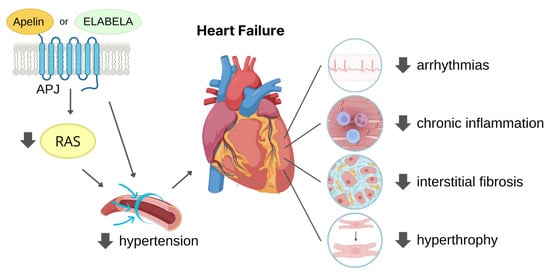
The widely expressed G protein-coupled apelin receptor (APJ) is activated by two bioactive endogenous peptides, apelin and ELABELA (ELA). The apelin/ELA-APJ-related pathway has been found involved in the regulation of many physiological and pathological cardiovascular processes. Increasing studies are deepening the role of the APJ pathway in limiting hypertension and myocardial ischaemia, thus reducing cardiac fibrosis and adverse tissue remodelling, outlining APJ regulation as a potential therapeutic target for heart failure prevention.
- apelin
- ELABELA/Toddler/Apela
- APLNR/APJ receptor
- peptide analogue
- cardiovascular disease
- myocardial infarction
- heart failure
- hypertension
- angiogenesis
- fibrosis
1. Introduction
2. APJ and Its Endogenous Agonists
2.1. APJ
2.2. Apelin

2.3. ELABELA
2.4. Physiological Ligand Activities in CV System
3. Cardioprotective Role of APJ Endogenous Ligands
3.1. Protection against Hypertension
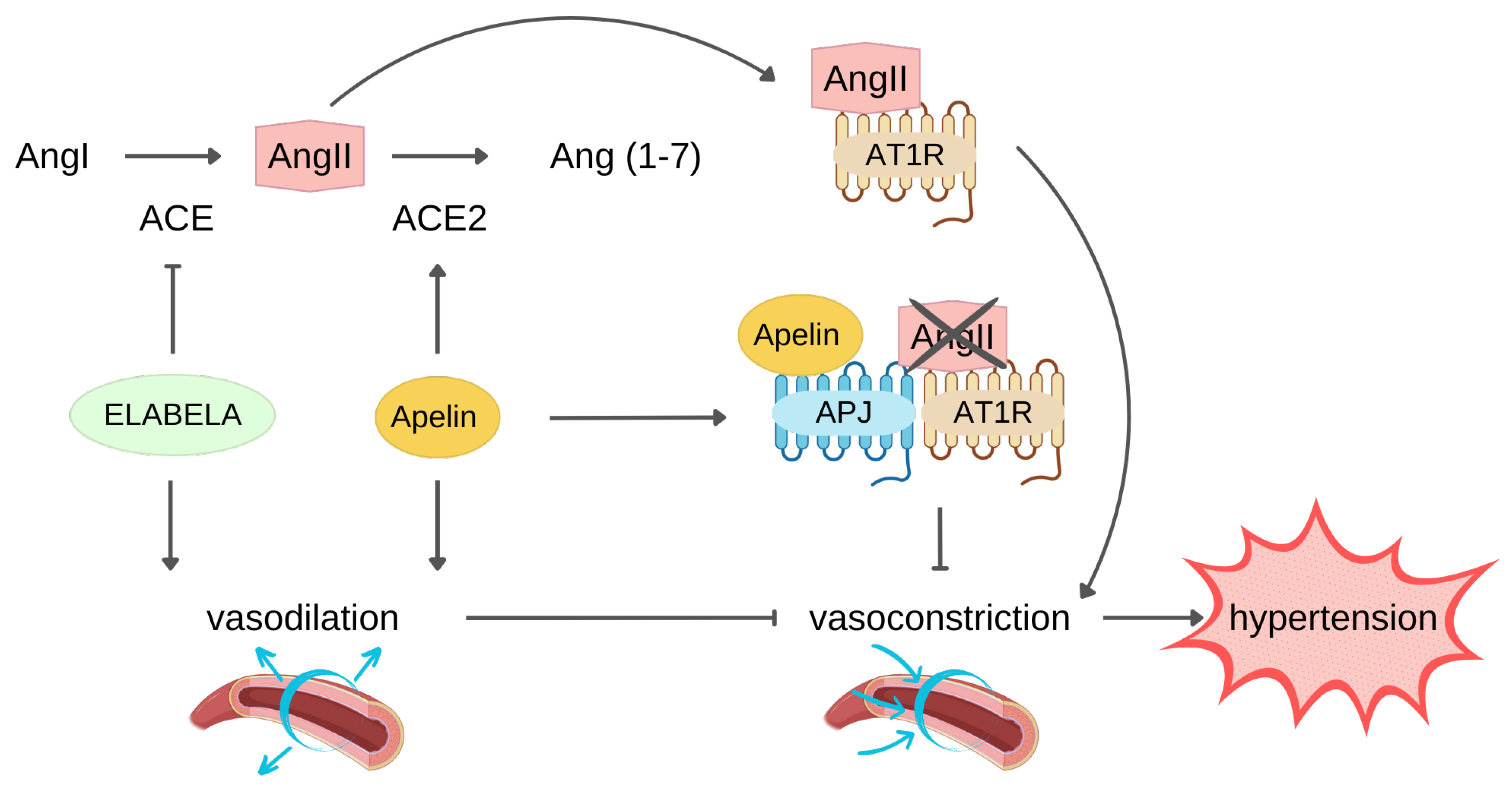
3.2. Protection against MI
4. APJ Peptide Agonists
4.1. APJ Endogenous Ligand Main Chain Modifications
|
APJ Ligand |
Substituted Residues |
Introduced Residues |
Effects |
Ref |
|---|---|---|---|---|
|
[Pyr1]-apelin-13 |
Leu5, Ser 6 |
- |
↑ plasma protein stability |
[88] |
|
Phe13 |
[(L-α-Me) Phe] |
↓ Pro12-Phe13 hydrolisis |
||
|
Pro12-Phe13 |
conformational constrained amino acids |
↑ binding affinity ↑ plasma protease stability |
[89] |
|
|
Aia -Phe 1Nal-α,α-dibenzylglycine |
↑ Gα12 pathways ↑ affinity and resistance to ACE2 cleavage ↑ in vitro and in vivo pharmacokinetics |
[90] |
||
|
Phe13 |
phenyl groups at the ortho-, meta-, and para-positions of Phe13 |
slight effect on APJ binding affinity |
||
|
(L-α-Me) Phe or p-benzoyl-L-Phe (Bpa) |
↑ APJ functional selectivity for Gαi proteins no effect on cardiac contractility in healthy rats ↑ binding affinity vs. [Pyr1]-apelin-13 ↑ resistance to ACE2 cleavage ↓ hypertrophy and fibrosis |
[91] |
||
|
N-Teminus (pGlu) |
palmitic acid, (at Pro12) Aib, Nle |
↑ half-life (29 h in rat plasma) |
[92] |
|
|
N-Terminus |
Alkylated dipeptide |
↑ affinity in the low nM range |
[93] |
|
|
extended and bulkier substituents |
binding affinity close to [Pyr1]-apelin-13 ↓ signalling potency for the β-arrestin 2 pathway |
|||
|
C-Terminus |
positive charge |
binding affinity close to [Pyr1]-apelin-13 partial agonism for β-arrestin2 recruitment |
||
|
1NaI12 |
amino-indoloazepinone-Orn |
↑affinity for APJ ↑ Gαi1 activation, ↑ stability ↓ hypotensive effects |
||
|
apelin-12 |
N-terminus Met10 |
(NαMe) Arg Nle10 |
↑ proteolytic stability ↑ cardiac function ↓ membrane damage |
|
|
apelin-17 |
Classical substituted |
P92 |
↑ plasma half-life sub-nanomolar affinity for APJ APJ internalization ↑ diuresis ↓ arterial blood pressure |
[96] |
|
LIT01-196 |
fluoroaddiction N-terminal |
|||
|
↓ blood pressure in normotensive and hypertensive rats |
[97] |
|||
|
Arg, Leu |
azapeptides |
↑ proteolytic stability vs. NEP |
[98] |
|
|
l-hArg, l-Cha |
- |
↑ proteolytic stability vs. NEP prolonged lowering blood pressure effect in mice |
[99] |
|
|
N-terminus |
palmitoylation PEGylation |
↑ plasma peptide half-life ↓ blood pressure in mice |
[30] |
|
|
aromatic head groups carbonate analogues |
↑↑ proteolytic stability |
[100] |
||
|
ELA |
Pro32 |
Tyr (OBn), Bpa, or 1Nal |
↑ binding affinity vs. ELA |
[101] |
|
N-terminus |
pyroglutamic acid (Pyr) residue to ELA(19-32) |
↓ arterial pressure ↓ inotropic effects on the heart ↑ half-life |
[38] |
|
Cyclized Peptide |
Modified Residues |
Cycle Position |
Effects |
Ref |
|---|---|---|---|---|
|
MM07 |
N-terminus |
N-terminal cycle (MM07) |
↑ vasodilatation ↑ cardiac function vs. [Pyr1]-apelin-13 |
[24] |
|
↑ cardiac function ↓ hypertrophy |
[102] |
|||
|
Macrocycle analogues |
G-P-M−P-F |
[X-P-Nle-P-X] [B1-P-Nle-P-X] [B1-P-Nle-P-Xd] |
↑ plasma peptide half-life ↑ binding affinity vs. apelin-13 powerful modulators of cardiovascular system |
[89] |
|
C-terminal |
C-thermal exocyclic residues endocyclic residues |
↑ G-protein activation on over-arresting signalling binding affinity close to [Pyr1]-apelin-13 |
[103] |
|
|
Internal position |
||||
|
β alanine spacer |
Same APJ affinity of apelin-13 ↓ hypotensive effect similar to apelin-13 |
|||
|
apelin-13′s His8 position |
Nγ-allyl-Nγ-nosyl-α,γ-diamino-butanoic acid Nπ-allyl-histidine linker |
↑ binding affinity ↑ potency |
[104] |
4.2. Antibody-Based/Bound APJ Agonists
|
Agonist |
Substituted Residues |
Introduced Residues |
Effects |
Ref |
|---|---|---|---|---|
|
JN241-9 |
between E104 and S105 to JN241 |
tyrosine |
agonist activity for cAMP β-arrestin recruitment |
[105] |
|
MM202 (apelin-modified) |
Met |
Nle and Fluroaddition-C-terminal |
high binding affinity vs [Pyr1]-apelin-13 = or ↑ potency than [Pyr1]-apelin-13 |
[106] |
|
MM202-AlbudAb |
= or ↑ potency than [Pyr1]-apelin-13 |
|||
|
MM202 and AlbudAb fusion |
↑ cardiac function ↓ heart rate |
|||
|
Fc-ELA-21 |
- |
Fc and ELA fusion |
↑ angiogenesis ↓ apoptosis ↑ cardiomyocyte proliferation ↓ heart fibrosis |
[107] |
4.3. Side Effect and Delivery Issues
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15051408
References
- Subramaniam, A.V.; Weston, S.A.; Killian, J.M.; Schulte, P.J.; Roger, V.L.; Redfield, M.M.; Blecker, S.B.; Dunlay, S.M. Development of Advanced Heart Failure: A Population-Based Study. Circ. Heart Fail. 2022, 15, e009218.
- Friedman, S.L. Fighting Cardiac Fibrosis with CAR T Cells. N. Engl. J. Med. 2022, 386, 1576–1578.
- O’Dowd, B.F.; Heiber, M.; Chan, A.; Heng, H.H.; Tsui, L.C.; Kennedy, J.L.; Shi, X.; Petronis, A.; George, S.R.; Nguyen, T. A Human Gene That Shows Identity with the Gene Encoding the Angiotensin Receptor Is Located on Chromosome 11. Gene 1993, 136, 355–360.
- Kleinz, M.J.; Skepper, J.N.; Davenport, A.P. Immunocytochemical Localisation of the Apelin Receptor, APJ, to Human Cardiomyocytes, Vascular Smooth Muscle and Endothelial Cells. Regul. Pept. 2005, 126, 233–240.
- Zhang, Z.; Tang, J.; Song, J.; Xie, M.; Liu, Y.; Dong, Z.; Liu, X.; Li, X.; Zhang, M.; Chen, Y.; et al. Elabela Alleviates Ferroptosis, Myocardial Remodeling, Fibrosis and Heart Dysfunction in Hypertensive Mice by Modulating the IL-6/STAT3/GPX4 Signaling. Free Radic. Biol. Med. 2022, 181, 130–142.
- Wu, Y.; Wang, X.; Zhou, X.; Cheng, B.; Li, G.; Bai, B. Temporal Expression of Apelin/Apelin Receptor in Ischemic Stroke and Its Therapeutic Potential. Front. Mol. Neurosci. 2017, 10, 1.
- Dawid, M.; Mlyczyńska, E.; Jurek, M.; Respekta, N.; Pich, K.; Kurowska, P.; Gieras, W.; Milewicz, T.; Kotula-Balak, M.; Rak, A. Apelin, APJ, and ELABELA: Role in Placental Function, Pregnancy, and Foetal Development—An Overview. Cells 2021, 11, 99.
- Chapman, N.A.; Dupré, D.J.; Rainey, J.K. The Apelin Receptor: Physiology, Pathology, Cell Signalling, and Ligand Modulation of a Peptide-Activated Class A GPCR. Biochem. Cell Biol. 2014, 92, 431–440.
- Chen, J.; Chen, X.; Li, S.; Jiang, Y.; Mao, H.; Zhang, R.; Ji, B.; Yan, M.; Cai, X.; Wang, C. Individual Phosphorylation Sites at the C-Terminus of the Apelin Receptor Play Different Roles in Signal Transduction. Redox Biol. 2020, 36, 101629.
- Scimia, M.C.; Hurtado, C.; Ray, S.; Metzler, S.; Wei, K.; Wang, J.; Woods, C.E.; Purcell, N.H.; Catalucci, D.; Akasaka, T.; et al. APJ Acts as a Dual Receptor in Cardiac Hypertrophy. Nature 2012, 488, 394–398.
- D’Aniello, C.; Fiorenzano, A.; Iaconis, S.; Liguori, G.L.; Andolfi, G.; Cobellis, G.; Fico, A.; Minchiotti, G. The G-Protein-Coupled Receptor APJ Is Expressed in the Second Heart Field and Regulates Cerberus–Baf60c Axis in Embryonic Stem Cell Cardiomyogenesis. Cardiovasc. Res. 2013, 100, 95–104.
- Sharma, B.; Ho, L.; Ford, G.H.; Chen, H.I.; Goldstone, A.B.; Woo, Y.J.; Quertermous, T.; Reversade, B.; Red-Horse, K. Alternative Progenitor Cells Compensate to Rebuild the Coronary Vasculature in Elabela- and Apj-Deficient Hearts. Dev. Cell 2017, 42, 655–666.e3.
- He, L.; Xu, J.; Chen, L.; Li, L. Apelin/APJ Signaling in Hypoxia-Related Diseases. Clin. Chim. Acta 2015, 451, 191–198.
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.-X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and Characterization of a Novel Endogenous Peptide Ligand for the Human APJ Receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476.
- Liu, J.; Liu, M.; Chen, L. Novel Pathogenesis: Regulation of Apoptosis by Apelin/APJ System. Acta Biochim. Biophys. Sin. 2017, 49, 471–478.
- Kawamata, Y.; Habata, Y.; Fukusumi, S.; Hosoya, M.; Fujii, R.; Hinuma, S.; Nishizawa, N.; Kitada, C.; Onda, H.; Nishimura, O.; et al. Molecular Properties of Apelin: Tissue Distribution and Receptor Binding. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2001, 1538, 162–171.
- De Falco, M.; De Luca, L.; Onori, N.; Cavallotti, I.; Artigiano, F.; Esposito, V.; De Luca, B.; Laforgia, V.; Groeger, A.M.; De Luca, A. Apelin Expression in Normal Human Tissues. In Vivo 2002, 16, 333–336.
- Shin, K.; Pandey, A.; Liu, X.-Q.; Anini, Y.; Rainey, J.K. Preferential Apelin-13 Production by the Proprotein Convertase PCSK3 Is Implicated in Obesity. FEBS Open Bio. 2013, 3, 328–333.
- Zhang, Y.; Maitra, R.; Harris, D.L.; Dhungana, S.; Snyder, R.; Runyon, S.P. Identifying Structural Determinants of Potency for Analogs of Apelin-13: Integration of C-Terminal Truncation with Structure–Activity. Bioorg. Med. Chem. 2014, 22, 2992–2997.
- Murza, A.; Belleville, K.; Longpré, J.-M.; Sarret, P.; Marsault, É. Stability and Degradation Patterns of Chemically Modified Analogs of Apelin-13 in Plasma and Cerebrospinal Fluid. Biopolymers 2014, 102, 297–303.
- Zhen, E.Y.; Higgs, R.E.; Gutierrez, J.A. Pyroglutamyl Apelin-13 Identified as the Major Apelin Isoform in Human Plasma. Anal Biochem 2013, 442, 1–9.
- Yang, P.; Kuc, R.E.; Brame, A.L.; Dyson, A.; Singer, M.; Glen, R.C.; Cheriyan, J.; Wilkinson, I.B.; Davenport, A.P.; Maguire, J.J. Apelin-13(1–12) Is a Biologically Active ACE2 Metabolite of the Endogenous Cardiovascular Peptide Apelin-13. Front. Neurosci. 2017, 11, 92.
- Japp, A.G.; Newby, D.E. Unlocking the Therapeutic Potential of Apelin. Hypertension 2016, 68, 307–309.
- Brame, A.L.; Maguire, J.J.; Yang, P.; Dyson, A.; Torella, R.; Cheriyan, J.; Singer, M.; Glen, R.C.; Wilkinson, I.B.; Davenport, A.P. Design, Characterization, and First-In-Human Study of the Vascular Actions of a Novel Biased Apelin Receptor Agonist. Hypertension 2015, 65, 834–840.
- Wang, W.; McKinnie, S.M.K.; Farhan, M.; Paul, M.; McDonald, T.; McLean, B.; Llorens-Cortes, C.; Hazra, S.; Murray, A.G.; Vederas, J.C.; et al. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17. Hypertension 2016, 68, 365–377.
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of Biological Peptides by Human Angiotensin-Converting Enzyme-Related Carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843.
- McKinnie, S.M.K.; Fischer, C.; Tran, K.M.H.; Wang, W.; Mosquera, F.; Oudit, G.Y.; Vederas, J.C. The Metalloprotease Neprilysin Degrades and Inactivates Apelin Peptides. ChemBioChem 2016, 17, 1495–1498.
- Zhong, J.-C.; Zhang, Z.-Z.; Wang, W.; McKinnie, S.M.K.; Vederas, J.C.; Oudit, G.Y. Targeting the Apelin Pathway as a Novel Therapeutic Approach for Cardiovascular Diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1942–1950.
- De Hert, E.; Bracke, A.; Pintelon, I.; Janssens, E.; Lambeir, A.-M.; Van Der Veken, P.; De Meester, I. Prolyl Carboxypeptidase Mediates the C-Terminal Cleavage of (Pyr)-Apelin-13 in Human Umbilical Vein and Aortic Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 6698.
- Fischer, C.; Lamer, T.; Wang, W.; McKinnie, S.M.K.; Iturrioz, X.; Llorens-Cortes, C.; Oudit, G.Y.; Vederas, J.C. Plasma Kallikrein Cleaves and Inactivates Apelin-17: Palmitoyl- and PEG-Extended Apelin-17 Analogs as Metabolically Stable Blood Pressure-Lowering Agents. Eur. J. Med. Chem. 2019, 166, 119–124.
- Galanth, C.; Hus-Citharel, A.; Li, B.; Llorens-Cortès, C. Apelin in the Control of Body Fluid Homeostasis and Cardiovascular Functions. Curr. Pharm. Des. 2012, 18, 789–798.
- Yang, P.; Read, C.; Kuc, R.E.; Buonincontri, G.; Southwood, M.; Torella, R.; Upton, P.D.; Crosby, A.; Sawiak, S.J.; Carpenter, T.A.; et al. Elabela/Toddler Is an Endogenous Agonist of the Apelin APJ Receptor in the Adult Cardiovascular System, and Exogenous Administration of the Peptide Compensates for the Downregulation of Its Expression in Pulmonary Arterial Hypertension. Circulation 2017, 135, 1160–1173.
- Ronkainen, V.; Ronkainen, J.J.; Hänninen, S.L.; Leskinen, H.; Ruas, J.L.; Pereira, T.; Poellinger, L.; Vuolteenaho, O.; Tavi, P. Hypoxia Inducible Factor Regulates the Cardiac Expression and Secretion of Apelin. FASEB J. 2007, 21, 1821–1830.
- Sheikh, A.Y.; Chun, H.J.; Glassford, A.J.; Kundu, R.K.; Kutschka, I.; Ardigo, D.; Hendry, S.L.; Wagner, R.A.; Chen, M.M.; Ali, Z.A.; et al. In Vivo Genetic Profiling and Cellular Localization of Apelin Reveals a Hypoxia-Sensitive, Endothelial-Centered Pathway Activated in Ischemic Heart Failure. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H88–H98.
- Guzelburc, O.; Demirtunc, R.; Altay, S.; Kemaloglu Oz, T.; Tayyareci, G. Plasma Apelin Level in Acute Myocardial Infarction and Its Relation with Prognosis: A Prospective Study. JRSM Cardiovasc. Dis. 2021, 10, 2048004020963970.
- Pauli, A.; Norris, M.L.; Valen, E.; Chew, G.-L.; Gagnon, J.A.; Zimmerman, S.; Mitchell, A.; Ma, J.; Dubrulle, J.; Reyon, D.; et al. Toddler: An Embryonic Signal That Promotes Cell Movement via Apelin Receptors. Science 2014, 343, 1248636.
- Chng, S.C.; Ho, L.; Tian, J.; Reversade, B. ELABELA: A Hormone Essential for Heart Development Signals via the Apelin Receptor. Dev. Cell 2013, 27, 672–680.
- Murza, A.; Sainsily, X.; Coquerel, D.; Côté, J.; Marx, P.; Besserer-Offroy, É.; Longpré, J.-M.; Lainé, J.; Reversade, B.; Salvail, D.; et al. Discovery and Structure–Activity Relationship of a Bioactive Fragment of ELABELA That Modulates Vascular and Cardiac Functions. J. Med. Chem. 2016, 59, 2962–2972.
- Couvineau, P.; Llorens-Cortes, C.; Iturrioz, X. Elabela/Toddler and Apelin Bind Differently to the Apelin Receptor. FASEB J. 2020, 34, 7989–8000.
- Huang, S.K.; Shin, K.; Sarker, M.; Rainey, J.K. Apela Exhibits Isoform- and Headgroup-Dependent Modulation of Micelle Binding, Peptide Conformation and Dynamics. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2017, 1859, 767–778.
- Wang, Z.; Yu, D.; Wang, M.; Wang, Q.; Kouznetsova, J.; Yang, R.; Qian, K.; Wu, W.; Shuldiner, A.; Sztalryd, C.; et al. Elabela-Apelin Receptor Signaling Pathway Is Functional in Mammalian Systems. Sci. Rep. 2015, 5, 8170.
- Perjés, Á.; Kilpiö, T.; Ulvila, J.; Magga, J.; Alakoski, T.; Szabó, Z.; Vainio, L.; Halmetoja, E.; Vuolteenaho, O.; Petäjä-Repo, U.; et al. Characterization of Apela, a Novel Endogenous Ligand of Apelin Receptor, in the Adult Heart. Basic Res. Cardiol. 2016, 111, 2.
- Kuba, K.; Sato, T.; Imai, Y.; Yamaguchi, T. Apelin and Elabela/Toddler; Double Ligands for APJ/Apelin Receptor in Heart Development, Physiology, and Pathology. Peptides 2019, 111, 62–70.
- Coquerel, D.; Chagnon, F.; Sainsily, X.; Dumont, L.; Murza, A.; Côté, J.; Dumaine, R.; Sarret, P.; Marsault, É.; Salvail, D.; et al. ELABELA Improves Cardio-Renal Outcome in Fatal Experimental Septic Shock. Crit. Care Med. 2017, 45, e1139–e1148.
- Kuba, K.; Zhang, L.; Imai, Y.; Arab, S.; Chen, M.; Maekawa, Y.; Leschnik, M.; Leibbrandt, A.; Markovic, M.; Schwaighofer, J.; et al. Impaired Heart Contractility in Apelin Gene–Deficient Mice Associated With Aging and Pressure Overload. Circ. Res. 2007, 101, e32–e42.
- Perjés, Á.; Skoumal, R.; Tenhunen, O.; Kónyi, A.; Simon, M.; Horváth, I.G.; Kerkelä, R.; Ruskoaho, H.; Szokodi, I. Apelin Increases Cardiac Contractility via Protein Kinase Cε- and Extracellular Signal-Regulated Kinase-Dependent Mechanisms. PLoS ONE 2014, 9, e93473.
- Seo, K.; Parikh, V.N.; Ashley, E.A. Stretch-Induced Biased Signaling in Angiotensin II Type 1 and Apelin Receptors for the Mediation of Cardiac Contractility and Hypertrophy. Front. Physiol. 2020, 11, 181.
- Berry, M.F.; Pirolli, T.J.; Jayasankar, V.; Burdick, J.; Morine, K.J.; Gardner, T.J.; Woo, Y.J. Apelin Has In Vivo Inotropic Effects on Normal and Failing Hearts. Circulation 2004, 110 (Suppl. S1), II187–II193.
- Folino, A.; Montarolo, P.G.; Samaja, M.; Rastaldo, R. Effects of Apelin on the Cardiovascular System. Heart Fail. Rev. 2015, 20, 505–518.
- Peyronnet, R.; Bollensdorff, C.; Capel, R.A.; Rog-Zielinska, E.A.; Woods, C.E.; Charo, D.N.; Lookin, O.; Fajardo, G.; Ho, M.; Quertermous, T.; et al. Load-Dependent Effects of Apelin on Murine Cardiomyocytes. Prog. Biophys. Mol. Biol. 2017, 130, 333–343.
- Wang, C.; Du, J.-F.; Wu, F.; Wang, H.-C. Apelin Decreases the SR Ca 2+ Content but Enhances the Amplitude of i Transient and Contractions during Twitches in Isolated Rat Cardiac Myocytes. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H2540–H2546.
- Mughal, A.; Sun, C.; O’Rourke, S.T. Activation of Large Conductance, Calcium-Activated Potassium Channels by Nitric Oxide Mediates Apelin-Induced Relaxation of Isolated Rat Coronary Arteries. J. Pharmacol. Exp. Ther. 2018, 366, 265–273.
- Sahinturk, S.; Demirel, S.; Ozyener, F.; Isbil, N. Apelin-13 Relaxes the Rat Thoracic Aorta via APJ, NO, AMPK, and Potassium Channels. Gen. Physiol. Biophys. 2021, 40, 427–434.
- Katugampola, S.D.; Maguire, J.J.; Matthewson, S.R.; Davenport, A.P. -(Pyr(1))Apelin-13 Is a Novel Radioligand for Localizing the APJ Orphan Receptor in Human and Rat Tissues with Evidence for a Vasoconstrictor Role in Man. Br. J. Pharmacol. 2001, 132, 1255–1260.
- Rikitake, Y. The Apelin/APJ System in the Regulation of Vascular Tone: Friend or Foe? J. Biochem. 2021, 169, 383–386.
- Sahinturk, S.; Demirel, S.; Ozyener, F.; Isbil, N. Vascular Functional Effect Mechanisms of Elabela in Rat Thoracic Aorta. Ann. Vasc. Surg. 2022, 84, 381–397.
- Helker, C.S.; Eberlein, J.; Wilhelm, K.; Sugino, T.; Malchow, J.; Schuermann, A.; Baumeister, S.; Kwon, H.-B.; Maischein, H.-M.; Potente, M.; et al. Apelin Signaling Drives Vascular Endothelial Cells toward a Pro-Angiogenic State. eLife 2020, 9, e55589.
- Wang, X.; Liang, G.; Guo, Q.; Cai, W.; Zhang, X.; Ni, J.; Tao, Y.; Niu, X.; Chen, S. ELABELA Improves Endothelial Cell Function via the ELA–APJ Axis by Activating the PI3K/Akt Signalling Pathway in HUVECs and EA.Hy926 Cells. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1953–1964.
- Roberts, E.M.; Newson, M.J.F.; Pope, G.R.; Landgraf, R.; Lolait, S.J.; O’Carroll, A.-M. Abnormal Fluid Homeostasis in Apelin Receptor Knockout Mice. J. Endocrinol. 2009, 202, 453–462.
- Hu, G.; Wang, Z.; Zhang, R.; Sun, W.; Chen, X. The Role of Apelin/Apelin Receptor in Energy Metabolism and Water Homeostasis: A Comprehensive Narrative Review. Front. Physiol. 2021, 12, 632886.
- Xu, J.; Chen, L.; Jiang, Z.; Li, L. Biological Functions of Elabela, a Novel Endogenous Ligand of APJ Receptor. J. Cell Physiol. 2018, 233, 6472–6482.
- Brubaker, P.H.; Kalra, S. Selected Abstracts From Recent Publications in Cardiopulmonary Disease Prevention and Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2016, 36, 140–144.
- Vaughan, A.S.; Coronado, F.; Casper, M.; Loustalot, F.; Wright, J.S. County-Level Trends in Hypertension-Related Cardiovascular Disease Mortality—United States, 2000 to 2019. J. Am. Heart Assoc. 2022, 11, e024785.
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left Ventricular Hypertrophy and Hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21.
- Arendse, L.B.; Danser AH, J.; Poglitsch, M.; Touyz, R.M.; Burnett, J.C.; Llorens-Cortes, C.; Ehlers, M.R.; Sturrock, E.D. Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure. Pharmacol. Rev. 2019, 71, 539–570.
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1–7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7). Physiol. Rev. 2018, 98, 505–553.
- Sainsily, X.; Coquerel, D.; Giguère, H.; Dumont, L.; Tran, K.; Noll, C.; Ionescu, A.L.; Côté, J.; Longpré, J.-M.; Carpentier, A.; et al. Elabela Protects Spontaneously Hypertensive Rats From Hypertension and Cardiorenal Dysfunctions Exacerbated by Dietary High-Salt Intake. Front. Pharmacol. 2021, 12, 709467.
- Wysocka, M.B.; Pietraszek-Gremplewicz, K.; Nowak, D. The Role of Apelin in Cardiovascular Diseases, Obesity and Cancer. Front. Physiol. 2018, 9, 557.
- Sato, T.; Suzuki, T.; Watanabe, H.; Kadowaki, A.; Fukamizu, A.; Liu, P.P.; Kimura, A.; Ito, H.; Penninger, J.M.; Imai, Y.; et al. Apelin Is a Positive Regulator of ACE2 in Failing Hearts. J. Clin. Investig. 2013, 123, 5203–5211.
- Zhang, Z.-Z.; Wang, W.; Jin, H.-Y.; Chen, X.; Cheng, Y.-W.; Xu, Y.-L.; Song, B.; Penninger, J.M.; Oudit, G.Y.; Zhong, J.-C. Apelin Is a Negative Regulator of Angiotensin II–Mediated Adverse Myocardial Remodeling and Dysfunction. Hypertension 2017, 70, 1165–1175.
- Abbasloo, E.; Najafipour, H.; Vakili, A. Chronic Treatment with Apelin, Losartan and Their Combination Reduces Myocardial Infarct Size and Improves Cardiac Mechanical Function. Clin. Exp. Pharmacol. Physiol. 2020, 47, 393–402.
- Tomek, J.; Bub, G. Hypertension-Induced Remodelling: On the Interactions of Cardiac Risk Factors. J. Physiol. 2017, 595, 4027–4036.
- Sato, T.; Kadowaki, A.; Suzuki, T.; Ito, H.; Watanabe, H.; Imai, Y.; Kuba, K. Loss of Apelin Augments Angiotensin II-Induced Cardiac Dysfunction and Pathological Remodeling. Int. J. Mol. Sci. 2019, 20, 239.
- Wang, W.; Shen, M.; Fischer, C.; Basu, R.; Hazra, S.; Couvineau, P.; Paul, M.; Wang, F.; Toth, S.; Mix, D.S.; et al. Apelin Protects against Abdominal Aortic Aneurysm and the Therapeutic Role of Neutral Endopeptidase Resistant Apelin Analogs. Proc. Natl. Acad. Sci. USA 2019, 116, 13006–13015.
- Song, J.; Zhang, Z.; Dong, Z.; Liu, X.; Liu, Y.; Li, X.; Xu, Y.; Guo, Y.; Wang, N.; Zhang, M.; et al. MicroRNA-122-5p Aggravates Angiotensin II-Mediated Myocardial Fibrosis and Dysfunction in Hypertensive Rats by Regulating the Elabela/Apelin-APJ and ACE2-GDF15-Porimin Signaling. J. Cardiovasc. Transl. Res. 2022, 15, 535–547.
- Lv, W.; Zhang, L.; Cheng, X.; Wang, H.; Qin, W.; Zhou, X.; Tang, B. Apelin Inhibits Angiotensin II-Induced Atrial Fibrosis and Atrial Fibrillation via TGF-Β1/Smad2/α-SMA Pathway. Front. Physiol. 2020, 11, 583570.
- Xu, C.; Wang, F.; Chen, Y.; Xie, S.; Sng, D.; Reversade, B.; Yang, T. ELABELA Antagonizes Intrarenal Renin-Angiotensin System to Lower Blood Pressure and Protects against Renal Injury. Am. J. Physiol. Renal Physiol. 2020, 318, F1122–F1135.
- Sato, T.; Sato, C.; Kadowaki, A.; Watanabe, H.; Ho, L.; Ishida, J.; Yamaguchi, T.; Kimura, A.; Fukamizu, A.; Penninger, J.M.; et al. ELABELA-APJ Axis Protects from Pressure Overload Heart Failure and Angiotensin II-Induced Cardiac Damage. Cardiovasc. Res. 2017, 113, 760–769.
- Hendrianus; Adiarto, S.; Prakoso, R.; Firdaus, I.; Indriani, S.; Rudiktyo, E.; Widyantoro, B.; Taofan, T.; Ambari, A.M.; Sukmawan, R. 10 A novel peptide elabela is associated with hypertension-related subclinical atherosclerosis. J. Hypertens. 2022, 40 (Suppl. S2), e3.
- Li, Y.; Yang, X.; Ouyang, S.; He, J.; Yu, B.; Lin, X.; Zhang, Q.; Tao, J. Declined Circulating Elabela Levels in Patients with Essential Hypertension and Its Association with Impaired Vascular Function: A Preliminary Study. Clin. Exp. Hypertens. 2020, 42, 239–243.
- Xie, H.; Luo, G.; Zheng, Y.; Hu, D.; Peng, F.; Xie, L. Lowered Circulating Apelin Is Significantly Associated with an Increased Risk for Hypertension: A Meta-Analysis. Clin. Exp. Hypertens. 2017, 39, 435–440.
- Baysal, S.S.; Pirat, B.; Okyay, K.; Bal, U.A.; Uluçam, M.Z.; Öztuna, D.; Müderrisoğlu, H. Treatment-Associated Change in Apelin Concentration in Patients with Hypertension and Its Relationship with Left Ventricular Diastolic Function. Anatol. J. Cardiol. 2017, 17, 125–131.
- Ma, Z.; Zhao, L.; Zhang, Y.; Zhong, J.; Yang, X. Declined ELABELA Plasma Levels in Hypertension Patients with Atrial Fibrillation: A Case Control Study. BMC Cardiovasc. Disord. 2021, 21, 390.
- Ma, Z.; Zhao, L.; Martin, S.; Zhang, Y.; Dong, Y.; Zhong, J.-C.; Yang, X.-C. Lower Plasma Elabela Levels in Hypertensive Patients With Heart Failure Predict the Occurrence of Major Adverse Cardiac Events: A Preliminary Study. Front. Cardiovasc. Med. 2021, 8, 638468.
- Yu, P.; Ma, S.; Dai, X.; Cao, F. Elabela Alleviates Myocardial Ischemia Reperfusion-Induced Apoptosis, Fibrosis and Mitochondrial Dysfunction through PI3K/AKT Signaling. Am. J. Transl. Res. 2020, 12, 4467–4477.
- Deng, J. Advanced Research on the Regulated Necrosis Mechanism in Myocardial Ischemia-Reperfusion Injury. Int. J. Cardiol. 2021, 334, 97–101.
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct Target Ther. 2022, 7, 48.
- Murza, A.; Besserer-Offroy, É.; Côté, J.; Bérubé, P.; Longpré, J.-M.; Dumaine, R.; Lesur, O.; Auger-Messier, M.; Leduc, R.; Sarret, P.; et al. C-Terminal Modifications of Apelin-13 Significantly Change Ligand Binding, Receptor Signaling, and Hypotensive Action. J. Med. Chem. 2015, 58, 2431–2440.
- Trân, K.; Murza, A.; Sainsily, X.; Coquerel, D.; Côté, J.; Belleville, K.; Haroune, L.; Longpré, J.-M.; Dumaine, R.; Salvail, D.; et al. A Systematic Exploration of Macrocyclization in Apelin-13: Impact on Binding, Signaling, Stability, and Cardiovascular Effects. J. Med. Chem. 2018, 61, 2266–2277.
- Trân, K.; Van Den Hauwe, R.; Sainsily, X.; Couvineau, P.; Côté, J.; Simard, L.; Echevarria, M.; Murza, A.; Serre, A.; Théroux, L.; et al. Constraining the Side Chain of C-Terminal Amino Acids in Apelin-13 Greatly Increases Affinity, Modulates Signaling, and Improves the Pharmacokinetic Profile. J. Med. Chem. 2021, 64, 5345–5364.
- Coquerel, D.; Delile, E.; Dumont, L.; Chagnon, F.; Murza, A.; Sainsily, X.; Salvail, D.; Sarret, P.; Marsault, E.; Auger-Messier, M.; et al. Gαi-Biased Apelin Analog Protects against Isoproterenol-Induced Myocardial Dysfunction in Rats. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H1646–H1656.
- Juhl, C.; Els-Heindl, S.; Schönauer, R.; Redlich, G.; Haaf, E.; Wunder, F.; Riedl, B.; Burkhardt, N.; Beck-Sickinger, A.G.; Bierer, D. Development of Potent and Metabolically Stable APJ Ligands with High Therapeutic Potential. ChemMedChem 2016, 11, 2378–2384.
- Théroux, L.; Van Den Hauwe, R.; Trân, K.; Fournier, J.; Desgagné, M.; Meneboo, N.; Lavallée, A.; Fröhlich, U.; Côté, J.; Hollanders, C.; et al. Signaling Modulation via Minimal C-Terminal Modifications of Apelin-13. ACS Pharmacol. Transl. Sci. 2023, 6, 290–305.
- Studneva, I.; Shulzhenko, V.; Veselova, O.; Pisarenko, O. Protective Effects of a Modified Apelin-12 and Dinitrosyl Iron Complexes in Experimental Cardioplegic Ischemia and Reperfusion. J. Physiol. Biochem. 2018, 74, 283–290.
- Sidorova, M.; Studneva, I.; Bushuev, V.; Pal’keeva, M.; Molokoedov, A.; Veselova, O.; Ovchinnikov, M.; Pisarenko, O. -Apelin-12: Optimization of Solid-Phase Synthesis and Evaluation of Biological Properties in Vitro and in Vivo. Peptides 2020, 129, 170320.
- Gerbier, R.; Alvear-Perez, R.; Margathe, J.; Flahault, A.; Couvineau, P.; Gao, J.; De Mota, N.; Dabire, H.; Li, B.; Ceraudo, E.; et al. Development of Original Metabolically Stable Apelin-17 Analogs with Diuretic and Cardiovascular Effects. FASEB J. 2017, 31, 687–700.
- Flahault, A.; Keck, M.; Girault-Sotias, P.-E.; Esteoulle, L.; De Mota, N.; Bonnet, D.; Llorens-Cortes, C. LIT01-196, a Metabolically Stable Apelin-17 Analog, Normalizes Blood Pressure in Hypertensive DOCA-Salt Rats via a NO Synthase-Dependent Mechanism. Front. Pharmacol. 2021, 12, 715095.
- McKinnie, S.M.K.; Wang, W.; Fischer, C.; McDonald, T.; Kalin, K.R.; Iturrioz, X.; Llorens-Cortes, C.; Oudit, G.Y.; Vederas, J.C. Synthetic Modification within the “RPRL” Region of Apelin Peptides: Impact on Cardiovascular Activity and Stability to Neprilysin and Plasma Degradation. J. Med. Chem. 2017, 60, 6408–6427.
- Fernandez, K.X.; Fischer, C.; Vu, J.; Gheblawi, M.; Wang, W.; Gottschalk, S.; Iturrioz, X.; Llorens-Cortés, C.; Oudit, G.Y.; Vederas, J.C. Metabolically Stable Apelin-Analogues, Incorporating Cyclohexylalanine and Homoarginine, as Potent Apelin Receptor Activators. RSC Med. Chem. 2021, 12, 1402–1413.
- Fischer, C.; Lamer, T.; Fernandez, K.; Gheblawi, M.; Wang, W.; Pascoe, C.; Lambkin, G.; Iturrioz, X.; Llorens-Cortes, C.; Oudit, G.Y.; et al. Optimizing PEG-Extended Apelin Analogues as Cardioprotective Drug Leads: Importance of the KFRR Motif and Aromatic Head Group for Improved Physiological Activity. J. Med. Chem. 2020, 63, 12073–12082.
- Trân, K.; Murza, A.; Sainsily, X.; Delile, E.; Couvineau, P.; Côté, J.; Coquerel, D.; Peloquin, M.; Auger-Messier, M.; Bouvier, M.; et al. Structure–Activity Relationship and Bioactivity of Short Analogues of ELABELA as Agonists of the Apelin Receptor. J. Med. Chem. 2021, 64, 602–615.
- Yang, P.; Read, C.; Kuc, R.E.; Nyimanu, D.; Williams, T.L.; Crosby, A.; Buonincontri, G.; Southwood, M.; Sawiak, S.J.; Glen, R.C.; et al. A Novel Cyclic Biased Agonist of the Apelin Receptor, MM07, Is Disease Modifying in the Rat Monocrotaline Model of Pulmonary Arterial Hypertension. Br. J. Pharmacol. 2019, 176, 1206–1221.
- Murza, A.; Sainsily, X.; Côté, J.; Bruneau-Cossette, L.; Besserer-Offroy, É.; Longpré, J.-M.; Leduc, R.; Dumaine, R.; Lesur, O.; Auger-Messier, M.; et al. Structure–Activity Relationship of Novel Macrocyclic Biased Apelin Receptor Agonists. Org. Biomol. Chem. 2017, 15, 449–458.
- Tran, K.; Sainsily, X.; Côté, J.; Coquerel, D.; Couvineau, P.; Saibi, S.; Haroune, L.; Besserer-Offroy, É.; Flynn-Robitaille, J.; Resua Rojas, M.; et al. Size-Reduced Macrocyclic Analogues of -Apelin-13 Showing Negative Gαi Bias Still Produce Prolonged Cardiac Effects. J. Med. Chem. 2022, 65, 531–551.
- Ma, Y.; Ding, Y.; Song, X.; Ma, X.; Li, X.; Zhang, N.; Song, Y.; Sun, Y.; Shen, Y.; Zhong, W.; et al. Structure-Guided Discovery of a Single-Domain Antibody Agonist against Human Apelin Receptor. Sci. Adv. 2020, 6, eaax7379.
- Read, C.; Yang, P.; Kuc, R.E.; Nyimanu, D.; Williams, T.L.; Glen, R.C.; Holt, L.J.; Arulanantham, H.; Smart, A.; Davenport, A.P.; et al. Apelin Peptides Linked to Anti-serum Albumin Domain Antibodies Retain Affinity in Vitro and Are Efficacious Receptor Agonists in Vivo. Basic Clin. Pharmacol. Toxicol. 2020, 126, 96–103.
- Xi, Y.; Yu, D.; Yang, R.; Zhao, Q.; Wang, J.; Zhang, H.; Qian, K.; Shi, Z.; Wang, W.; Brown, R.; et al. Recombinant Fc-Elabela Fusion Protein Has Extended Plasma Half-Life Andmitigates Post-Infarct Heart Dysfunction in Rats. Int. J. Cardiol. 2019, 292, 180–187.
- Holt, L.J.; Basran, A.; Jones, K.; Chorlton, J.; Jespers, L.S.; Brewis, N.D.; Tomlinson, I.M. Anti-Serum Albumin Domain Antibodies for Extending the Half-Lives of Short Lived Drugs. Protein Eng. Des. Sel. 2008, 21, 283–288.
- Sahoo, S.; Kariya, T.; Ishikawa, K. Targeted Delivery of Therapeutic Agents to the Heart. Nat. Rev. Cardiol. 2021, 18, 389–399.
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/MTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128.
- Girault-Sotias, P.-E.; Gerbier, R.; Flahault, A.; de Mota, N.; Llorens-Cortes, C. Apelin and Vasopressin: The Yin and Yang of Water Balance. Front. Endocrinol. 2021, 12, 735515.
- Jahangirian, H.; Ghasemian Lemraski, E.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A Review of Drug Delivery Systems Based on Nanotechnology and Green Chemistry: Green Nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978.
- Martinho, N.; Damgé, C.; Reis, C.P. Recent Advances in Drug Delivery Systems. J. Biomater. Nanobiotechnol. 2011, 2, 510–526.
- Serpooshan, V.; Sivanesan, S.; Huang, X.; Mahmoudi, M.; Malkovskiy, A.V.; Zhao, M.; Inayathullah, M.; Wagh, D.; Zhang, X.J.; Metzler, S.; et al. -Apelin-13 Delivery via Nano-Liposomal Encapsulation Attenuates Pressure Overload-Induced Cardiac Dysfunction. Biomaterials 2015, 37, 289–298.
- de Villiers, M.M.; Aramwit, P.; Kwon, G.S. (Eds.) Nanotechnology in Drug Delivery; Springer New York: New York, NY, USA, 2009.
- Fang, J.; Koh, J.; Fang, Q.; Qiu, H.; Archang, M.M.; Hasani-Sadrabadi, M.M.; Miwa, H.; Zhong, X.; Sievers, R.; Gao, D.; et al. Injectable Drug-Releasing Microporous Annealed Particle Scaffolds for Treating Myocardial Infarction. Adv. Funct. Mater. 2020, 30, 2004307.
- Almas, T.; Haider, R.; Malik, J.; Mehmood, A.; Alvi, A.; Naz, H.; Satti, D.I.; Zaidi, S.M.J.; AlSubai, A.K.; AlNajdi, S.; et al. Nanotechnology in Interventional Cardiology: A State-of-the-Art Review. IJC Heart Vasc. 2022, 43, 101149.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
