Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Plants produce a variety of secondary metabolites, making them an area of interest in the search for new phytochemicals to cope with antimicrobial resistance (AMR). A great part of agri-food waste is of plant origin, constituting a promising source of valuable compounds with different bioactivities, including those against antimicrobial resistance. Many types of phytochemicals, such as carotenoids, tocopherols, glucosinolates, and phenolic compounds, are widely present in plant by-products, such as citrus peels, tomato waste, and wine pomace.
- agri-food wastes
- phytochemicals
- multidrug resistance
- bacterial infection
- antibacterial activity
1. Introduction
Phytochemicals are natural chemical compounds found in plant foods, such as fruits, vegetables, legumes, whole grains, nuts, seeds, and herbs. These compounds act as a natural defence system for plants, protecting them from infections and microbial invasions and giving them colour, aroma, and flavour [1]. Phytochemicals have emerged as safe alternatives to conventional antibiotics to treat antibiotic-resistant pathogen-originated infections, as well as an alternative to chemical additives to foodborne bacteria [2]. Many phytochemicals have demonstrated their potential as bactericidal agents and have proved to inhibit the vital events for the sustenance and resistance of the pathogen, including efflux pumps, replication machinery, and cell permeability, among others [3]. Phytochemicals are grouped according to their structural characteristics into four large groups: nitrogen alkaloids, phenolic compounds, terpenoids, and organosulfur compounds [1].
Agri-food wastes comprise peels, seeds, shells, pomace, and leaves. These residues are important substrates for phytochemicals, including polyphenols, carotenoids, essential oils, tocopherols, and terpenes. In addition to their antibiotic activities, phytochemicals found in agri-food wastes can be easily managed via their valorisation to produce value-added products, food additives, therapeutics, or other environmental applications due to their antioxidant, therapeutic, and nutritional properties [4][5][6][7] (Figure 1).
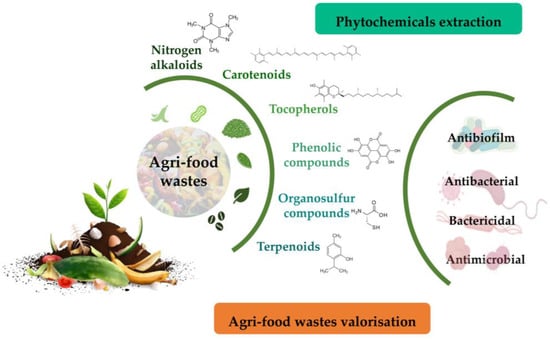
Figure 1. Overall strategy to unveil phytochemicals with antimicrobial activity from food wastes as a strategy for their valorisation.
Several studies have shown the antibacterial potential of phytochemicals found in agri-food wastes (Table 1). For instance, Carmo and collaborators [8] isolated coumarins (bergapten, xanthotoxin, dimethyl allyl xanthyletin) and an imidazole alkaloid from the crude extract of leaves and bark of Pilocarpus pennatifolius Lemaire. The extracts and pure compounds were tested against different strains of bacteria and fungi, which showed promising antimicrobial and antifungal activities. The alkaloid identified showed a minimal inhibitory concentration of 1.56 μg·mL−1 against Enterococcus fecalis, and 1.56 μg·mL−1 and 6.25 μg·mL−1 against Salmonella enteritidis and Pseudomonas aeruginosa, respectively. The extracts of the studied species proved to be an alternative source in the search for new antimicrobial agents for the treatment of diseases caused by bacteria.
Table 1. Antibacterial potential of phytochemicals found in agri-food wastes.
| Phytochemicals | Agri-Food Waste | Target Pathogen | Action | Ref. |
|---|---|---|---|---|
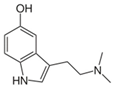 Nitrogen alkaloids |
||||
| Imidazole | Pilocarpus pennatifolius (jaborandi) leaves | Gram-positive (Enterococcus fecalis) and Gram-negative (Salmonella enteritidis, P. aeruginosa) | Antibacterial | [8] |
| Trigonelline, caffeine | Arabica coffee leaves | Gram-positive (S. aureus) and Gram-negative (E. coli, P. aeruginosa) | Antibacterial and bactericidal | [9] |
| Caffeine, quinine | Coffee silverskin extracts | Gram-positive (S. aureus) and Gram-negative (E. coli, P. aeruginosa) | Antibiofilm | [10] |
| Peganine, harmol, harmine, β-carboline, quinazoline alkaloids | Peganum harmala seeds | Ralstonia solanacearum Phylotype II, Pectobacterium cartovorum subsp. Cartovorum, Erwinia amylovora, Burkholderia gladioli pv. allicola | Antibacterial | [11] |
| Solanidine, α-chaconine, α-solanine | Potato peels | Lactobacillus reuteri, Lactobacillus acidophilus, Lactobacillus rhamnosus, E. coli | Antimicrobial | [12] |
| α-Solanine, α-chaconine | Potato sprouts | Gram-positive bacteria (S. aureus, Bacillus subtilis, Enterococcus hirae) and Gram-negative (E. coli, P. aeruginosa) | Antimicrobial | [13] |
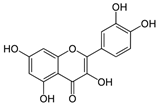 Phenolic compounds |
||||
| Ellagic acid, trans-fertaric acid, quercetin, kaempferol | Grape pomace and lees | Gram-positive (S. aureus, Bacillus subtilis, Bacillus cereus) and Gram-negative (E. coli) | Bactericidal | [14] |
| Quercetin and its glucosides (quercetin aglycone, quercetin-4′-O-monoglucoside, quercetin-3,4′-O-diglucoside, anthocyanin) |
Skinned onions | Gram-positive (S. aureus, Bacillus cereus) and Gram-negative (E. coli, P. aeruginosa) | Antibacterial and antibiofilm | [15] |
| Sinensetin, 4′,5,6,7-tetramethoxyflavone, nobiletin, tangeretin, 3,3′,4′,5,6,7-hexamethoxyflavone, 3,3′,4′,5,6,7,8-heptamethoxyflavone, eriocitrin, nairutin, hesperidin | Orange peels | E. coli, S. aureus, Bacillus subtilis | Antibacterial | [16] |
| Hesperidin, eriocitrin, diosmin | Lemon peels | S. aureus | Antibacterial | [17] |
| Pelargonidin-diglucoside, gallotannin, ellagitannin, cyanidin-glucoside, pelargonidin-glucoside, catechin, p-coumaroyl-glucoside, p-coumaroyl-ester, p-coumaroyl-glucoside, quercetin-rutinoside, ellagic acid, quercetin-glucoside, quercetin-glucuronide, methyl-ellagic acid-pentose, and kaempferol-glucuronide. | Camellia oleifera seeds | E. coli, S. aureus, Bacillus subtilis | Antibacterial | [18] |
| Chlorogenic acid, caffeic acid, coumaric acid | Apple, lime, grapes, pomegranate, and papaya wastes | Bacillus subtilis, E. coli | Antibacterial activity of pigments | [19] |
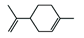 Terpenoids |
||||
| Limonene, linalyl acetate, linalool, β-pinene, γ-terpinene | Citrus peels (orange, lemon, and bergamot) | S. aureus | Bactericidal | [20] |
| Limonene, β-myrcene, linalool, α-pinene, β-pinene | Orange peels | Gram-positive (S. aureus, Listeria monocytogenes) and Gram-negative (E. coli, P. aeruginosa) | Bactericidal | [21] |
| Caffeic acid, p-coumaric acid, salicylic acid | Yellow passion fruit pulp and seeds | Gram-positive (S. aureus, Bacillus cereus) and Gram-negative (E. coli, Salmonella enteritidis) | Antibacterial | [22] |
| D-limonene, lauric acid, 1-methyl-1,4-cyclohexadiene, methyl linoleate, myristic acid, (E,E,E)-2,6,10-trimethyl-2,6,9,11-dodecanetetraen-1-al, palmitic acid, β-myrcene | Orange peels | Cutibacterium acnes (formerly Propionibacterium acnes) | Antibacterial | [23] |
 Organosulfur compounds |
||||
| Glucoraphanin, sulforaphane, sulforaphane nitrile | Punica granatum L. peel | Gram-positive (S. aureus, Enterococcus faecalis) and Gram-negative (P. aeruginosa, Klebsiella pneumoniae) | Antibacterial | [24] |
| Allyl isothiocyanate | Lepidium latifolium flower, leaf, stem, and root | Gram-positive (Listeria monocytogenes, S. aureus) and Gram-negative (Salmonella Typhimurium, E. coli, P. aeruginosa) | Antibacterial a (time-killing and growth kinetic assays) | [25] |
| Lucoraphanin, sulforaphane, sulforaphane nitrile | Broccoli (raw, cooked, and cooked broccoli plus mustard seeds as a source of myrosinase) | E. coli | Antibacterial | [26] |
Overall, phytochemicals found in agri-food wastes have a significant potential to be used as alternative antibiotics and food additives. Additionally, their valorisation can lead to the production of value-added products with beneficial properties.
2. Nitrogen Alkaloids
Alkaloids are a type of organic nitrogen heterocyclic compound that have a wide range of chemical structures based on the rings in the molecule [1]. Nicotine, morphine, caffeine, and mescaline are some of the well-known alkaloids. Plants produce alkaloids as a defence mechanism against insects and herbivores. These compounds also have antibacterial properties against a range of microorganisms, such as Mycobacterium fortuitum, Mycobacterium tuberculosis, Mycobacterium smegmatis, E. coli, S. aureus, Salmonella typhimurium, Klebsiella pneumonia, and P. aeruginosa [1][3].
The coffee industry generates a significant amount of by-products that can be used as a source of bioactive compounds [9][10]. Researchers have evaluated the antibacterial activity of arabica coffee leaves and found that the extracts contain the alkaloids trigonelline and caffeine [9]. These extracts were found to be effective against E. coli.
3. Phenolic Compounds
Plant polyphenols, also known as phenolic compounds, are organic compounds that contain at least one phenol group and have an aromatic ring with one or more hydroxyl groups in their molecular structure [2], and they are classified into flavonoids and non-flavonoids based on their structural characteristics [1][3] secondary metabolites that play a crucial role in plant physiology, including defence against herbivores and pathogens and mechanical support for the plant. [1] have shown antimicrobial properties against a wide range of microorganisms, and they can sensitize multidrug-resistant strains to bacteriostatic or bactericidal antibiotics, making them promising natural antimicrobial agents [3]. Additionally, polyphenols have been established as chemopreventive and therapeutic agents due to their potential health-benefiting properties, including antioxidant, antiallergic, anti-inflammatory, anticancer, antihypertensive, and antimicrobial features [1][2][3]. Sharma et al. [15] investigated the biological activities of polyphenols in skinned fresh and ageing onions. The authors found that the antibiofilm activity against E. coli, P. aeruginosa, S. aureus, and Bacillus cereus increased with ageing onions as the levels of quercetin and total phenolic content also increased upon aging in the studied varieties.
3.1. Flavonoids
Plant flavonoids, which have a 2-phenyl-benzo-γ-pyrane nucleus with two benzene rings, have demonstrated promising antimicrobial activities and antioxidant properties [1][3]. Many classes of flavonoids, including flavonols, flavanols, flavanones, isoflavonoids, chalcones, and dihydrochalcones, have been identified as allelochemicals that inhibit microbial growth. Flavonoids are also known to inhibit quorum sensing and biofilm formation, as well as act as resistant-reversal agents [3]. Catechins and proanthocyanidins possess antioxidant properties and have been proposed to neutralize bacterial toxic factors originating from V. cholerae, V. vulnificus, S. aureus, Bacillus anthracis, and C. botulinum. Additionally, citrus flavonoids, such as apigenin, kaempferol, quercetin, and naringenin, are effective antagonists of cell–cell signalling [2][27]. Chrysin and kaempferol restrict the DNA gyrase activity, which is an essential enzyme in DNA replication in E. coli, while aglycone flavonoids, such as myricetin, hesperetin, and phloretin, inhibit biofilm formation in Staphylococcus strains [3].
3.2. Non-Flavonoids
Phenolic acids, including benzoic, phenylacetic, and phenylpropionic acids, have been discovered to have inhibitory effects on both pathogenic and non-pathogenic bacteria and fungi. These include E. coli, Lactobacillus spp., S. aureus, P. aeruginosa, and Candida albicans [2][3].
Hydroxycinnamic acids, such as caffeic, coumaric, ferulic, and sinapic acids, have also been found to inhibit the growth of Bacillus cereus, S. aureus, and Pseudomonas fluorescens [2]. Ferulic acid and gallic acid have also demonstrated antibacterial properties against various bacterial isolates. Both acids damage the cell walls of E. coli, P. aeruginosa, and S. aureus, leading to local damage and cellular material leakage [3]; gallic acid has been shown to exhibit strong antibacterial potential against Enterococcus faecalis, Streptococcus pneumonia, P. aeruginosa, Moraxella catarrhalis, S. aureus, Enterococcus faecalis, E. coli, and Streptococcus agalactiae strains [3].
4. Terpenoids
Terpenoids are a diverse group of organic compounds that are similar to terpenes. They consist of mono- and sesquiterpenoids [1], which are the main components of essential oils. Essential oils are volatile plant products [3] that can be extracted from various plant parts, such as flowers and fruits. They contain a mixture of low-mass plant natural products or phytochemicals, including myrcene, o-cimene, citral, geraniol, eugenol, carvacrol, linalool, citronellal, carvone, limonene, terpinenes, menthol, and menthone [1][3].
Essential oils have strong antimicrobial properties and are commonly used in traditional medicine. They are considered safe for consumption and vital host tissues. However, their stability is crucial for their quality and pharmacological potency [3]. Essential oils are known for their remarkable antibacterial activities against both Gram-positive and negative pathogens, including bactericidal and re-potentiating or re-sensitizing of antibiotics potentials against pathogenic microbes. They have also demonstrated their potential in targeting and disturbing the most prevalent drug-resistance-determining mechanisms of microbes, namely the cell wall, cell membrane and permeability, drug efflux pumps, mobile genetic elements, quorum sensing, and biofilm [3].
Citrus fruits are the main source of essential oils [1][20][21][23]. For example, Djenane [20] evaluated the chemical composition of citrus peel (orange, lemon, and bergamot) essential oils. The essential oils analysed were mainly composed of limonene (77.4%) for orange essential oil; linalyl acetate (37.3%) and linalool (23.4%) for bergamot essential oil; and limonene (51.4%), β-pinene (17.0%), and γ-terpinene (13.5%) for lemon essential oil. The in vitro antimicrobial activity of the essential oils was evaluated against S. aureus, which revealed that lemon essential oil had more antibacterial effects than the other essential oils.
5. Organosulfur Compounds
Organosulfur compounds, also known as thiols, are present in various plants and vegetables. These compounds include glucosinolates and allyl sulphides, which contain sulfur in their structure. Glucosinolates are found in cruciferous vegetables of the Brassicales order while allyl sulphides are abundant in garlic [1].
Glucosinolates play a vital role in plant defence against microbial pathogens and insect herbivores. They act as signalling molecules that initiate pathways such as stomatal closure, apoptosis, and callose accumulation [28]. A study by Blažević et al. [25] investigated the glucosinolate profile and antibacterial activity of Lepidium latifolium L. against food spoilage bacteria. The results showed that allyl isothiocyanate, a compound found in the plant, was highly effective against E. coli.
This entry is adapted from the peer-reviewed paper 10.3390/metabo13050634
References
- Martillanes, S.; Rocha-Pimienta, J.; Delgado-Adámez, J. Agrifood by-Products as a Source of Phytochemical Compounds. In Descriptive Food Science; Díaz, A.V., García-Gimeno, R.M., Eds.; IntechOpen: London, UK, 2018.
- Panda, L.; Duarte-Sierra, A. Recent Advancements in Enhancing Antimicrobial Activity of Plant-Derived Polyphenols by Biochemical Means. Horticulturae 2022, 8, 401.
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726.
- Gong, C.; Singh, A.; Singh, P.; Singh, A. Anaerobic Digestion of Agri-Food Wastes for Generating Biofuels. Indian J. Microbiol. 2021, 61, 427–440.
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods 2021, 10, 707.
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515.
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-Food Industry Waste as Resource of Chemicals: The Role of Membrane Technology in Their Sustainable Recycling. Sustainability 2022, 14, 1483.
- do Carmo, G.; Fernandes, T.S.; Pedroso, M.; Ferraz, A.; Neto, A.T.; Silva, U.F.; Mostardeiro, M.A.; Back, D.F.; Dalcol, I.I.; Morel, A.F. Phytochemical and antimicrobial study of Pilocarpus pennatifolius Lemaire. Fitoterapia 2018, 131, 1–8.
- Mesquita Junior, G.A.; da Costa, Y.F.G.; Mello, V.; Costa, F.F.; Rodarte, M.P.; Costa, J.C.D.; Alves, M.S.; Vilela, F.M.P. Chemical characterisation by UPLC-Q-ToF-MS/MS and antibacterial potential of Coffea arabica L. leaves: A coffee by-product. Phytochem. Anal. 2022, 33, 1036–1044.
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128.
- Shaheen, H.A.; Issa, M.Y. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic. 2020, 264, 108940.
- Friedman, M.; Huang, V.; Quiambao, Q.; Noritake, S.; Liu, J.; Kwon, O.; Chintalapati, S.; Young, J.; Levin, C.E.; Tam, C.; et al. Potato Peels and Their Bioactive Glycoalkaloids and Phenolic Compounds Inhibit the Growth of Pathogenic Trichomonads. J. Agric. Food. Chem. 2018, 66, 7942–7947.
- Miedzianka, J.; Peksa, A.; Nems, A.; Drzymala, K.; Zambrowicz, A.; Kowalczewski, P. Trypsin inhibitor, antioxidant and antimicrobial activities as well as chemical composition of potato sprouts originating from yellow- and colored-fleshed varieties. J. Environ. Sci. Health B 2020, 55, 42–51.
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J. Microencapsul. 2018, 35, 229–240.
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518–528.
- Guo, C.; Shan, Y.; Yang, Z.; Zhang, L.; Ling, W.; Liang, Y.; Ouyang, Z.; Zhong, B.; Zhang, J. Chemical composition, antioxidant, antibacterial, and tyrosinase inhibition activity of extracts from Newhall navel orange (Citrus sinensis Osbeck cv. Newhall) peel. J. Sci. Food Agric. 2020, 100, 2664–2674.
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior Antibacterial Activity of Integral Lemon Pectin Extracted via Hydrodynamic Cavitation. ChemistryOpen 2020, 9, 628–630.
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2020, 29, 17–25.
- Gupta, N.; Poddar, K.; Sarkar, D.; Kumari, N.; Padhan, B.; Sarkar, A. Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 2019, 244, 138–143.
- Djenane, D. Chemical Profile, Antibacterial and Antioxidant Activity of Algerian Citrus Essential Oils and Their Application in Sardina pilchardus. Foods 2015, 4, 208–228.
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: Changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. J. Food Meas. Charact. 2019, 14, 862–875.
- Pereira, M.G.; Maciel, G.M.; Haminiuk, C.W.I.; Bach, F.; Hamerski, F.; Scheer, A.D.; Corazza, M.L. Effect of Extraction Process on Composition, Antioxidant and Antibacterial Activity of Oil from Yellow Passion Fruit (Passiflora edulis Var. Flavicarpa) Seeds. Waste Biomass Valorization 2019, 10, 2611–2625.
- Hou, H.S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.L.; Yang, Z.H.; Quan, C. Extraction of essential oil from Citrus reticulate Blanco peel and its antibacterial activity against Cutibacterium acnes (formerly Propionibacterium acnes). Heliyon 2019, 5, e02947.
- Dawoud, T.M.; Akhtar, N.; Okla, M.K.; Shah, A.N.; Shah, A.A.; Abdel-Mawgoud, M.; AbdElgayed, G.; Al-Hashimi, A.; AbdElgawad, H. Seed Priming with Pomegranate Peel Extract Improves Growth, Glucosinolates Metabolism and Antimicrobial Potential of Brassica oleraceae Varieties. J. Plant Growth Regul. 2022, 42, 3043–3055.
- Blazevic, I.; Dulovic, A.; Maravic, A.; Cikes Culic, V.; Montaut, S.; Rollin, P. Antimicrobial and Cytotoxic Activities of Lepidium latifolium L. Hydrodistillate, Extract and Its Major Sulfur Volatile Allyl Isothiocyanate. Chem. Biodivers. 2019, 16, e1800661.
- Abukhabta, S.; Khalil Ghawi, S.; Karatzas, K.A.; Charalampopoulos, D.; McDougall, G.; Allwood, J.W.; Verrall, S.; Lavery, S.; Latimer, C.; Pourshahidi, L.K.; et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur. J. Nutr. 2021, 60, 1263–1276.
- Bouyahya, A.; Chamkhi, I.; Balahbib, A.; Rebezov, M.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Benali, T.; El Omari, N. Mechanisms, Anti-Quorum-Sensing Actions, and Clinical Trials of Medicinal Plant Bioactive Compounds against Bacteria: A Comprehensive Review. Molecules 2022, 27, 1484.
- Pacifico, D.; Lanzanova, C.; Pagnotta, E.; Bassolino, L.; Mastrangelo, A.M.; Marone, D.; Matteo, R.; Lo Scalzo, R.; Balconi, C. Sustainable Use of Bioactive Compounds from Solanum Tuberosum and Brassicaceae Wastes and by-Products for Crop Protection—A Review. Molecules 2021, 26, 2174.
This entry is offline, you can click here to edit this entry!
