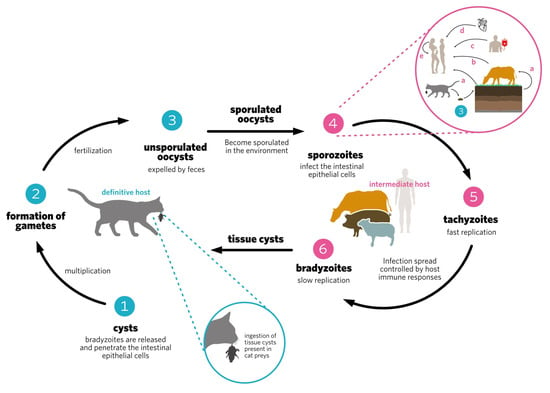
Nanoparticles include particles ranging in size from nanometers to micrometers, whose physicochemical characteristics are optimized to make them appropriate delivery vehicles for drugs or immunogens important in the fight and/or prevention of infectious diseases. There has been a rise in the use of nanoparticles in preventive vaccine formulations as immunostimulatory adjuvants, and as vehicles for immunogen delivery to target immune cells. Toxoplasma is important worldwide, and may cause human toxoplasmosis. In immunocompetent hosts, infection is usually asymptomatic, but in immunocompromised patients it can cause serious neurological and ocular consequences, such as encephalitis and retinochoroiditis. Primary infection during pregnancy may cause abortion or congenital toxoplasmosis. Currently, there is no effective human vaccine against this disease. Evidence has emerged from several experimental studies testing nanovaccines showing them to be promising tools in the prevention of experimental toxoplasmosis.
- nanoparticles
- Toxoplasma gondii
- adjuvant
- immune system
- toxoplasmosis
1. Introduction
2. Benefits of Using Nanoparticles in Vaccination
3. Recent Advances in the Use of Nanoparticles for T. gondii Vaccination
This entry is adapted from the peer-reviewed paper 10.3390/vaccines11040733
References
- Dubey, J.P. Outbreaks of clinical toxoplasmosis in humans: Five decades of personal experience, perspectives and lessons learned. Parasites Vectors 2021, 14, 263.
- Deng, H.; Devleesschauwer, B.; Liu, M.; Li, J.; Wu, Y.; van der Giessen, J.W.B.; Opsteegh, M. Seroprevalence of Toxoplasma gondii in pregnant women and livestock in the mainland of China: A systematic review and hierarchical meta-analysis. Sci. Rep. 2018, 8, 6218.
- Delgado, I.L.S.; Zúquete, S.; Santos, D.; Basto, A.P.; Leitão, A.; Nolasco, S. The Apicomplexan Parasite Toxoplasma gondii. Encyclopedia 2022, 2, 189–211.
- Milne, G.; Webster, J.P.; Walker, M. Toward Improving Interventions Against Toxoplasmosis by Identifying Routes of Transmission Using Sporozoite-specific Serological Tools. Clin. Infect. Dis. 2020, 71, e686–e693.
- Elsheikha, H. Congenital toxoplasmosis: Priorities for further health promotion action. Public Health 2008, 122, 335–353.
- Wang, Z.-D.; Wang, S.-C.; Liu, H.-H.; Ma, H.-Y.; Li, Z.-Y.; Wei, F.; Zhu, X.-Q.; Liu, Q. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: A systematic review and meta-analysis. Lancet HIV 2017, 4, e177–e188.
- Liu, T.; Gao, P.; Bu, D.; Liu, D. Association between Toxoplasma gondii infection and psychiatric disorders: A cross-sectional study in China. Sci. Rep. 2022, 12, 15092.
- Silva, M.d.; Teixeira, C.; Gomes, P.; Borges, M. Promising Drug Targets and Compounds with Anti-Toxoplasma gondii Activity. Microorganisms 2021, 9, 1960.
- Brito, C.; Silva, T.M.; Castro, M.M.; Wyrwas, W.; Oliveira, B.; Fonseca, B.M.; Oliveira, P.; Roberts, C.W.; Teixeira, N.; Borges, M. Toxoplasma gondii infection reduces serum progesterone levels and adverse effects at the maternal-foetal interface. Parasite Immunol. 2020, 42, e12690.
- Hasan, T.; Nishikawa, Y. Advances in vaccine development and the immune response against toxoplasmosis in sheep and goats. Front. Veter-Sci. 2022, 9, 951584.
- Peplow, M. Nanotechnology offers alternative ways to fight COVID-19 pandemic with antivirals. Nat. Biotechnol. 2021, 39, 1172–1174.
- Rasmussen, M.K.; Kardjilov, N.; Oliveira, C.L.P.; Watts, B.; Villanova, J.; Botosso, V.F.; Sant’Anna, O.A.; Fantini, M.C.A.; Bordallo, H.N. 3D visualisation of hepatitis B vaccine in the oral delivery vehicle SBA-15. Sci. Rep. 2019, 9, 6106.
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Han, Y.; Kato, K. Nanoparticles show potential to retard bradyzoites in vitro formation of Toxoplasma gondii. Folia Parasitol. 2019, 66, 001.
- Dacoba, T.G.; Olivera, A.; Torres, D.; Crecente-Campo, J.; Alonso, M.J. Modulating the immune system through nanotechnology. Semin. Immunol. 2017, 34, 78–102.
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224.
- Petkar, K.C.; Patil, S.M.; Chavhan, S.S.; Kaneko, K.; Sawant, K.K.; Kunda, N.K.; Saleem, I.Y. An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery. Pharmaceutics 2021, 13, 455.
- Luo, M.; Samandi, L.; Wang, Z.; Chen, Z.; Gao, J. Synthetic nanovaccines for immunotherapy. J. Control. Release 2017, 263, 200–210.
- Smith, D.M.; Simon, J.K.; Baker, J.R., Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605.
- Henriksen-Lacey, M.; Christensen, D.; Bramwell, V.W.; Lindenstrøm, T.; Agger, E.M.; Andersen, P.; Perrie, Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J. Control. Release 2010, 145, 102–108.
- Hayat, S.M.G.; Darroudi, M. Nanovaccine: A novel approach in immunization. J. Cell. Physiol. 2019, 234, 12530–12536.
- Djurisic, S.; Jakobsen, J.C.; Petersen, S.B.; Kenfelt, M.; Gluud, C. Aluminium adjuvants used in vaccines versus placebo or no intervention. Cochrane Database Syst. Rev. 2017, 2017, CD012805.
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511.
- Mbow, M.L.; De Gregorio, E.; Valiante, N.M.; Rappuoli, R. New adjuvants for human vaccines. Curr. Opin. Immunol. 2010, 22, 411–416.
- Swaminathan, G.; Thoryk, E.A.; Cox, K.S.; Meschino, S.; Dubey, S.A.; Vora, K.A.; Celano, R.; Gindy, M.; Casimiro, D.R.; Bett, A.J. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine 2016, 34, 110–119.
- Lin, J.; Alexander-Katz, A. Cell Membranes Open “Doors” for Cationic Nanoparticles/Biomolecules: Insights into Uptake Kinetics. ACS Nano 2013, 7, 10799–10808.
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. An Overview of Antibody Conjugated Polymeric Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2020, 12, 802.
- Lim, S.S.-Y.; Othman, R.Y. Recent Advances in Toxoplasma gondii Immunotherapeutics. Korean J. Parasitol. 2014, 52, 581–593.
- Chu, K.-B.; Quan, F.-S. Advances in Toxoplasma gondii Vaccines: Current Strategies and Challenges for Vaccine Development. Vaccines 2021, 9, 413.
- Wang, T.; Yin, H.; Li, Y.; Zhao, L.; Sun, X.; Cong, H. Vaccination with recombinant adenovirus expressing multi-stage antigens of Toxoplasma gondii by the mucosal route induces higher systemic cellular and local mucosal immune responses than with other vaccination routes. Parasite 2017, 24, 12.
- Li, Y.; Zhou, H. Moving towards improved vaccines for Toxoplasma gondii. Expert Opin. Biol. Ther. 2018, 18, 273–280.
- Ahmadpour, E.; Sarvi, S.; Soteh, M.B.H.; Sharif, M.; Rahimi, M.T.; Valadan, R.; Tehrani, M.; Khalilian, A.; Montazeri, M.; Ramandi, M.F.; et al. Enhancing immune responses to a DNA vaccine encoding Toxoplasma gondii GRA14 by calcium phosphate nanoparticles as an adjuvant. Immunol. Lett. 2017, 185, 40–47.
- Sun, H.-C.; Huang, J.; Fu, Y.; Hao, L.-L.; Liu, X.; Shi, T.-Y. Enhancing Immune Responses to a DNA Vaccine Encoding Toxoplasma gondii GRA7 Using Calcium Phosphate Nanoparticles as an Adjuvant. Front. Cell. Infect. Microbiol. 2021, 11, 787635.
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142.
- Luo, F.; Zheng, L.; Hu, Y.; Liu, S.; Wang, Y.; Xiong, Z.; Hu, X.; Tan, F. Induction of Protective Immunity against Toxoplasma gondii in Mice by Nucleoside Triphosphate Hydrolase-II (NTPase-II) Self-amplifying RNA Vaccine Encapsulated in Lipid Nanoparticle (LNP). Front Microbiol 2017, 8, 605.
- Hasan, T.; Kawanishi, R.; Akita, H.; Nishikawa, Y. Toxoplasma gondii GRA15 DNA Vaccine with a Liposomal Nanocarrier Composed of an SS-Cleavable and pH-Activated Lipid-like Material Induces Protective Immunity against Toxoplasmosis in Mice. Vaccines 2021, 10, 21.
- Rahimi, M.T.; Sarvi, S.; Sharif, M.; Abediankenari, S.; Ahmadpour, E.; Valadan, R.; Ramandie, M.F.; Hosseini, S.-A.; Daryani, A. Immunological evaluation of a DNA cocktail vaccine with co-delivery of calcium phosphate nanoparticles (CaPNs) against the Toxoplasma gondii RH strain in BALB/c mice. Parasitol. Res. 2016, 116, 609–616.
- Dimier-Poisson, I.; Carpentier, R.; N’Guyen, T.T.L.; Dahmani, F.; Ducournau, C.; Betbeder, D. Porous nanoparticles as delivery system of complex antigens for an effective vaccine against acute and chronic Toxoplasma gondii infection. Biomaterials 2015, 50, 164–175.
- Yu, Z.; Zhou, T.; Luo, Y.; Dong, L.; Li, C.; Liu, J.; Luo, J.; Yan, R.; Xu, L.; Song, X.; et al. Modulation Effects of Toxoplasma gondii Histone H2A1 on Murine Macrophages and Encapsulation with Polymer as a Vaccine Candidate. Vaccines 2020, 8, 731.
- Roozbehani, M.; Falak, R.; Mohammadi, M.; Hemphill, A.; Razmjou, E.; Meamar, A.R.; Masoori, L.; Khoshmirsafa, M.; Moradi, M.; Gharavi, M.J. Characterization of a multi-epitope peptide with selective MHC-binding capabilities encapsulated in PLGA nanoparticles as a novel vaccine candidate against Toxoplasma gondii infection. Vaccine 2018, 36, 6124–6132.
- Ducournau, C.; Nguyen, T.T.; Carpentier, R.; Lantier, I.; Germon, S.; Précausta, F.; Pisella, P.-J.; Leroux, H.; Van Langendonck, N.; Betbeder, D.; et al. Synthetic parasites: A successful mucosal nanoparticle vaccine against Toxoplasma congenital infection in mice. Futur. Microbiol. 2017, 12, 393–405.
- Ducournau, C.; Moiré, N.; Carpentier, R.; Cantin, P.; Herkt, C.; Lantier, I.; Betbeder, D.; Dimier-Poisson, I. Effective Nanoparticle-Based Nasal Vaccine Against Latent and Congenital Toxoplasmosis in Sheep. Front. Immunol. 2020, 11, 2183.
- Dodangeh, S.; Fasihi-Ramandi, M.; Daryani, A.; Valadan, R.; Asgarian-Omran, H.; Hosseininejad, Z.; Chegeni, T.N.; Pagheh, A.S.; Javidnia, J.; Sarvi, S. Protective efficacy by a novel multi-epitope vaccine, including MIC3, ROP8, and SAG1, against acute Toxoplasma gondii infection in BALB/c mice. Microb. Pathog. 2021, 153, 104764.
- Nabi, H.; Rashid, I.; Ahmad, N.; Durrani, A.; Akbar, H.; Islam, S.; Bajwa, A.A.; Shehzad, W.; Ashraf, K.; Imran, N. Induction of specific humoral immune response in mice immunized with ROP18 nanospheres from Toxoplasma gondii. Parasitol. Res. 2016, 116, 359–370.
- Naeem, H.; Sana, M.; Islam, S.; Khan, M.; Riaz, F.; Zafar, Z.; Akbar, H.; Shehzad, W.; Rashid, I. Induction of Th1 type-oriented humoral response through intranasal immunization of mice with SAG1-Toxoplasma gondii polymeric nanospheres. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 1025–1034.
- Allahyari, M.; Golkar, M.; Fard-Esfahani, P.; Dimier-Poisson, I.; Mévélec, M.-N. Co-delivery of PLGA nanoparticles loaded with rSAG1 antigen and TLR ligands: An efficient vaccine against chronic toxoplasmosis. Microb. Pathog. 2022, 162, 105312.
- Khorshidvand, Z.; Khosravi, A.; Mahboobian, M.M.; Larki-Harchegani, A.; Fallah, M.; Maghsood, A.H. Novel naltrexone hydrochloride nanovaccine based on chitosan nanoparticles promotes induction of Th1 and Th17 immune responses resulting in protection against Toxoplasma gondii tachyzoites in a mouse model. Int. J. Biol. Macromol. 2022, 208, 962–972.
- Gaafar, M.R.; El-Mansoury, S.T.; Eissa, M.M.; Shalaby, T.I.; Younis, L.K.; Rashed, H.A. Effect of alginate nanoparticles on the immunogenicity of excretory-secretory antigens against acute toxoplasmosis in murine model. Acta Trop. 2022, 225, 106215.
- Abdollahi, S.H.; Shahmabadi, H.E.; Arababadi, M.K.; Askari, N.; Falahati-Pour, S.K. The immune response against Toxoplasma gondii in BALB/c mice induced by mannose-modified nanoliposome of excreted/secreted antigens. Parasitol. Res. 2021, 120, 2855–2861.
- Azadi, Y.; Ahmadpour, E.; Hamishehkar, H.; Daryani, A.; Spotin, A.; Mahami-Oskouei, M.; Barac, A.; Rajabi, S.; Alizadeh, P.; Montazeri, M. Quantification of Toxoplasma gondii in the tissues of BALB/c mice after immunization with nanoliposomal excretory-secretory antigens using Real-Time PCR. Comp. Immunol. Microbiol. Infect. Dis. 2018, 59, 52–56.
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83.
- Chu, K.-B.; Quan, F.-S. Virus-Like Particle Vaccines Against Respiratory Viruses and Protozoan Parasites. Curr. Top. Microbiol. Immunol. 2021, 433, 77–106.
- Lee, D.H.; Lee, S.H.; Kim, A.R.; Quan, F.S. Virus-Like Nanoparticle Vaccine Confers Protection against Toxoplasma gondii. PLoS ONE 2016, 11, e0161231.
- Kang, H.-J.; Lee, S.H.; Kim, M.J.; Chu, K.B.; Lee, D.H.; Chopra, M.; Choi, H.J.; Park, H.; Jin, H.; Quan, F.-S. Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii Infection. Pharmaceutics 2019, 11, 342.
