pneumonia prophylaxis in renal transplant recipients. Am. J. Transpl. 2018, 18, 462–466.
- Heaslet, H.; Harris, M.; Fahnoe, K.; Sarver, R.; Putz, H.; Chang, J.; Subramanyam, C.; Barreiro, G.; Miller, J.R. Structural comparison of chromosomal and exogenous dihydrofolate reductase from staphylococcus aureus in complex with the potent inhibitor trimethoprim. Proteins 2009, 76, 706–717.
- Zhou, W.; Scocchera, E.W.; Wright, D.L.; Anderson, A.C. Antifolates as effective antimicrobial agents: New generations of trimethoprim analogs. Medchemcomm 2013, 4, 908.
- Quazi, S.; Malik, J.; Suman, K.S.; Capuzzo, A.M.; Haider, Z. Discovery of potential drug-like compounds against viral protein (VP40) of marburg virus using pharmacophoric based virtual screening from ZINC database. bioRxiv 2021.
- Lombardo, M.N.; G-Dayanandan, N.; Wright, D.L.; Anderson, A.C. Crystal structures of trimethoprim-resistant DfrA1 rationalize potent inhibition by propargyl-linked antifolates. ACS Infect. Dis. 2016, 2, 149–156.
- Rashid, U.; Ahmad, W.; Hassan, S.F.; Qureshi, N.A.; Niaz, B.; Muhammad, B.; Imdad, S.; Sajid, M. Design, synthesis, antibacterial activity and docking study of some new trimethoprim derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 5749–5753.
- Pedrola, M.; Jorba, M.; Jardas, E.; Jardi, F.; Ghashghaei, O.; Viñas, M.; Lavilla, R. Multicomponent reactions upon the known drug trimethoprim as a source of novel antimicrobial agents. Front. Chem. 2019, 7, 475.
- Ghashghaei, O.; Caputo, S.; Sintes, M.; Revés, M.; Kielland, N.; Estarellas, C.; Luque, F.J.; Aviñó, A.; Eritja, R.; Serna-Gallego, A.; et al. Multiple multicomponent reactions: Unexplored substrates, selective processes, and versatile chemotypes in biomedicine. Chemistry 2018, 24, 14513–14521.
- Dolce, D.; Neri, S.; Grisotto, L.; Campana, S.; Ravenni, N.; Miselli, F.; Camera, E.; Zavataro, L.; Braggion, C.; Fiscarelli, E.V.; et al. Methicillin-resistant staphylococcus aureus eradication in cystic fibrosis patients: A randomized multicenter study. PLoS ONE 2019, 14, e0213497.
- Lo, D.K.; Muhlebach, M.S.; Smyth, A.R. Interventions for the eradication of meticillin-resistant staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst. Rev. 2018, 7, CD009650.
- Xhemali, X.; Smith, J.R.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Compton, M.; Singh, N.B.; Jahanbakhsh, S.; Rybak, M.J. Evaluation of dalbavancin alone and in combination with β-lactam antibiotics against resistant phenotypes of staphylococcus aureus. J. Antimicrob. Chemother. 2019, 74, 82–86.
- Dömling, A.; Achatz, S.; Beck, B. Novel anti-tuberculosis agents from MCR libraries. Bioorg. Med. Chem. Lett. 2007, 17, 5483–5486.
- Nutt, R.F.; Joullie, M.M. Four-component condensation: A new versatile method for the synthesis of substituted prolyl peptides. J. Am. Chem. Soc. 1982, 104, 5852–5853.
- Akritopoulou-Zanze, I. Isocyanide-based multicomponent reactions in drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 324–331.
- Fan, X.; Zhang, X.; Bories, C.; Loiseau, P.M.; Torrence, P.F. The ugi reaction in the generation of new nucleosides as potential antiviral and antileishmanial agents. Bioorg. Chem. 2007, 35, 121–136.
- Musonda, C.C.; Taylor, D.; Lehman, J.; Gut, J.; Rosenthal, P.J.; Chibale, K. Application of multicomponent reactions to antimalarial drug discovery. Part 1. Parallel synthesis and antiplasmodial activity of new 4-aminoquinoline ugi adducts. Bioorg. Med. Chem. Lett. 2004, 14, 3901–3905.
- Short, K.M.; Ching, B.W.; Mjalli, A.M.M. Exploitation of the ugi 4CC reaction: Preparation of small molecule combinatorial libraries via solid phase. Tetrahedron 1997, 53, 6653–6679.
- Musonda, C.C.; Gut, J.; Rosenthal, P.J.; De Souza, C.; Chibale, R.C. Application of multicomponent reactions to antimalarial drug discovery. Part 2. New antiplasmodial and antitrypanosomal 4-aminoquinoline g-and d-lactams via a ‘catch and release’ protocol. Bioorg. Med. Chem. 2006, 14, 5605–5615.
- Linderman, R.J.; Binet, S.; Petrich, S.R. Enhanced diastereoselectivity in the asymmetric ugi reaction using a new “convertible” isonitrile. J. Org. Chem. 1999, 64, 8058.
- Cohen, E. Chitin biochemistry: Synthesis and inhibition. Annu. Rev. Entomol. 1987, 32, 71–93.
- Plant, A.; Thompson, P.; Williams, D.M. Application of the ugi reaction for the one-pot synthesis of uracil polyoxin C analogues. J. Org. Chem. 2009, 74, 4870–4873.
- Neves Filho, R.A.W.; Stark, S.; Westermann, B.; Wessjohann, L.A. The multicomponent approach to N-methyl peptides: Total synthesis of antibacterial (−)-viridic acid and analogues. Beilstein J. Org. Chem. 2012, 8, 2085–2090.
- Brase, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990.
- Quazi, S. Role of artificial intelligence and machine learning in bioinformatics: Drug discovery and drug repurposing. Preprints 2021, 2021050346.
- Iarani, G.M.; Moradi, R.; Mahammadkhani, L. Application of multicomponent reactions in the total synthesis of natural peptides. Org. Chem. 2019, 18–40.
- Okandeji, B.O.; Greenwald, D.M.; Wroten, J.; Sello, J.K. Synthesis and evaluation of inhibitors of bacterial drug efflux pumps of the major facilitator superfamily. Bioorg. Med. Chem. 2011, 19, 7679–7689.
- Okamoto, K.; Sakagami, M.; Feng, F.; Togame, H.; Takemoto, H.; Ichikawa, S.; Matsuda, A. Total synthesis of pacidamycin D by Cu (I)-catalyzed oxy enamide formation. Org. Lett. 2011, 13, 5240–5243.
- Winn, M.; Goss, R.J.; Kimura, K.I.; Bugg, T.D. Antimicrobial nucleoside antibiotics targeting cell wall assembly: Recent advances in structure–function studies and nucleoside biosynthesis. Nat. Prod. Rep. 2010, 27, 279–304.
- Fernandes, P.B.; Swanson, R.N.; Hardy, D.J.; Hanson, C.W.; Coen, L.; Rasmussen, R.R.; Chen, R.H. Pacidamycins, a novel series of antibiotics with anti-pseudomonas aeruginosa activity. III. Microbiologic profile. J. Antibiot. 1989, 42, 521–526.
- Yan, Y.-M.; Rao, Y.; Ding, M.-W. One-pot synthesis of multisubstituted benzimidazoles via sequential ugi and catalytic aza-wittig reaction starting from 2-aminobenzoyl azides. J. Org. Chem. 2016, 81, 1263–1268.
- Mavrova, A.T.; Yancheva, D.; Anastassova, N.; Anichina, K.; Zvezdanovic, J.; Djordjevic, A.; Markovic, D.; Smelcerovic, A. Synthesis, electronic properties, antioxidant and antibacterial activity of some new benzimidazoles. Bioorg. Med. Chem. 2015, 23, 6317–6326.
- Pan, T.; He, X.; Chen, B.; Chen, H.; Geng, G.; Luo, H.; Zhang, H.; Bai, C. Development of benzimidazole derivatives to inhibit HIV-1 replication through protecting APOBEC3G protein. Eur. J. Med. Chem. 2015, 95, 500–513.
- Vasantha, K.; Basavarajaswamy, G.; Rai, M.V.; Boja, P.; Pai, V.R.; Shruthi, N.; Bhat, M. Rapid ‘one-pot’synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015, 25, 1420–1426.
- Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. Antibacterial self-healing hydrogel via the ugi reaction. ACS Appl. Polym. Mater. 2019, 2, 404–410.
