Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Marine products are among the most promising sources of biologically active molecules. Aplysinopsins, tryptophan-derived marine natural products, were isolated from different natural marine sources including sponges, stony corals (hard corals) especially genus scleractinian, as well as sea anemone, in addition to one nudibranch. Aplysinopsins were reported to be isolated from different marine organisms related to various geographic areas such as Pacific, Indonesia, Caribbean, and Mediterranean regions.
- aplysinopsin
- sources
- synthesis
- bioactivity
1. Different Sources and Chemical Structures of Aplysinopsins
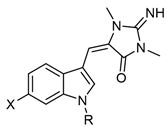
The chemical backbone of the natural aplysinopsins include a simple configuration of monomeric aplysinopsin-type structures and their brominated derivatives at the A ring, variation in the structure of the C ring, the presence and configuration of the C-8-C-1′ double bond, the oxidation state of the 2-aminoimidazoline fragment and N-alkylated at the B ring (Figure 1), in addition to the aplysinopsin dimers form.
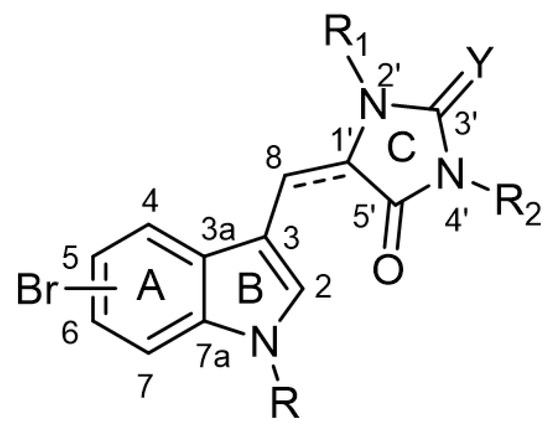
Figure 1. The chemical configuration of monomeric aplysinopsin-type structures shows detailed segmentation of the rings, carbon numbering, and type of bonds.
Aplysinopsin, (E)-5-((1H-indol-3-yl)methylene)-2-imino-1,3-dimethylimidazo-li-din-4-one (1), was first isolated from the sponge genus Thorecta of the Australian Great Barrier Reef by Kazlauskas et al. [3]. Sequentially, aplysinopsin and its derivatives have been reported in many other marine organisms from various geographic areas (Table 1, Table 2, Table 3 and Table 4) [2].
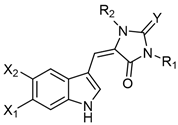
Table 1. Monomeric aplysinopsin-type structures and their brominated derivatives.
 |
||||||
| Aplysinopsin Derivatives | X1 | X2 | R1 | R2 | Y | Source [Ref.] |
| Aplysinopsin (1) | H | H | Me | Me | NH | Thorecta sp. sponge Great Barrier Reef Australia [3], Verongia spengelli sponge Florida Keys [4], Dercitus sp. sponge Caribbean [5], Smenospongia aurea sponge Caribbean [24], Astroides calycularis anthozoan Mediterranean [25], Tubastraea aurea Japan scleractinian coral [11], Tubastraea sp. scleractinian coral Philippines [12], Radianthus kuekenthali sea anemone Japan [26], Aplysina sp. sponge Japan [27], Tubastraea faulkneri scleractinian coral Australia [13], Tubastraea sp. scleractinian Japane [14], Smenospongia sp. sponge Indo-Pacific reefs [8], and Verongula rigida sponge Florida Keys [9]. |
| Isoaplysinopsin (2) | H | H | H | Me | NMe | Aplysina sp. sponge Japan [27] and Smenospongia aurea sponge Jamaica [7]. |
| 2′-de-N-methylaplysinopsin (3) | H | H | H | Me | H | Dercitus sp. sponge Caribbean [5], Tubastrea coccinea coral Hawaii [10], Phestilla melanobrachia mollusk [10], Dendrophyllia sp. scleractinian coral Philippines [12], Smenospongia aurea sponge Jamaica [7], Verongula rigida sponge Florida Keys [9]. |
| Methylaplysinopsin (4) |
H | H | Me | Me | NMe | Aplysinopsis reticulata sponge Australia [2] and Smenospongia aurea sponge Jamaica [7]. |
| 4′-Demethyl-3′-N-methylaplysinopsin (5) | H | H | Me | H | NMe | Dendrophyllia sp. scleractinian coral Philippines [12] and Smenospongia aurea sponge Jamaica [7]. |
| N-3′-ethyl-aplysinopsin (6) | H | H | Me | Et | NMe | Smenospongia aurea sponge Jamaica [7]. |
| 3′-Deimino-2′,4′-bis(demethyl)-3′-oxo-aplysinopsin (7) | H | H | H | H | O | Leptopsammia pruvoti scleractinian coral France [12]. |
| 3′-Deimino-3′oxoaplysinopsin (8) |
H | H | Me | Me | O | Thorecta sp. sponge Great Barrier Reef Australia [3] and Tubastraea sp. scleractinian coral Philippines [12]. |
| 6-Bromo-2′-de-N-methylaplysinopsin (9) |
Br | H | Me | H | NH | Dercitus sp. sponge Caribbean [5], Tubastrea coccinea coral Hawaii [10], Phestilla melanobrachia mollusk [10], Dendrophyllia sp. scleractinian coral Philippines [12], Tubastraea faulkneri scleractinian coral Australia [13], Smenospongia aurea sponge Jamaica [7], Hyrtios erecta sponge Japan [6]. |
| 6-Bromoaplysinopsin (10) | Br | H | Me | Me | NH | Tubastrea coccinea coral Hawaii [10], Smenospongia aurea sponge Caribbean [24], Astroides calycularis anthozoan Mediterranean [25], Radianthus kuekenthali sea anemone Japan [26], Tubastraea faulkneri scleractinian coral Australia [13], Smenospongia aurea sponge Jamaica [7], Smenospongia aurea sponge Florida Keys [9]. |
| 6-Bromo-4′-de-N-methylaplysinopsin (11) | Br | H | H | Me | NH | Smenospongia aurea sponge Caribbean [24]. |
| 6-Bromo-4′-demethyl- 3′-N-methyl-aplysinopsin (12) |
Br | H | H | Me | NMe | Dendrophyllia sp. scleractinian coral Philippines [12]. |
| 5,6-Dibromo-2′-demethylaplysinopsin (13) | Br | Br | Me | H | NH | Hyrtios erecta sponge Japan [6]. |
| 6-Bromo-3′-deimino-2′,4′-bis(demethyl)-3′- Oxoaplysinopsin (14) |
Br | H | H | H | O | Smenospongia aurea sponge Caribbean [28] and Leptopsammia pruvoti scleractinian coral France [12]. |
| 6-Bromo-3′-deimino-3′-oxoaplysinopsin (15) |
Br | H | Me | Me | O | Astroides calycularis anthozoan Mediterranean [25] and Tubastraea sp. scleractinian coral Philippines [12]. |
Table 2. Aplysinopsins substituted at the nitrogen atom.
Table 3. Aplysinopsins with a single C-8-C-1′ bond.
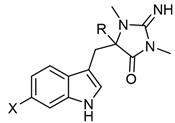 Aplysinopsin DerivativesRXSources [Ref.]1′,8-Dihydroaplysinopsin (19)HHTubastrea coccinea coral Hawaii [10], Radianthus kuekenthali sea anemone Japan [26], and Thorectandra sp. sponge Indo-Pacific reefs [8].6-Bromo-1′,8-dihydroaplysinopsin (20)BrH6-Bromo-1-hydroxy-1′,8-dihydroaplysinopsin (21)BrOHThorectandra sp. sponge Indo-Pacific reefs [8].6-Bromo-1-methoxy-1′,8-dihydroaplysinopsin (22)BrOCH36-Bromo-1-ethoxy-1′,8-dihydroaplysinopsin (23)BrOCH2CH3
Aplysinopsin DerivativesRXSources [Ref.]1′,8-Dihydroaplysinopsin (19)HHTubastrea coccinea coral Hawaii [10], Radianthus kuekenthali sea anemone Japan [26], and Thorectandra sp. sponge Indo-Pacific reefs [8].6-Bromo-1′,8-dihydroaplysinopsin (20)BrH6-Bromo-1-hydroxy-1′,8-dihydroaplysinopsin (21)BrOHThorectandra sp. sponge Indo-Pacific reefs [8].6-Bromo-1-methoxy-1′,8-dihydroaplysinopsin (22)BrOCH36-Bromo-1-ethoxy-1′,8-dihydroaplysinopsin (23)BrOCH2CH3Table 4. Aplysinopsin dimers.
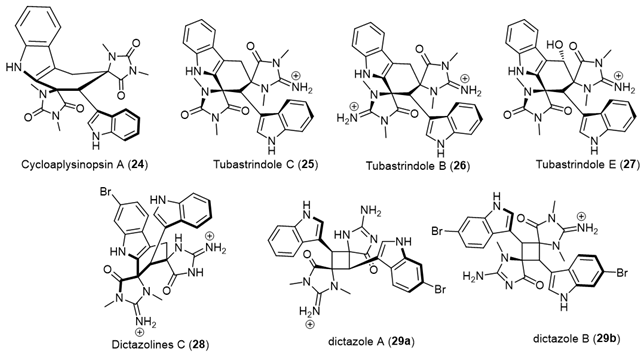 Aplysinopsin DimersSources [Ref.]Cycloaplysinopsin (24)Scleractinian corals of the family Dendrophylliidae from Comoro islands and hard coral Tubastraea sp., from the Great Hanish in the Archipelago of the Hanish Islands, Yemen, tropical Indo-Pacific (Comoros, Philippines) scleractinian corals of the family Dendrophylliidae [29,30].Tubastrindoles A–C (25)Stony Coral, Tubastraea sp. scleractinian Japane [14].Tubastrindole B (26)Australian Marine Sponge, a specimen of the sponge Ianthella cf. flabelliformis [31].Tubastrindoles D–H (27)Stony Coral, Tubastraea aurea Odomari area, Kagoshima Prefecture, Japan [32].Dictazolines A and BMarine Sponge Smenospongia cerebriformis, Hospital Point on Solarte Isle, Boca del Toro, on the northwest coast of Panama [33].Dictazolines C–E (28) and
Aplysinopsin DimersSources [Ref.]Cycloaplysinopsin (24)Scleractinian corals of the family Dendrophylliidae from Comoro islands and hard coral Tubastraea sp., from the Great Hanish in the Archipelago of the Hanish Islands, Yemen, tropical Indo-Pacific (Comoros, Philippines) scleractinian corals of the family Dendrophylliidae [29,30].Tubastrindoles A–C (25)Stony Coral, Tubastraea sp. scleractinian Japane [14].Tubastrindole B (26)Australian Marine Sponge, a specimen of the sponge Ianthella cf. flabelliformis [31].Tubastrindoles D–H (27)Stony Coral, Tubastraea aurea Odomari area, Kagoshima Prefecture, Japan [32].Dictazolines A and BMarine Sponge Smenospongia cerebriformis, Hospital Point on Solarte Isle, Boca del Toro, on the northwest coast of Panama [33].Dictazolines C–E (28) andDictazoles A and B (29)Marine Sponge Smenospongia cerebriformis, Panamanian sponge [34].
2. Biological Activity
Aplysinopsins possess an array of biological activities (Table 5).
Table 5. Biological activities of aplysinopsins.
| Aplysinopsin Derivatives | Activity [Ref.] |
|---|---|
| Aplysinopsin (1) | - CNS permeable scaffold for dual inhibition of cholinesterase and BACE-1 inhibition [21,47]. - Possess monoamine oxidase (MAO) inhibitory activity (IC50 of 5.6 nM) [19]. - Antiplasmodial activity (IC50: 0.43 µg/mL) [48]. - Antineoplastic activity (IC50 values against L-1210 and KB cells, respectively, 2.3 and 6.4 µg/mL [4,27]. - Inhibit the growth of Staphylococcus epidermidis [8]. - An inhibitor of development of fertilized sea urchin eggs at 2.5 µg/mL [11]. - Induce symbiosis between sea anemone and anemonefish [26]. |
| Isoaplysinopsin (2) | - Showed cytotoxic against murine lymphoma L-1210 (IC50 11.5 µg/mL) and human epidermoid carcinoma KJ3 (31% inhibition at 20 µg/mL) cells [27]. |
| Methylaplysinopsin (4) | - Antidepressant activity by enhancing serotonin activity in the central nervous system [49,50]. - Inhibition of monoamine oxidase (MAO) [50]. - Showed cytotoxicity (IC50 values against L-1210 and KB cells of 3.5 and 6.7 µg/mL, respectively) [27]. |
| N-3′-ethyl-aplysinopsin (6) | - Serotonin receptors modulator with Ki value 1.7 µM to the 5-HT2A and 3.5 µM to 5-HT2C serotonin subtypes [7]. |
| 6-Bromo-2′-de-N-methylaplysinopsin (9) | - Serotonin receptors modulator (showed significant selectivity to the 5-HT2C serotonin subtype over the 5-HT2A subtype) [7]. - Antiplasmodial. - Inhibitor of nitric oxide synthase (nNOS). |
| 6-Bromoaplysinopsin (10) | - Serotonin receptors modulator (showed highest affinity to 5-HT2C with a Ki value similar to that of serotonin 0.33 µM) [7]. |
| 5,6-Dibromo-2′-demethylaplysinopsin (13) | - Inhibitor of nitric oxide synthase (nNOS) [6]. |
| 6-Bromo-3′-deimino-3′-oxoaply-sinopsin (15) | - Antiplasmodial activity [30]. |
| 1′,8-Dihydroaplysinopsin (19) | - Induce symbiosis between sea anemone and anemonefish [26]. |
| 6-Bromo-1′,8-dihydroaplysinopsin (20) 6-Bromo-1-hydroxy-1′,8-dihydroaplysinopsin (21) 6-Bromo-1-methoxy-1′,8-dihydroaplysinopsin (22) 6-Bromo-1-ethoxy-1′,8-dihydroaplysinopsin (23) |
- Inhibit the growth of Staphylococcus epidermidis [8]. |
This entry is adapted from the peer-reviewed paper 10.3390/md21050268
This entry is offline, you can click here to edit this entry!