Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Solar energy is free from noise and environmental pollution. It could be used to replace non-renewable sources such as fossil fuels, which are in limited supply and have negative environmental impacts. The first generation of solar cells was made from crystalline silicon. They were relatively efficient, however very expensive because they require a lot of energy to purify the silicon. The production of solar cells has been improved since the first generation (thin-film solar cells, dye-sensitized solar cells, perovskite solar cells, and organic solar cells).
- solar energy
- thin films
- organic solar cells
- crystalline silicon
- dye-sensitized solar cells
- power conversion efficiency
- renewable energy
- perovskite solar cell
1. Introduction
The process of the conversion of energy from the most abundant source, the sun, into electricity is termed as the photovoltaic effect [1]. Semiconductor materials are the basic requirement for the process of the conversion of photons into electrons [2]. These materials can be divided into organic and inorganic [3] substances.
Photovoltaic solar-cell technologies can be divided into three distinct generations [4]. The first generation was crystalline silicon. This technology currently dominates the global solar-cell market due to it has good performance and stability. The second generation is based on thin films of amorphous silicon and inorganic semiconductor elements such as copper indium gallium selenide (CIGS), cadmium telluride (CdTe), and copper indium selenide (CuInSe2). Moreover, the third generation is based on organic semiconductors. Organic solar cells can either be based on dye-sensitized solar cells [5], multiple junctions based on group IV and group III–V elements, and hybrid solar cells that contain inorganic quantum dots or nanomaterials with organic materials [6]. The potential of organic solar cells for practical applications [7] is demonstrated by recent enhancements in the power-conversion efficiency (PCE).
Organic–inorganic hybrid perovskites (OIHPs) are a group of materials which show magnificent potential for high performance and low production costs in photovoltaic technology [8]. Any material which has an identical crystal structure with calcium titanium oxide (CaTiO3) is recognized as a “perovskite” structure, as named by the Russian Mineralogist Lev Alek-sevich von Perrovski (1839). These perovskites have a stoichiometry of ABX3 three-dimensional structure, where the “A” and “B”-sites are occupied by larger and smaller cations, respectively, and the anion resides at the “X”-site. The 3D crystal structure of ABX3 consists of corner-shared BX6 octahedrals and “A” cations occupy the cavity between them [9][10]. Materials with perovskite structures are quite abundant in nature and can be found in an enormous number of compounds that show a wide range of physical properties, applications, and importance.
Perovskite solar cells can have a conventional (regular) or an inverted PSC structure, depending on the architecture. TiO2 and n-type semiconductors are used in the normal configuration, whereas p-type poly (3,4-ethylene dioxythiophene)-poly(styrene sulfonate) (PEDOT: PSS) are used in the inverted configuration. Typical mesoscopic perovskite solar cell structures consist of a glass surface with a transparent conducting oxide (TCO), such as in FTO (F:SnO2), in which an anode is deposited on the glass substrate, a dense (compact) layer of TiO2 is a hole-blocking layer, and a mesoporous layer of TiO2 serves as an electron-transporting layer (ETL). Moreover, a layer of the perovskite material is used for light absorption, followed by a hole-transporting layer, followed by metal contacts to complete the device’s structure. This type of solar cell has an organic material with a perovskite structure in its absorbing layer, most typically a lead–metal hybrid (Methylammonium lead iodide perovskite, MAPbI3). Methylammonium lead iodide is an ambipolar semiconductor that may transmit both electrons and holes to the collecting electrodes. Because of this, perovskite solar cells can operate without a hole or electron conductor. The Spiro-OMeTAD polymer composition is the HTL material most frequently utilized in perovskite solar cells.
Perovskite solar cells’ superior absorption capabilities and low exciton binding energy are two of their most notable features. Light absorption is the initial physical process in solar systems. When a photon activates an active material with an energy higher than its bandgap, an exciton (electron-hole pairs) is produced. Excitons must reach the contacts after being photogenerated in the perovskite to be retrieved. The charge-transport capabilities of the perovskite play a role in this process. Compared with conventional solar cells, perovskite serves as an absorber in thin-film solar cells to offer effective transport of excitons. The remarkable PCE of these perovskite solar cells is produced by the material’s outstanding charge-transport properties. They possess long charge-carrier diffusion lengths surpassing 5 m and the associated lifetimes of 1 s in both single-crystal and polycrystalline films. These diffusion lengths guarantee that the produced charge can be recovered even from thicker films that absorb the input light completely. However, the interfacial contacts with the charge extraction layer also have a role in the device’s ultimate quality. After absorption and charge transmission, the photovoltaic process requires the extraction of the photogenerated charge. As a result, the optimal extraction interface should provide the least possible voltage, current, and FF loss. An optimal selective contact should not absorb light to prevent the reduction of light intensity in the perovskite. Similarly, there should not be any energetic losses during the absorber injection (no interfacial recombination). These contact materials need to be selective to enable one kind of carrier to be injected, but they also need to lower series resistance.
One of two major processes can result in charge separation in perovskite. One involves injecting photogenerated electrons into an n-type semiconductor such as TiO2, while the other involves injecting holes into a p-type material that transports holes, such as spiro-OMeTAD. To stop holes from reaching the TCO substrate (anode) and short-circuiting the cell, a hole-blocking layer is utilized between the TCO-conducting substrate and the mesoscopic scaffold and/or perovskite layer. On the other hand, using a mesoporous TiO2 layer greatly lessens the hysteresis behavior of PSC even if a hybrid organic–inorganic perovskite absorber can transfer photogenerated electrons to the conductive substrate itself.
Dye-sensitized solar cells (DSSC) were first discovered by professor Michael Gratzel in 1991. The DSSC consists of a working electrode [11], a counter electrode, and an electrolyte. Dyes (natural organic materials and synthetic materials) are attached to the TiO2 films, serve as the working electrodes. The DSSC consisting of ruthenium (II) polypyridyl complex (N3 dye) [12] showed a power-conversion efficiency of about 10%. A platinum conductive glass served as the counter electrode [11]. The working principle of DSSC converts sunlight into electricity through the creation of exciton. The semiconductor material is placed on a conductive transparent plate to form a thin layer. A dye monolayer layer is placed on the surface of the semiconductor. Photoexcitation experienced by the dye produces electrons excited from the valence band to the conduction band of the dye, which then enter the conduction band of the metal oxide. This electron injection is performed through the connection between titanium and the carboxyl group in the dye. This process produces a positively charged dye and a negatively charged TiO2 particle. The electrons then exit through the external circuit to the opposite electrode. This flow of electrons is used as electrical energy. The electron vacancies in the valence band of the dye are replaced by electrons from the electrolyte. The electrolyte used in DSSC is usually an organic solvent containing a redox system such as an iodide–triiodide pair. The regeneration of the sensitizer by iodide occurs by donating electrons in the valence band of the oxidized dye. The iodide is regenerated by reducing the triiodide at the opposite electrode by utilizing electrons from the external circuit. This process takes place continuously as a cycle so that a continuous current is produced.
Metal chalcogenide films are considered as important materials for solar-cell applications [13]. Several types of films (metal sulfide, metal selenide, and metal telluride) have been prepared using a vacuum method or non-vacuum deposition technique [14]. These films could be cheaper to produce, show good quality materials, and have the most potential for the future.
2. Organic Solar Cells
2.1. Advantages of Organic Solar Cells over Inorganic Solar Cells
The advantages of organic solar cells (OSC) over inorganic solar cells are threefold [15][16]. Firstly, in terms of the materials, limited silicon wafer technology leads to heavy, rigid, fragile, and bulky materials that are only available in a couple of colors. Conversely, the materials for OSC are light-weight, thin, flexible, and color-tunable, and can be customized to any design. Secondly, the fabrication process of inorganic solar cells is expensive, complicated, energy-intensive, must be conducted at high temperatures, and has limitations in the possible sizes of fabrications. On the other hand, OSC technology is cheap, simple, non-energy consuming, can be fabricated at ambient temperature, has no limitations in the size of fabrication, and has high speed production besides various possibilities of printing and coating fabrication techniques (such as inkjet, screen, and spray). Finally, the applications of inorganic solar cell are limited only to rooftops and open fields compared with the unlimited possibilities of OSC applications such as windows, screens, smart glasses, automotives, and the charging of mobile devices and laptops [17][18][19].
2.2. Challenges for Organic Solar Cell Technology
Silicon is the most widely used material for the commercial photovoltaic market due to its high-power conversion efficiency [20][21][22]. Inorganic solar cells are associated with certain challenges such their its high-cost and complicated and energy-intensive fabrication protocols. Organic solar cells are gaining attention due to their simple fabrication protocols. OSC technology has not yet gained much attention commercially because of its low power-conversion efficiency (PCE) and limited lifetime [23][24]. The heterojunction generated by contact of donor and acceptor material is the basis of OSC. There are a number of challenges in OSC technology such as limited absorption efficiency of donor materials, hindered exciton diffusion, charge dissociation, and transport of charges. Different approaches to overcome these challenges could be improved in exciton diffusion length, crystal structure, and the collection of photogenerated carriers. These improvements can be realized by the application of polymer-based nanocomposites, plasmonics, and tandem architectures. Moreover, hybrid nanocomposites (composed of both organic and inorganic materials) can be a synergistic combination to take advantage of the unique properties of both components [25][26][27]. The operation of hybrid solar cells has more similarities with OSC. Hybrid solar cells are composed of n-type inorganic material along with p-type semiconductor polymer and are capable of achieving high PCE.
2.3. Materials for Organic Solar Cells
Organic materials for OSC can be derivative of small molecules or semiconducting polymers [28]. Organic semiconductors have conjugated organic moieties such as dendrimers, pigments, dyes, polymers, and small molecules, which can be a good choice for OSC. Any OSC requires two types of materials: an electron pair donor and an electron pair acceptor. Conjugated polymers are employed as donor materials. Initially, poly(1,4-phenylenevinylene) (PPV) and its derivatives gained considerable attention due to their appropriate optoelectronic properties [29][30]. Later, polythiophene and its derivative, especially poly(3-hexylthiophene), P3HT, began to be used widely (as a donor material) owing to its stability, high carrier mobility, and solubility [31][32][33]. The structures of some important donor materials for OSC technology are listed in Figure 1. Although fullerene and its derivatives dominated OSC technology in the initial phase [34][35][36][37][38], small molecules such as 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone)-5,5,11,11-tetrakis(4hexylphenyl)-dithieno [2,3-d:2′,3′-d′]-s-indaceno [1,2-b:5,6-b′]dithiophene (ITIC), IHIC, and PCDTBT (termed as non-fullerene acceptors, NFA) have attracted considerable attention recently due to their superior properties [39][40][41][42]. Moreover, donor–acceptor conjugated polymers such as poly [2,6-(4,4-bis-(2-ethylhexyl)-4H-cyclopenta [2,1-b;3,4-b′]dithiophene)-alt-4,7(2,1,3-benzothiadiazole)], PCPDTBT [43], and poly [2,7-(9,9-dioctylcarbazole)-alt-4,7-bis(thiophen-2-yl)benzo-2,1,3-thiadiazole], PCzDTBT [44] have been employed. The structures of some important fullerene and non-fullerene acceptor materials are depicted in Figure 1.
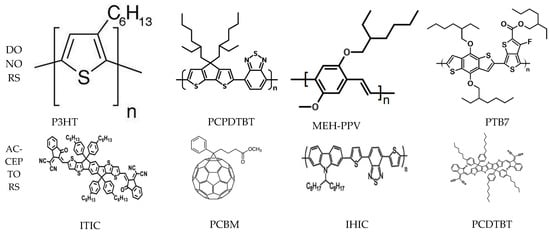
Figure 1. The structures of some important donor and acceptor materials of OSC.
The engineering of the input donor and acceptor materials can help in improving the optoelectronic properties of these materials, which, in turn, improve the performance of OSC. One of the major limitations of the donor materials for OSC is their limited capacity of absorbing the whole wavelength range of the solar spectrum. The absorption efficiency can be improved by increasing the HOMO level, decreasing the LUMO level, or by compressing the band gap between HOMO–LUMO levels [45][46]. Polymers have higher molar mass and possess longer conjugation lengths, and hence the energy band gap is lower [47][48][49]. Voltametric p-type doping can also help in improving the optoelectronic properties through broadening of the absorption spectrum [50][51][52][53]. Regioregularity of polymers facilitates the mobility of excitons and thus improve the performance of OSC [54][55][56]. Physiochemical properties of OSC materials can be easily tuned by changing their chemistry, which is a unique advantage compared with conventional inorganic solar cells. For instance, the introduction of flexible side-chains such as alkyl or alkoxy leads to an improvement of solubility while the introduction of electron-donating or -withdrawing substituents can help in the alignment of electronic energy levels. The improvement of the optoelectronic properties of polymers by chemical modifications is shown for several polymers such as poly(p-phenylene vinylene) [57] and P3HT [58][59][60]. Electron-withdrawing groups such as Fluorine on the polymer chain improve the charge transference by tuning of the LUMO level [61], while electron-donating substituents on the polymer chain enhance charge-carrier mobility by minimizing the structural defects due to an increase in the HOMO energy level [62][63][64].
Moreover, large donor–acceptor interfaces as provided by nanostructures render effective exciton dissociation and efficient charge transport [65][66][67][68][69][70][71][72]. Conjugated polymer-based metallic nanoparticles can be prepared by several methods [73][74][75]. Different metals can have peculiar and dissimilar effects on the optoelectronic properties of the nanocomposites and their performance in the context of solar cells [76][77][78][79][80]. The incorporation of silver (Ag) and gold (Au) nano particles in polymer bulk heterojunction solar cells enhances their performance owing to the improved absorption of sunlight. In the context of OSC, conjugated polymer nanowires are mostly used due to their ability to create percolation pathways for both electrons and holes, leading to higher device efficiency [81][82][83][84]. The applications of polymer nanowires have several advantages such as (a) better control over morphology, (b) appropriate matching of the exciton diffusion lengths to the widths and lengths of polymer nanowires, (c) large interfacial area between donor and acceptor, (d) existence of electrically bi-continuous morphology, (e) achievement of high absorption coefficient and high carrier mobilities, (f) possibility of manufacturing devices with high surface areas, and (g) the avoidance of blend phase-separation difficulties [85][86]. Thermal annealing of the P3HT/PCBM mixture at 120 °C for 60 min renders P3HT nanowires with improved crystallinity of P3HT and enhances the demixing between P3HT and PCBM [87].
3. Perovskite Solar Cells
3.1. The Meaning of Perovskite
Originally, the term “perovskite” referred to the calcium titanate crystal structure, which was identified in 1839 by German mineralogist Gustav Rose and given the name by Russian mineralogist Lev Perovski. Compounds with perovskite structures typically contain the formula ABX3, where ‘A’ and ‘B’ stand for cations and ‘X’ is an anion joining the two cations. These metal halide perovskites, consisting of lead (Pb2+), methyl-ammonium CH3NH3+, an organic cation, and a halide anion such as iodide (I) or bromide (Br), have drawn much attention as prospective perovskite solar-cell materials in recent years. Figure 2 displays three-dimensional (3D) and two-dimensional (2D) schematic designs as well as the usual unit cell arrangement of a perovskite compound. Usually, structures can be built from a wide variety of unique component combinations. By changing the composition of these substances, researchers may produce perovskite crystals with a variety of properties, including physical, optical, magnetic, and electrical.
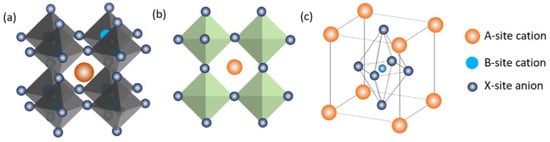
Figure 2. (a) 3D and (b) 2D schematic diagrams and (c) the schematic diagram of a perovskite unit cell [88].
In 1958, Chr. Kn. Moller first discovered halide perovskites for cesium lead halides [89]. Following this, it was also observed that small organic molecules with effective radii less than 260 pm (such as MA (CH3NH3), FA (CH(NH)2)) could also accommodate the inside of the lead halide octahedrons from the analysis of Goldschimdt’s tolerance factor/octahedral factor; additionally, Dieter Weber, in 1978, successfully synthesized MAPbX3 (X = halogens (I, Br, Cl or Mixtures of them) and superhalogen (BF4, PF6)) for the first time [90]. Thereafter, researchers started working on layered 2D perovskite systems (commonly, (R-NH3)2MX4), where the extended inorganic lead halide octahedral cage is separated by large organo-ammonium cations [91]. The resurgent hybrid perovskite was initiated by the introduction of three-dimensional hybrid perovskites (CH3NH3PbI3 and CH3NH3PbBr3) as absorbers [92] in mesoporous solar cells by Miyasaka and co-workers in 2009. Since then, researchers across the globe have used them as absorber materials in photovoltaic cells and achieved PCE as high as 25.7% in sa ingle junction [93] and 29.8% in monolithic perovskite/Si tandem solar cells. Superior optoelectronic properties such as low bandgap with direct nature, high optical absorption both in visible and IR regions, ultrahigh diffusion length, high defect tolerance, large and balanced electron-hole mobilities, high photoluminescence quantum yield, and low surface recombination velocity make them attractive candidates for solar-cell application [94][95].
Nowadays, the various structures of perovskite solar cells such as mesoporous, planar, and inverted structures have been developed. In the mesoporous solar cells, the device structure was composed of glass/fluorine-doped tin oxide (FTO)/dense electron transport layer (ETL)/mesoporous oxide layer/perovskite layer (~500 nm)/hole transport layer (HTL)/metal electrode [96][97][98]. The mesoporous TiO2 is used as an ETL material allowing perovskites to penetrate into the pores to form an interconnected absorbing layer. Additionally, titanium dioxide (TiO2) also prevents the electron-hole recombination in the FTO by blocking the holes. Experimental results revealed that other oxide materials such as zinc oxide (ZnO), aluminum oxide (Al2O3), and zirconium dioxide (ZrO2) were also used as mesoporous oxide layers. In this structure, Spiro-OMeTAD (2,2′,7,7′-Tetrakis [N, N-di(4-methoxyphenyl) amino]-9,9′-spirobifluorene) is the most used HTL and noble metals such as Au, Ag, and Pt are used as the counter electrodes. In the case of planar structure, the mesoporous ETL is removed and only two sharp interfaces are observed between perovskites and two transport layers (ETL and HTL). As a result, the rapid and effective separation of electrons and holes are observed through the ETL and HTL, respectively [99]. Due to the minimum number of interfaces, planar heterojunction structures help to understand the operating mechanisms of light absorption and electron-hole separation precisely and optimize the structure for the development of highly efficient laminated perovskite photovoltaic cells. Besides the two abovementioned structures, perovskite solar cells without HTL have become an interesting research project due to their advantages of easy and simple device fabrication and high stability [100]. These perovskite materials served the purpose of both light absorbers and hole transport channels. Using device simulation, Minemoto and Murata showed that the built-in electric field was not affected if the work function of metal electrodes was close to the valence band maximum of the light-absorbing materials for the case of HTL-free perovskite solar cells [101].
Usually, the perovskite layer is spin-coated on the substrate via either a single-step or two-step process. In the single-step process, perovskite precursor (a mixture of CH3NH3I and PbI2 dissolved in DMF:DMSO) is spin-coated onto the FTO substrate in one step and kept at 1000 C to achieve a uniform perovskite film. In recent times, an anti-solvent method is effective, in which the perovskite precursor solution is prepared in the polar solvent and quenched during the process of spin coating by a non-polar solvent. It is often observed that precise control over the volume of quenching solvent and timing is very much essential to achieve uniform morphology and optimal device performance. In the two-step process, lead iodide (PbI2) was the first to be spin-coated and then transferred into a vessel where 1 molar CH3NH3I was dissolved in isopropanol. Other than these two frequently used methods, OIHPs can also be deposited via a ‘vacuum-assisted solution process’ (VASP) in which perovskite films are coated and annealed in a chamber filled with the organic component vapor [102].
3.2. Advantages and the Challenges of Perovskite Solar Cells
Perovskite photovoltaics have a high light-absorption coefficient (104 cm−1), which results in an impressive photocurrent generation [103], long carrier diffusion length, and solution processability [104][105][106]. Perovskite solar cells are cheaper to produce, owing to their inexpensive and naturally abundant materials (lead, iodine, carbon, and hydrogen) [107]. These solar cells show better defect tolerance than other solar semiconductors on the market [108], which increases their reusability and lowers production costs. Despite their benefits, the commercialization of perovskite solar cells is hampered by another factor. Water by itself (moisture) and other polar solvents provide a serious problem for perovskite solar cells. These solvents can alter the solvated phases of a perovskite and occasionally hydrate a perovskite to produce a monohydrate phase. Polar solvents can considerably be prevented from harming perovskite solar cells by modifying deposition techniques, according to reports. However, environmental moisture is unavoidable [109].
It is observed that various external factors such as oxygen, light, and heat have expedited the degradation, which can be slowed by choosing a proper composition of the perovskite material (such as FA0.85MA0.1Cs0.05PbI2.7Br0.3, which show better stability and power conversion efficiency) [110][111][112][113]. In addition, it was noted that the deposition of more hydrophobic and UV-stable ETL/HTL has improved the stability of the device (replacing TiO2 with SnO2). The combination of Ruddlesden–Popper (RP) 2D-layered perovskites with conventional 3D perovskites has improved the stability of the device as 2D perovskite passivate/encapsulate the device [114].
On the other hand, the use of an excessive amount of lead in the perovskite component is also another big challenge in its commercialization. As an alternative, tin-based perovskites are studied, but the PCE of such devices [115] is poor (PCE ~13%). Current-voltage hysteresis between the forward and reverse bias is another major issue. Although the reason for hysteresis is still under debate, it is commonly ascribed to the combination of ion migration with high levels of charge-carrier recombination. To achieve a truly low cost-per-watt and dominate the photovoltaic market, solar cells based on OIHPs should satisfy the much-heralded trio of high-power-conversion efficiency (PCE), yearlong lifetimes, and ultra-low manufacturing costs. Certainly, OIHP-based solar-cell devices are quite farther ahead than other thin-film technologies, as they have shown enormous potential for achieving this.
4. Dye-Sensitized Solar Cells
Dye-sensitized solar cells (DSSC) are solar cells made of semiconductors that are coated with a dye to increase the efficiency of sunlight [116]. This type of solar cell was first introduced by Michael Gratzel. DSSCs are composed of a working electrode consisting of fluorine tin oxide (FTO) glass, titanium dioxide (TiO2), dye, an electrolyte consisting of an I¯/I3¯ redox pair, and a counter electrode consisting of platinum. DSSCs work in the visible region. DSSC components have undergone various developments over the years to increase their efficiency.
4.1. Advantages and Limitations of Dye-Sensitized Solar Cells
Some of the advantages offered by dye-sensitized solar cells, including their relatively low cost of fabrication, operability under scattered light conditions, and the variable shape of the cell, which can be made opaque or opaque or optically transparent, thereby providing more value from an artistic point of view [117]. Organic solar cells have dyes derived from organic and synthetic organic materials. Examples of dyes from organic materials are mangosteen, juwet fruit, water henna, nail henna, blueberries, binahong leaves, carrots, kenikir, and mangosteen peel. Synthetic organic dyes such as ruthenium complex (N719) could produce a higher efficiency of 10.4–11.1% [118][119]. However, the cost of producing ruthenium complex dyes is still relatively high, thus encouraging the development of new dye sensitizers (complexes of osmium, rhenium, iron, and iridium) [120].
The advantages of using synthetic, organic dyes include their higher conversion efficiency (an efficiency increase of up to 30% in synthetic dyes, with organic dyes being 5%), increased chemical and thermal stability, the color being difficult to degrade compared with organic dyes, and having a higher electron movement than organic dyes. There have been many studies using natural organic dyes and ruthenium dyes with a wide variety of solvents, including research conducted [121] using nano-particle TiO2 paste, ruthenium dye (N719), and counter electrodes (platinum) to produce the greatest efficiency of 0.121%. Hardani and co-workers [122] made DSSC using TiO2 doping carbon nanotubes with various concentrations and ruthenium (N719) as a dye, and the resulting efficiency was 1.3%. Another research group [123] used TiO2 nanoparticles as the active electrode and ruthenium complex (N719) as a dye and the greatest efficiency reached 2.17%.
The solid electrolyte based on PEG polymer gel (polyethylene glycol) containing redox coupling is used (to replace liquid electrolyte) to reduce electrolyte degradation. During the experiment, the doctor blade/slip-casting and spin-coating techniques were used for TiO2 coating. In comparison with other processes, this doctor blade/slip-casting method is incredibly straightforward and uncomplicated. The spin-coating technique is used to deposit homogeneous thin films on a flat substrate. While, the doctor blade/slip-casting technique is a technique for coating the TiO2 suspension on semiconductor glass utilizing a stir rod/spatula by rolling it on the glass surface to flatten the dripping TiO2 suspension. The substrate’s center, which either rotates slowly or not at all, receives a modest amount of coating material. The coating substance is subsequently dispersed throughout the substrate using centrifugal force as the substrate is rotated quickly. The device used for spin coating is called a “coater” or “spinner”. Spin coating is a straightforward TiO2-deposition technology that produces films with regulated thickness and great uniformity by rotating the TiO2 paste at a specific speed.
4.2. Definition and Fundamental of Dye-Sensitized Solar Cells
DSSC generally uses indium tin oxide (ITO) or fluorine-doped tin oxide (FTO) as a substrate. The oxide substrate layer functions as a current collector and the substrate material itself as a sealing layer between the cells in the DSSC and the outside air [124].
Titanium dioxide (TiO2) is the material of choice used as a working electrode or photoelectrode in DSSC. The three modifications of TiO2 include anatase, rutile, and brookite, but only anatase and rutile are stable [125]. Brookite is difficult to produce and therefore is not considered in DSSC applications [126]. The particle sizes of anatase and rutile increase[127] with increasing temperature. Figure 3, showing different growth rates, shows that rutile has a much higher growth rate than anatase. The growth rate of anatase is flat at 800 °C.
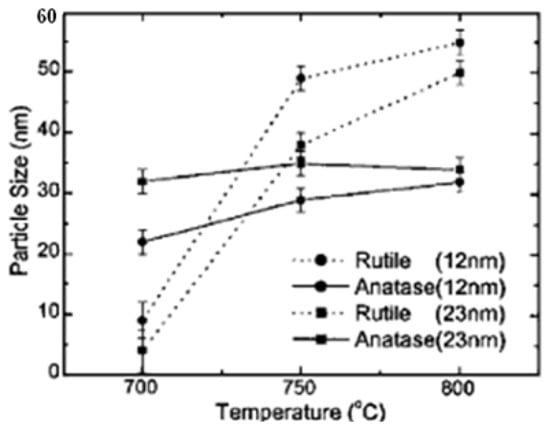
Figure 3. Changes in the particle size of anatase and rutile as a function of annealing temperature [127].
The absorbance spectrum defines the possibility of a useful semiconductor for photocatalysts having a band gap (Eg) proportional to the photon energy of the visible or ultraviolet spectrum (Eg < 3.5 eV). Most authors have determined that the energy gap of rutile is 3.0 eV and that of anatase is 3.2 eV. A larger active surface area and a more effective photocatalyst [128] can be observed in the anatase phase.
The photocatalytic process is based on the dual ability of a semiconductor material (TiO2) to absorb photons and carry out transformation reactions at the material junction simultaneously. Figure 4 shows several photoexcitation pathways [129] followed by electron and hole de-excitation. The enlarged portion of Figure 4 shows electrons excited from the valence band (Ev) to the conduction band (Ec) due to photons with energy (hv) equal to or greater than the band gap of the semiconductor.
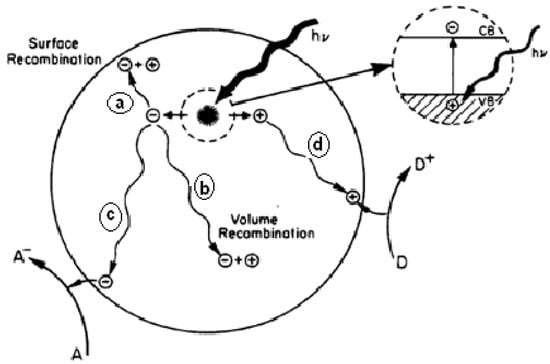
Figure 4. The schematic of photoexcitation followed by de-excitation on the semiconductor’s surface [129].
From Figure 4, it is assumed that the semiconductor remains intact, and the charge transfer is uniform to the adsorbed organic or inorganic molecules. The excited electron-hole pair can go through several paths as follows: the semiconductor can donate electrons on the surface to reduce the adsorbed acceptor (A/A−), for example, oxygen (lane c). Holes can migrate to the surface and electrons from donors (D/D+) can join holes (c lanes). Competition for electron transfer to adsorbed molecules by the separate recombination of electrons and holes occurs in the volume of the semiconductor particles (band b) or on the semiconductor’s surface (lane a).
Electron paramagnetic resonance spectroscopy (EPR) showed that the detected electrons were either captured as Ti3+ or as electrons in the conduction band, while the holes were trapped in the O− free-oxygen center resulting from the O2− lattice in the valence band. The reaction is written as follows [130]:
5. Thin-Film Solar Cells
Currently, there are different types of thin-film materials that have been and are employed in solar-cell applications. Technically speaking, these materials have several advantages such as low production cost, being environmentally friendly, and the formation of films can be conducted in various substrates [131].
5.1. CdTe Film Solar Cells
Cadmium telluride (CdTe) films show high degradation durability, near-optimum band-gap value (1.45 eV), a low production cost, and high direct absorption coefficient (104 cm−1), and can be used to replace silicon-based solar cells. Generally, CdTe-based solar cells consist of glass, transparent conducting oxide (serving as the front contact), a CdS window layer, a CdTe absorber layer, and back contact. However, this type of solar cell has many problems such as glass breakage (during the production process) and poor thermal conductivity (causing very poor performance). The preparation of CdTe films onto flexible metal foils and polymer substrate can solve these problems. The power conversion efficiency was shown to be 11% for devices prepared on polyimide foils [132], indicating the superstrate and substrate configurations. Several deposition methods have been reported to produce cadmium-tellurium thin films (Table 1). The photovoltaic properties of the obtained films were studied.
Table 1. The growth of CdTe films using various deposition methods and the photovoltaic properties.
| Deposition Technique | Photovoltaic Properties | Reference | |||
|---|---|---|---|---|---|
| Voc (mV) | Isc (mA/cm2) | PEC (%) | FF (%) | ||
| Electro deposition | 723 | 18.7 | 8.6 | 64 | [133] |
| Electro deposition | 700 | 18.1 | 6 | - | [134] |
| Electro deposition | 250–290 | 4.12–6.35 | 0.28–0.92 | 25–52 | [135] |
| Thermal-evaporation method | 519 | 18.19 | 6.4 | 45.6 | [136] |
| Evaporation method | 863 | 27.1 | 18.7 | 80.5 | [137] |
| Glancing angle-deposition method | 774 | 23 | 11 | 61.9 | [138] |
| Vapor-transport deposition | 806–839 | 20.2–21.6 | 12.5–13.5 | 73.3–76.9 | [139] |
| Vapor-transport deposition | 683–839 | 22.2–22.9 | 9.2–14.4 | 60.6–75 | [140] |
| Sputtering method | - | - | 11.2 | - | [141] |
| Radio-frequency sputtering | - | - | 14 | - | [142] |
| Laser-ablation method | 600 | 13 | 3 | 40 | [143] |
| Pulsed-laser deposition | - | - | 6.68 | - | [144] |
5.2. Cu(In,Ga)Se2 Film Solar Cells
The Cu(In,Ga)Se2 (CIGS) films are already available in the global solar panel market due to their excellent radiation tolerance, high absorption coefficient (105 cm−1), suitable band gap value (1.04 eV to 1.65 eV), and long-term stability. However, these films are very expensive because of the indium and gallium (poor abundance). Several deposition techniques have been reported for the preparation of CIGS films and the photovoltaic parameters were studied as well (Table 2). Thin films could be deposited onto rigid glass substrates, and successfully reached power conversion efficiencies of up to 20.3%. However, the current focus of the production of films onto flexible substrates (metal foils and polyimide films) is because of their significantly lower cost. Polyimide films are highly desirable due to electrical insulation and enabling direct monolithic interconnection.
Table 2. The growth of CIGS films using various deposition methods and the photovoltaic properties.
| Deposition Technique | Photovoltaic Properties | Reference | |||
|---|---|---|---|---|---|
| Voc (mV) | Isc (mA/cm2) | PEC (%) | FF (%) | ||
| Co-evaporation method | - | - | 6.46–9.78 | - | [145] |
| chemical bath deposition method | 760 | 0.059 | 0.011 | 25.1 | [146] |
| Co-sputtering method | 551–553 | 22.7–29.5 | 9.1–11.1 | 68.4–72.3 | [147] |
| Vacuum evaporation method | 712 | 34.8 | 18.7 | 75.7 | [148] |
| Chemical vapor deposition | - | - | 9.91 | 64.75 | [149] |
| Spin-coating method | - | - | 10 | - | [150] |
| Sputtering and evaporation method | - | - | 20.4 | - | [151] |
| Vacuum evaporation | - | - | 17.1 | - | [152] |
| Co-precipitation method | 168.2 | 8.11 | 0.4 | 27.6 | [153] |
| Inkjet printing method | 386 | 29.78 | 5.04 | 44 | [154] |
| Evaporation method | - | - | 10.6 | - | [155] |
| Electro-deposition method | - | - | 9.4 | - | [156] |
| Electro-deposition method | 666 | 30.5 | 15.4 | 75.6 | [157] |
| Electro-deposition method | 413 | 32.5 | 6.6 | 49 | [158] |
| Electro-deposition method | 741 | 23.2 | 10.2 | 59.6 | [159] |
| Electro-deposition method | - | - | 4 | - | [160] |
| Electro-deposition method | - | - | 4.5 | - | [161] |
| Electro-deposition method | - | - | 1.63 | - | [162] |
| Electro-deposition method | - | 0.143–0.376 | 0.079–0.403 | 0.384–0.526 | [163] |
| DC-magnetron sputtering method | 553 | 30.18 | 11.28 | 68 | [164] |
5.3. Cu2ZnSnS4 Film Solar Cells
Copper–zinc–tin sulfide (Cu2ZnSnS4) films are becoming increasingly prominent for several reasons. These materials could replace indium and gallium in CIGS films, as their constituents are Earth-abundant and do not contain cadmium (toxic material). The experimental results showed that CZTS-based solar cells have an excellent absorption coefficients (more than 104 cm−1), tunable band gap values (1.45 eV to 1.6 eV), and greater stability in the kesterite phase (Figure 5) when compared with stannite and wurtzite. Researchers have reported that vacuum and non-vacuum deposition techniques have been used to produce CZTS films (Table 3). Further, they concluded that high-quality films could be synthesized using the vacuum-deposition method; however, this entails increased production costs.
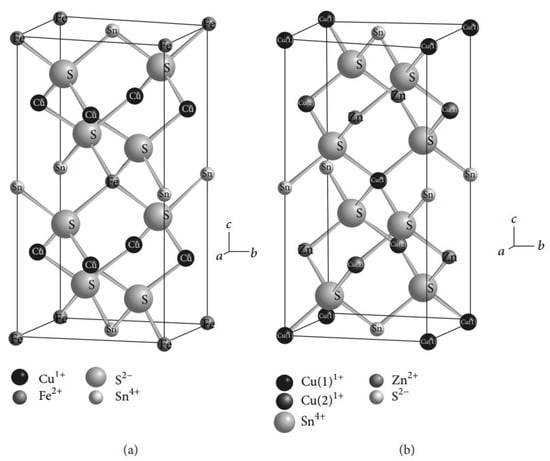
Figure 5. Crystal structures of (a) stannite and (b) kesterite [165].
Table 3. The growth of CZTS films using various deposition methods and the photovoltaic properties.
| Deposition Technique | Photovoltaic Properties | Reference | |||
|---|---|---|---|---|---|
| Voc (mV) | Isc (mA/cm2) | PEC (%) | FF (%) | ||
| Pulsed-laser deposition | 376 | 38.9 | 5.85 | 40 | [166] |
| Chemical route | 521 | 19.13 | 6.17 | 62 | [167] |
| Nanoparticle ink technique | - | - | 2.1 | - | [168] |
| Electro-deposition method | 563 | 14.8 | 3.4 | 41 | [169] |
| Pulsed hybrid electro-deposition method | 350 | 3.9 | 0.59 | 43 | [170] |
| Sputtering method | - | - | 2.95–3.59 | - | [171] |
| SILAR method | - | - | 0.76–1.24 | - | [172] |
| reactive-pulsed dc magnetron co-sputtering | 667 | 19.6 | 7.9 | 60 | [173] |
| Electro-deposition method | 204 | 7.6 | 0.55 | 35.68 | [174] |
| Spin-coating method | - | - | 0.5–2.4 | - | [175] |
| magnetron sputtering method | 666 | 7.9 | 2.26 | 43.39 | [176] |
| Spin-coating method | 556 | 13.5 | 3.02 | 40.3 | [177] |
| Spray pyrolysis | 246–421 | 3.9–10.3 | 0.4–1.4 | 32.5–39 | [178] |
| Spin-coating method | 520 | 112.52 | 0.28 | 26.75 | [179] |
| Pulsed-laser deposition | - | - | 6.62 | - | [180] |
| Sulfurization technique | 610 | 12 | 4.5 | 62 | [181] |
5.4. CuInX (X = S, Se, and Te) Film Solar Cells
Several deposition methods have been used to produce CuInSe2, CuInTe2, and CuInS2 films (Table 4). It is noted that the non-vacuum deposition method offers an attractive cost-saving opportunity, higher deposition speed, and less waste of chemicals when compared with the vacuum-deposition technique. The obtained films could be employed for solar-cell applications because of long-term stability under solar radiation, excellent absorption coefficients (105 cm−1) in the visible light portion, and suitable band gap values (1.5 eV).
Table 4. The growth of CuInX (X = S, Se, and Te) films using various deposition methods and the photovoltaic properties.
| Deposition Technique | Photovoltaic Properties | Reference | |||
|---|---|---|---|---|---|
| Voc (mV) | Isc (mA/cm2) | PEC (%) | FF (%) | ||
| Electrostatic spray deposition | 230 | 21.72 | 1.75 | 34 | [182] |
| Spray-coating method | - | - | 1.1–1.9 | - | [183] |
| Electro-deposition method | - | - | 1.6 | - | [184] |
| Solution-coating process | 420 | 36.39 | 7.72 | 50.5 | [185] |
| Spin-coating method | - | - | 4.19 | - | [186] |
| Electro-deposition method | 280 | 4.06 | 0.41 | 36.72 | [187] |
| Pulsed electro-deposition | 440 | 33.5 | 5.2 | 38 | [188] |
| Chemical liquid-deposition | - | - | 2 | - | [189] |
| Doctor blade-coating method | - | - | 2 | - | [190] |
| Solvo-thermal method | 130 | 20.72 | 0.735 | 27.3 | [191] |
| Spin-coating method | 780 | - | 6.31 | - | [192] |
| Thermal co-evaporation method | - | - | 10.2 | - | [193] |
| Ink-based deposition | 320 | 18.49 | 2.15 | 37 | [194] |
| Ink-based deposition | - | - | 2.11 | - | [195] |
| Co-precipitation method | - | - | 0.83–1.27 | - | [196] |
| Colloidal synthetic method | 439–471 | - | 0.65–1.45 | - | [197] |
| Solvothermal method | 268 | 4 | 0.75 | 56 | [198] |
| Electro-deposition method | 480 | 20 | 4.13 | 43 | [199] |
| Electro-deposition method | - | - | 3.4–3.8 | - | [200] |
5.5. Other Metal Chalcogenide Film Solar Cells
Several types of metal sulfide, metal telluride, and metal selenide films have been prepared using different deposition methods. The photovoltaic behavior of these films was studied (Table 5) as reported by many researchers.
Table 5. The growth of different types of thin films using various deposition methods and the photovoltaic properties.
| Deposition Technique | Thin Films | Photovoltaic Properties | Reference | |||
|---|---|---|---|---|---|---|
| Voc (mV) | Isc (mA/cm2) | PEC (%) | FF (%) | |||
| Spin-coating method | Cu2ZnSn(S,Se)4 | 361 | 32 | 6.4 | 55.4 | [201] |
| Radio frequency sputtering | Cu2ZnSn(S,Se)4 | 349 | 30.6 | 4.93 | 46.13 | [202] |
| Dip technique | CdSe | - | - | 1.01 | 33.44 | [203] |
| Rapid thermal-evaporative method | CdSe | 222–501 | 0.17–6.45 | 0.02–1.88 | 50–58.1 | [204] |
| Thermal-evaporative method | Sb2S3 | 330–470 | 0.66–7.8 | 0.08–1.57 | 39.01–42.45 | [205] |
| Hydrothermal method | In2S3 | 320 | 23.43 | 2.39 | 31.82 | [206] |
| Hydrothermal method | Cu2SnS3 | 596 | 10.7 | 1.58 | 24.8 | [207] |
| Sol–gel method | Cu2SnS3 | 276 | 11.6 | 1.12 | 35 | [208] |
| Pulsed-chemical vapor deposition | SnS | 244 | 19.4 | 2.04 | 42.97 | [209] |
| Radio-frequency sputtering | SnS | - | - | 0.38 | - | [210] |
| Radio-frequency sputtering | SnS | 450 | 17.13 | 5.24 | 68 | [211] |
| Solution processed | CuSbS2 | - | - | 1.72 | - | [212] |
| Mechanochemical and doctor-blade technique | Cu4SnS4 | 300 | 29.2 | 2.34 | 26 | [213] |
| chemical vapor deposition | PbS | 520 | 1.93 | 0.29 | 29 | [214] |
| Nebulizer-spray method | PbS | - | - | 0.39 | - | [215] |
| Chemical-bath deposition | Ni3Pb2S2 | 610 | 9.9 | 2.7 | 47 | [216] |
| Chemical-bath deposition | ZnTe | 150 | 25.6 | 0.49 | 24.86 | [217] |
This entry is adapted from the peer-reviewed paper 10.3390/app13042051
References
- Mohd, R.; Santosh, B.; Suvarna, S.; Pooja, V.; Anil, T. A review paper on electricity generation from solar energy. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 1884–1889.
- Ho, S.M. Power conversion efficiency in thin film solar cell: A review. Int. J. Chem. Sci. 2016, 14, 143–151.
- Ho, S.M. A review of metal oxide thin films in solar cell applications. Int. J. Thin Film Sci. Technol. 2022, 11, 37–45.
- Ahmad, K.S.; Naqvi, S.N.; Jaffri, S.B. Systematic review elucidating the generations and classifications of solar cells contributing towards environmental sustainability integration. Rev. Inorg. Chem. 2021, 41, 21–39.
- Hong, Z.; Dou, L.; Li, G.; Yang, Y. Tandem Solar Cell—Concept and Practice in Organic Solar Cells. In Progress in High-Efficient Solution Process Organic Photovoltaic Devices: Fundamentals, Materials, Devices and Fabrication; Yang, Y., Li, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 315–346.
- Kippelen, B.; Brédas, J.L. Organic photovoltaics. Energy Environ. Sci. 2009, 2, 251–261.
- Renee, K.; Diaz, A.; Eva, O. A new tetracyclic lactam building block for thick, broad band gap photovoltaics. J. Am. Chem. Soc. 2014, 136, 11578–11581.
- Kim, J.; Lee, J.; Jung, H.; Shin, H. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918.
- Nandi, P.; Giri, C.; Joseph, B.; Rath, S. CH3NH3PbI3, A Potential Solar Cell Candidate: Structural and Spectroscopic Investigations. J. Phys. Chem. A 2016, 120, 9732–9739.
- Lee, W.; Seo, S.; Nandi, P.; Jung, H. Dynamic Structural Property of Organic-Inorganic Metal Halide Perovskite. iScience 2021, 24, 101959.
- Carmen, C.; Pascasio, D.; Latini, A.; Matteo, B. Nanostructured semiconductor materials for dye sensitized solar cells. J. Nanomater. 2017, 2017, 5323164.
- Wood, J.; Summers, H.; Gibson, E. Increased photocurrent in a tandem dye-sensitized solar cell by modifications in push–pull dye-design. Chem. Commun. 2015, 51, 3915–3918.
- Majd, S.; Ahed, Z.; Mna, H. Assessment of flexible pristine CdS film electrodes in photo electrochemical light to electricity conversions. Mater. Chem. Phys. 2022, 293, 126967.
- Anuar, K.; Saravanan, N.; Ho, S.M.; Noraini, K. XRD and AFM studies of ZnS thin films produced by electrodeposition method. Arab. J. Chem 2010, 3, 243–249.
- Li, Y.; Huang, W.; Zhao, D.; Wang, L.; Jiao, Z.; Huang, Q.; Wang, P.; Sun, M.; Yuan, G. Recent Progress in Organic Solar Cells: A Review on Materials from Acceptor to Donor. Molecules 2022, 27, 1800.
- Mohammad, A. Introduction to Organic Solar Cells. Sustain. Energy 2014, 2, 85–90.
- Paula, T.; Fatima, M. Recent advances in polymer structures for organic solar cells: A review. AIMS Energy 2022, 10, 149–176.
- Hu, Z.; Wang, J.; Ma, X. A critical review on semitransparent organic solar cells. Nano Energy 2020, 78, 105376.
- Yeh, N.; Yeh, P. Organic solar cells: Their developments and potentials. Renew. Sust. Energ. Rev. 2013, 21, 421–431.
- Amin, N. Introduction of inorganic solar cells. In Comprehensive Guide on Organic and Inorganic Solar Cells: Fundamental Concepts to Fabrication Methods; Akhtaruzzaman, M., Selvanathan, V., Eds.; Academic Press: Cambridge, UK, 2022; pp. 57–63.
- Zakutayev, A.; Major, J.D.; Hao, X.; Walsh, A. Emerging inorganic solar cell efficiency tables (version 2). J. Phys. Energy 2021, 3, 032003.
- Wong, L.H.; Zakutayev, A.; Major, J.D.; Hao, X. Emerging inorganic solar cell efficiency tables (Version 1). J. Phys. Energy 2019, 1, 032001.
- Abdulrazzaq, O.A.; Saini, V.; Bourdo, S.; Dervishi, E. Organic Solar Cells: A Review of Materials, Limitations, and Possibilities for Improvement. Part. Sci. Technol. 2013, 31, 427–442.
- Dey, S. Recent Progress in Molecular Design of Fused Ring Electron Acceptors for Organic Solar Cells. Small 2019, 15, 1900134.
- Michaels, H.; Benesperi, I.; Freitag, M. Challenges and prospects of ambient hybrid solar cell applications. Chem. Sci. 2021, 12, 5002–5015.
- Khokhar, M.Q.; Hussain, S.Q.; Chowdhury, S.; Zahid, M.A. High-efficiency hybrid solar cell with a nano-crystalline silicon oxide layer as an electron-selective contact. Energy Convers. Manag. 2022, 252, 115033.
- Van, P.; Anh, N.; Cham, T.; Tu, L.T. Enhanced power conversion efficiency of an n-Si/PEDOT:PSS hybrid solar cell using nanostructured silicon and gold nanoparticles. RSC Adv. 2022, 12, 10514–10521.
- Ameri, T.; Li, N.; Brabec, C.J. Highly efficient organic tandem solar cells: A follow up review. Energy Environ. Sci. 2013, 6, 2390–2413.
- Shaheen, S.; Brabec, J.; Sariciftci, N. 2.5% efficient organic plastic solar cells. Appl. Phys. Lett. 2001, 78, 841.
- Seyler, H.; Wong, W.; Jones, D.; Holmes, A. Continuous Flow Synthesis of Fullerene Derivatives. J. Org. Chem. 2011, 76, 3551–3556.
- Liang, Z.; Cheng, X.; Jiang, Y.; Yu, J. P3HT-Based Organic Solar Cells with a Photoresponse to 1000 nm Enabled by Narrow Band Gap Nonfullerene Acceptors with High HOMO Levels. ACS Appl. Mater. Interfaces 2021, 13, 61487–61495.
- Andersen, T.; Weyhe, A.; Tao, Q.; Zhao, F. Novel cost-effective acceptor:P3HT based organic solar cells exhibiting the highest ever reported industrial readiness factor. Mater. Adv. 2020, 1, 658–665.
- Chatterjee, S.; Jinnai, S.; Ie, Y. Nonfullerene acceptors for P3HT-based organic solar cells. J. Mater. Chem. A 2021, 9, 18857–18886.
- Abdallaoui, M.; Sengouga, N.; Chala, A. Comparative study of conventional and inverted P3HT: PCBM organic solar cell. Opt. Mater. 2020, 105, 109916.
- Benduhn, J.; Tvingstedt, K.; Piersimoni, F.; Ullbrich, S. Intrinsic non-radiative voltage losses in fullerene-based organic solar cells. Nat. Energy 2017, 2, 17053.
- Xiao, T.; Cui, W.; Anderegg, J.; Shinar, J. Simple routes for improving polythiophene:fullerene-based organic solar cells. Org. Electron. 2011, 12, 257–262.
- Ganesamoorthy, R.; Sathiyan, G.; Sakthivel, P. Review: Fullerene based acceptors for efficient bulk heterojunction organic solar cell applications. Sol. Energy Mater. Sol. Cells 2017, 161, 102–148.
- Duan, L.; Yi, H.; Zhang, Y.; Haque, F. Comparative study of light- and thermal-induced degradation for both fullerene and non-fullerene-based organic solar cells. Sustain. Energy Fuels 2019, 3, 723–735.
- Firdaus, Y.; Le, M.; Khan, J.I.; Kan, Z. Key Parameters Requirements for Non-Fullerene-Based Organic Solar Cells with Power Conversion Efficiency >20%. Adv. Sci. 2019, 6, 1802028.
- Sorrentino, R.; Kozma, E.; Luzzati, S.; Po, R. Interlayers for non-fullerene-based polymer solar cells: Distinctive features and challenges. Energy Environ. Sci. 2021, 14, 180–223.
- Xue, P.; Cheng, P.; Han, R.; Zhan, X. Printing fabrication of large-area non-fullerene organic solar cells. Mater. Horiz. 2022, 9, 194–219.
- Armin, A.; Li, W.; Sandberg, J.; Xiao, Z. A History and Perspective of Non-Fullerene Electron Acceptors for Organic Solar Cells. Adv. Energy Mater. 2021, 11, 2003570.
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497–500.
- Leclerc, N.; Michaud, A.; Sirois, K.; Morin, J. Synthesis of 2,7-Carbazolenevinylene-Based Copolymers and Characterization of Their Photovoltaic Properties. Adv. Funct. Mater. 2006, 16, 1694–1704.
- Gao, Y.; Liu, M.; Zhang, Y.; Liu, Z. Recent Development on Narrow Bandgap Conjugated Polymers for Polymer Solar Cells. Polymers 2017, 9, 39.
- Kroon, R.; Zgeng, T.; Jing, W. Relating open circuit voltage losses to the active layer morphology and contact selectivity in organic solar cells. J. Mater. Chem. A 2018, 6, 12574–12581.
- Ballantyne, A.M.; Chen, L.; Dane, J.; Hammant, T. The Effect of Poly (3-hexylthiophene) Molecular Weight on Charge Transport and the Performance of Polymer: Fullerene Solar Cells. Adv. Funct. Mater. 2008, 18, 2373–2380.
- Schilinsky, P.; Asawapirom, U.; Scherf, U.; Biele, M. Influence of the molecular weight of poly (3-hexylthiophene) on the performance of bulk heterojunction solar cells. Chem. Mater. 2005, 17, 2175–2180.
- Trznadel, M.; Pron, A.; Zagorska, M.; Chrzaszcz, R. Effect of molecular weight on spectroscopic and spectroelectrochemical properties of regioregular poly (3-hexylthiophene). Macromolecules 1998, 31, 5051–5058.
- Jenkins, J.L.; Lee, P.A.; Nebesny, K.W.; Ratcliff, E.L. Systematic electrochemical oxidative doping of P3HT to probe interfacial charge transfer across polymer–fullerene interfaces. J. Mater. Chem. A 2014, 2, 19221–19231.
- Ratcliff, E.L.; Lee, P.A.; Armstrong, N.R. Work function control of hole-selective polymer/ITO anode contacts: An electrochemical doping study. J. Mater. Chem. 2010, 20, 2672–2679.
- Skompska, M.; Szkurłat, A. The influence of the structural defects and microscopic aggregation of poly (3-alkylthiophenes) on electrochemical and optical properties of the polymer films: Discussion of an origin of redox peaks in the cyclic voltammograms. Electrochim. Acta 2001, 46, 4007–4015.
- Bobade, R.S. Polythiophene composites: A review of selected applications. J. Polym. Eng. 2011, 31, 209–215.
- Loewe, R.S.; Ewbank, P.C.; Liu, J.; Zhai, L. Regioregular, head-to-tail coupled poly (3-alkylthiophenes) made easy by the GRIM method: Investigation of the reaction and the origin of regioselectivity. Macromolecules 2001, 34, 4324–4333.
- Chen, T.; Wu, X.; Rieke, R.D. Regiocontrolled synthesis of poly (3-alkylthiophenes) mediated by Rieke zinc: Their characterization and solid-state properties. J. Am. Chem. Soc. 1995, 117, 233–244.
- Ansari, M.A.; Mohiuddin, S.; Kandemirli, F.; Malik, M.I. Synthesis and characterization of poly(3-hexylthiophene): Improvement of regioregularity and energy band gap. RSC Adv. 2018, 8, 8319–8328.
- Brédas, J.L.; Heeger, A.J. Influence of donor and acceptor substituents on the electronic characteristics of poly(paraphenylene vinylene) and poly(paraphenylene). Chem. Phys. Lett. 1994, 217, 507–512.
- Hou, J.; Tan, Z.; Yan, Y.; He, Y. Synthesis and Photovoltaic Properties of Two-Dimensional Conjugated Polythiophenes with Bi(thienylenevinylene) Side Chains. J. Am. Chem. Soc. 2006, 128, 4911–4916.
- Zhang, M.; Guo, X.; Yang, Y.; Zhang, J. Downwards tuning the HOMO level of polythiophene by carboxylate substitution for high open-circuit-voltage polymer solar cells. Polym. Chem. 2011, 2, 2900–2906.
- Ansari, M.A.; Hafeez, A.; Mustafa, M.; Wijesundera, R. Molecular tailoring of donor and acceptor materials of organic solar cells for improvement of their optoelectronic properties. Mater. Sci. Semicond. Process. 2022, 150, 106919.
- Zhou, H.; Yang, L.; Stuart, A.C.; Price, S. Development of Fluorinated Benzothiadiazole as a Structural Unit for a Polymer Solar Cell of 7% Efficiency. Angew. Chem. Int. 2011, 50, 2995–2998.
- Eakins, G.L.; Alford, J.S.; Tiegs, B.J.; Breyfogle, B.E. Tuning HOMO–LUMO levels: Trends leading to the design of 9-fluorenone scaffolds with predictable electronic and optoelectronic properties. J. Phys. Org. Chem. 2011, 24, 1119–1128.
- Perepichka, D.F.; Bryce, M. Molecules with Exceptionally Small HOMO–LUMO Gaps. Angew. Chem. Int. 2005, 44, 5370–5373.
- Huang, Y.; Guo, X.; Liu, F.; Huo, L. Improving the Ordering and Photovoltaic Properties by Extending π–Conjugated Area of Electron-Donating Units in Polymers with D-A Structure. Adv. Mater. 2012, 24, 3383–3389.
- Kroon, R.; Lenes, M.; Hummelen, J.C.; Blom, P.W.M.; de Boer, B. Small Bandgap Polymers for Organic Solar Cells (Polymer Material Development in the Last 5 Years). Polym. Rev. 2008, 48, 531–582.
- Takakazu, Y.; Ken, S.; Akio, Y. Preparation and Characterization of Poly(thienylene)s. Bull. Chem. Soc. Jpn. 1983, 56, 1497–1502.
- Deshmukh, M.A.; Park, S.; Hedau, B.S.; Ha, T. Recent progress in solar cells based on carbon nanomaterials. Sol. Energy 2021, 220, 953–990.
- Anrango, C.; Pavón, K.; Frontana, B.A. Recent Advances in Hole-Transporting Layers for Organic Solar Cells. Nanomaterials 2022, 12, 443.
- Wei, Z.; Chen, L.; Liu, K.; Liu, S. Two-Dimensional Nanomaterials for Boosting the Performance of Organic Solar Cells. Coatings 2021, 11, 1530.
- Zhu, Y.; Chen, C.; Cheng, P.; Ma, J. Recent advances in hydrothermal synthesis of facet-controlled CeO2-based nanomaterials. Dalton Trans. 2022, 51, 6506–6518.
- Lee, J.M.; Kim, S.O. Enhancing Organic Solar Cells with Plasmonic Nanomaterials. ChemNanoMat 2016, 2, 19–27.
- Sreedhar, R.; Mehdizadeh, H.; Ompong, D.; Setsoafia, D. Characterising Exciton Generation in Bulk-Heterojunction Organic Solar Cells. Nanomaterials 2021, 11, 209.
- Kurokawa, N.; Yoshikawa, H.; Hirota, N.; Hyodo, K. Size-dependent spectroscopic properties and thermochromic behavior in poly(substituted thiophene) nanoparticle. Chemphyschem 2004, 5, 1609–1615.
- Szymanski, C.; Wu, C.; Hooper, J.; Salazar, M.A. Single Molecule Nanoparticles of the Conjugated Polymer MEH−PPV, Preparation and Characterization by Near-Field Scanning Optical Microscopy. J. Phys. Chem. B 2005, 109, 8543–8546.
- Wu, C.; Szymanski, C.; McNeill, J. Preparation and Encapsulation of Highly Fluorescent Conjugated Polymer Nanoparticles. Langmuir 2006, 22, 2956–2960.
- Liu, Y.; Zhao, J.; Li, Z. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293.
- He, Z.; Xiao, B.; Liu, F. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179.
- Lu, L.; Luo, Z.; Xu, T.; Yu, L. Cooperative Plasmonic Effect of Ag and Au Nanoparticles on Enhancing Performance of Polymer Solar Cells. Nano Lett. 2013, 13, 59–64.
- Jbira, E.; Derouiche, H.; Missaoui, K. Enhancing effect of silver nanoparticles (AgNPs) interfacial thin layer on silicon nanowires (SiNWs)/PEDOT: PSS hybrid solar cell. Sol. Energy 2020, 211, 1230–1238.
- Thomas, A.; Vinayakan, R.; Ison, V.V. An inverted ZnO/P3HT:PbS bulk-heterojunction hybrid solar cell with a CdSe quantum dot interface buffer layer. RSC Adv. 2020, 10, 16693–16699.
- Lee, H.Y.; Kim, S. Nanowires for 2D material-based photonic and optoelectronic devices. Nanophotonics 2022, 11, 2571–2582.
- Yang, J.; Yu, F.; Chen, A.; Zhao, S. Synthesis and application of silver and copper nanowires in high transparent solar cells. Adv. Powder Mater. 2022, 1, 100045.
- Wu, F.; Liu, Y.; Zhang, J.; Duan, S. Recent Advances in High-Mobility and High-Stretchability Organic Field-Effect Transistors: From Materials, Devices to Applications. Small Methods 2021, 5, 2100676.
- Wang, L.; Wen, L.; Tong, Y.; Wang, S. Photo-rechargeable batteries and supercapacitors: Critical roles of carbon-based functional materials. Carbon Energy 2021, 3, 225–252.
- Xin, H.; Kim, F.S.; Jenekhe, S.A. Highly Efficient Solar Cells Based on Poly(3-butylthiophene) Nanowires. J. Am. Chem. Soc. 2008, 130, 5424–5425.
- Xin, H.; Reid, O.G.; Ren, G.; Kim, F.S. Polymer Nanowire/Fullerene Bulk Heterojunction Solar Cells: How Nanostructure Determines Photovoltaic Properties. ACS Nano 2010, 4, 1861–1872.
- Yang, X.; Loos, J.; Veenstra, S.C.; Verhees, W. Nanoscale Morphology of High-Performance Polymer Solar Cells. Nano Lett. 2005, 5, 579–583.
- Yu, Y.; Xia, J.; Liang, Y. Basic understanding of perovskite solar cells and passivation mechanism. AIP Adv. 2022, 12, 055307.
- Moller, K. Crystal Structure and Photoconductivity of Cæsium Plumbohalides. Nature 1958, 182, 1436.
- Weber, D. CH3NH3PbX3, a Pb(II)-System with Cubic Perovskite Structure. Z. Naturforschung B 1978, 33, 1443–1445.
- Arend, H.; Huber, W.; Mischgofsky, F.; Richter, K. Layer Perovskites of the (CnH2n+1NH3)2MX4 and NH3(CH2)mNH3MX4 Families with M = Cd, Cu, Fe, Mn OR Pd and X = Cl OR Br: Importance, Solubilities and Simple Growth Techniques. J. Cryst. Growth 1978, 43, 213–223.
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051.
- NREL. Best Research-Cell Efficiences. 2022. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 30 November 2022).
- Nandi, P.; Giri, C.; Swain, D. Room Temperature Growth of CH3NH3PbCl3 Single Crystals by Solvent Evaporation Method. CrystEngComm 2019, 21, 656–661.
- Nandi, P.; Topwal, D.; Park, N.G.; Shin, H. Organic-Inorganic Hybrid Lead Halides as Absorbers in Perovskite Solar Cells: A Debate on Ferroelectricity. J. Phys. D Appl. Phys. 2020, 53, 493002.
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry, R. Sequential Deposition as a Route to High-Performance Perovskite-Sensitized Solar Cells. Nature 2013, 499, 316–319.
- Nandi, P.; Giri, C.; Bansode, U.; Topwal, D. CH3NH3PbI3 Based Solar Cell: Modified by Antisolvent Treatment. AIP Conf. Proc. 2017, 1832, 080065.
- Nandi, P.; Giri, C.; Bansode, U.; Topwal, D. Study on CH3NH3PbI3 Based Perovskite Solar Cell: An Approach to Antisolvent Treatment under Ambient Condition. Recent Trends Chem. Mater. Sci. 2021, 3, 24–30.
- Liu, D.; Kelly, T.L. Perovskite Solar Cells with a Planar Heterojunction Structure Prepared Using Room-Temperature Solution Processing Techniques. Nat. Photonics 2014, 8, 133–138.
- Shi, J.; Dong, J.; Lv, S.; Xu, Y. Hole-Conductor-Free Perovskite Organic Lead Iodide Heterojunction Thin-Film Solar Cells: High Efficiency and Junction Property. Appl. Phys. Lett. 2014, 104, 63901.
- Minemoto, T.; Murata, M. Impact of Work Function of Back Contact of Perovskite Solar Cells without Hole Transport Material Analyzed by Device Simulation. Curr. Appl. Phys. 2014, 14, 1428–1433.
- Mohamad, N.; Arzaee, A.; Nawas, M.; Teridi, M. Superiority of Two-Step Deposition over One-Step Deposition for Perovskite Solar Cells Processed in High Humidity Atmosphere. Opt. Mater. 2021, 118, 111288.
- Meng, L.; You, J.; Guo, T. Recent Advances in the Inverted Planar Structure of Perovskite Solar Cells. Acc. Chem. Res. 2016, 49, 155–165.
- Park, N. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72.
- Tablero, C. Absorption coefficients data of lead iodine perovskites using 14 different organic cations. Data Br. 2019, 27, 104636.
- Zhang, P.; Li, M.; Chen, W. A Perspective on Perovskite Solar Cells: Emergence, Progress, and Commercialization. Front. Chem. 2022, 10, 802890.
- Meng, L.; You, J.; Yang, Y. Addressing the stability issue of perovskite solar cells for commercial applications. Nat. Commun. 2018, 9, 5265.
- Ball, J.; Petrozza, A. Defects in perovskite-halides and their effects in solar cells. Nat. Energy 2016, 1, 16149.
- Askar, M.; Bernard, G.; Wiltshire, B. Multinuclear Magnetic Resonance Tracking of Hydro, Thermal, and Hydrothermal Decomposition of CH3NH3PbI3. J. Phys. Chem. C 2017, 121, 1013–1024.
- Nandi, P.; Mahana, S.; Welter, E.; Topwal, D. Probing the Role of Local Structure in Driving the Stability of Halide Perovskites CH3NH3PbX3. J. Phys. Chem. C 2021, 125, 24655–24662.
- Kim, Y.; Nandi, P.; Lee, D.; Shin, H. Stabilization of 3-D Trigonal Phase in Guanidinium (C(NH2)3) Lead Triiodide (GAPbI3) Films. Appl. Surf. Sci. 2021, 542, 148575.
- Nandi, P.; Li, Z.; Kim, Y.; Ahn, T. Stabilizing Mixed Halide Lead Perovskites against Photoinduced Phase Segregation by A-Site Cation Alloying. ACS Energy Lett. 2021, 6, 837–847.
- Nandi, P.; Giri, C.; Swain, D.; Manju, U. Temperature Dependent Photoinduced Reversible Phase Separation in Mixed-Halide Perovskite. ACS Appl. Energy Mater. 2018, 1, 3807–3814.
- Jeong, S.; Seo, S.; Yang, H.; Park, H. Cyclohexylammonium-Based 2D/3D Perovskite Heterojunction with Funnel-Like Energy Band Alignment for Efficient Solar Cells (23.91%). Adv. Energy Mater. 2021, 11, 2102236.
- Cao, J.; Yan, F. Recent Progress in Tin-Based Perovskite Solar Cells. Energy Environ. Sci. 2021, 14, 1286–1325.
- Hadipour, A.; Boer, D.; Blom, P. Device operation of organic tandem solar cells. Org. Electron. 2008, 9, 617–624.
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153.
- Nazeeruddin, M.; Edgar, M.; Robin, H.; Nick, V. Redox regulation in ruthenium(II) polypyridyl complexes and their application in solar energy conversion. J. Chem. Soc. Dalton Trans. 1997, 23, 4571–4578.
- Chiba, Y.; Islam, A.; Watanabe, Y.; Komiya, R. Dye-sensitized solar cells with conversion efficiency of 11.1%. Jpn. J. Appl. Phys. 2006, 45, L638.
- Sarto, P.; Yukie, I. Clean and renewable energy from dye sensitized solar cells using fruit extracts. In Proceedings of the RIO 3—World Climate & Energy Event, Rio de Janeiro, Brazil, 1–5 December 2003; pp. 91–96.
- Lia, M.; Jojo, H.; Putri, N. Performance analysis of flexible DSSC with binder addition. AIP Conf. Proc. 2016, 1725, 020050.
- Hardani, H.; Ridwan, H.; Suhada, A. Ruthenium (N719) Optimization to Improve Dye Sensitized Solar Cell Efficiency. Int. J. Thin Film Sci. Technol. 2022, 11, 47–53.
- Hardani, I.S.; Mustariani, B.; Dewi, Y.K. Efficient TiO2 nanoparticle-ruthenium sensitizers with high open-circuit voltage (Voc) for high-performance dye-sensitized solar cells. J. Phys. Conf. Ser. 2021, 1816, 012005.
- Gao, F.; Bard, A.; Kispert, L. Photocurrent generated on a carotenoid-sensitized TiO2 nanocrystalline mesoporous electrode. J. Photochem. Photobiol. A Chem. 2000, 130, 49–56.
- Kalyanasundaram, K.; Gratzel, M. Applications of functionalized transition metal complexes in photonic and optoelectronic devices. Coord. Chem. Rev. 1998, 177, 347–414.
- Hardani, H.; Alpiana, H.; Lily, M. Making prototype dye sensitized solar cells (DSSC) based on TiO2 nanopory using extration of mangsteen peel (Garnicia mangostana). Indones. Phys. Rev. 2018, 1, 55–65.
- Qadir, M.; Sun, K.; Sahito, I.; Arbab, A. Composite multi-functional over layer: A novel design to improve the photovoltaic performance of DSSC. Sol. Energy Mater. Sol. Cells 2015, 140, 141–149.
- Wang, S.; Ding, Z.; Chang, X.; Xu, J. Modified Nano-TiO2 Based Composites for Environmental Photocatalytic Applications. Catalysts 2020, 10, 759.
- Linsebigler, A.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758.
- Berger, T.; Sterrer, M.; Diwald, O.; Knözinger, E.; Panayotov, D. Light-induced charge separation in anatase TiO2 particles. J. Phys. Chem. B 2005, 109, 6061–6068.
- Gupta, D.; Vishnu, C.; Kumar, R. Sputter deposition of 2D MoS2 thin films—A critical review from a surface and structural perspective. Inorg. Chem. Commun. 2022, 144, 109848.
- Xavier, M.; Pantoja, E.; Romeo, A.; Tiwari, A. CdTe/CdS solar cells on flexible substrates. Sol. Energy 2004, 77, 831–838.
- Fulop, G.; Doty, M.; Betz, J.; Liu, H. High-efficiency electrodeposited cadmium telluride solar cells. Appl. Phys. Lett. 1982, 40, 327.
- Lepiller, C.; Cowache, P.; Gibson, N.; Ozsan, E.; Lincot, D. Fast electrodeposition route for cadmium telluride solar cells. Thin Solid Films 2000, 361–362, 118–122.
- Qian, L.; Fu, W.; Chi, K.; Yang, L.; Lv, P.; Zhou, L. Effects of CTAB concentrations on properties of electrodeposited cadmium telluride films. CrystEngComm 2014, 16, 5227–5233.
- Suha, A.; Hamid, M.; Hussein, A.; Shihab, A. Enhanced efficiency of CdTe photovoltaic by thermal evaporation vacuum. Energy Proc. 2019, 157, 635–643.
- Amit, M.; Jason, M.; Ali, A.; Sampath, S.; John, M. Polycrystalline CdTe photovoltaics with efficiency over 18% through improved absorber passivation and current collection. Sol. Energy Mater. Sol. Cells 2018, 176, 9–18.
- Dipendra, A.; Junda, M.; Ebin, B.; Randall, J.; Nikolas, P. Glancing angle deposited CdTe: Nanostructured films and impact on solar cell performance. Surf. Coat. Technol. 2020, 381, 125127.
- Ebin, B.; Fadhil, K.; Adam, B.; Heben, J.; Randy, J. Wet chemical etching of cadmium telluride photovoltaics for enhanced open-circuit voltage, fill factor, and power conversion efficiency. J. Mater. Res. 2019, 34, 3988–3997.
- Kamala, K.; Ebin, B.; Indra, S.; Suman, R.; Manoj, K.; Heben, J. Semi-Transparent p-Type Barium Copper Sulfide as a Back Contact Interface Layer for Cadmium Telluride Solar Cells. Sol. Energy Mater. Sol. Cells 2020, 218, 110764.
- Gupta, A.; Viral, P.; Alvin, D. High efficiency ultra-thin sputtered CdTe solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 2263–2271.
- Akhlesh, G.; Alvin, D. All-sputtered 14% CdS∕CdTe thin-film solar cell with ZnO:Al transparent conducting oxide. Appl. Phys. Lett. 2004, 85, 684.
- Susumu, K.; Ezumi, H. Preparation of CdS/CdTe solar cell by laser ablasion. Sol. Energy Mater. Sol. Cells 1994, 35, 299–303.
- Chao, D.; Ming, Z.; Li, B.; Feng, L.; Wu, J. Preparation and characterization of pulsed laser deposited CdTe thin films at higher FTO substrate temperature and in Ar + O2 atmosphere. Mater. Sci. Eng. B 2013, 178, 801–806.
- Min, S.; Jo, J.; Cho, A.; Yun, H.; Kim, K.; Ahn, S. Semi-transparent photovoltaics using ultra-thin Cu(In,Ga)Se2 absorber layers prepared by single-stage co-evaporation. Sol. Energy 2019, 181, 276–284.
- Yen, Y.; Lin, Y.; Chang, S.; Hong, H.; Tuan, H.; Chueh, Y. Investigation of bulk hybrid heterojunction solar cells based on Cu(In,Ga)Se2 nanocrystals. Nanoscale Res. Lett. 2013, 8, 329.
- Jheng, B.; Liu, P.; Wu, M. Efficiency enhancement of non-selenized Cu(In,Ga)Se2 solar cells employing scalable low-cost antireflective coating. Nanoscale Res. Lett. 2014, 9, 331.
- Chirila, A.; Fabian, P.; Stephan, B.; Uhl, R.; Verma, R.; Shiro, N.; Lukas, K. Highly efficient Cu(In,Ga)Se2 solar cells grown on flexible polymer films. Nat. Mater. 2011, 10, 857–861.
- Sim, J.; Kang, S.; Nandi, R.; Jeong, K.; Lee, C. Implementation of graphene as hole transport electrode in flexible CIGS solar cells fabricated on Cu foil. Sol. Energy 2018, 162, 357–363.
- Mitzi, B.; Yuan, M.; Jay, S.; Alex, G. Hydrazine-based deposition route for device-quality CIGS films. Thin Solid Films 2009, 517, 2158–2162.
- Binetti, S.; Mereu, R.; Donne, A.; Meschia, M.; Garattini, P. Fabricating Cu(In,Ga)Se2 solar cells on flexible substrates by a new roll-to-roll deposition system suitable for industrial applications. Semicond. Sci. Technol. 2015, 30, 105006.
- Bloesch, P.; Uhl, A.; Tiwari, A.; Bilger, G.; Verma, R. Cu(In,Ga)Se2 solar cell grown on flexible polymer substrate with efficiency exceeding 17%. Prog. Photovolt. 2011, 19, 560–564.
- Fraga, D.; Carda, J.; Calvet, I.; Barrachina, E. Developing CIGS solar cells on glass-ceramic substrates. Mater. Lett. 2018, 221, 104–106.
- Wei, W.; Su, Y.; Chang, C. Inkjet printed chalcopyrite CuInxGa1−xSe2 thin film solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 2616–2620.
- Xu, C.; Zhang, H.; Parry, J.; Perera, S.; Zeng, H. A single source three-stage evaporation approach to CIGS absorber layer for thin film solar cells. Sol. Energy Mater Sol. Cells 2013, 117, 357–362.
- Raghu, N.; Arturo, M. CuIn1−xGaxSe2-based photovoltaic cells from electrodeposited precursor films. Sol. Energy Mater. Sol. Cells 2003, 76, 331–337.
- Bhattacharya, N.; Hiltner, F.; Noufi, N.; Sites, R.; Batchelor, W. 15.4% CuIn1-xGaxSe2 based photovoltaic cells from solution based precursor films. Presented at the European Materials Research Society Meeting, Strasbourg, France, 1–4 June 1999. NREL/CP-590-26611.
- Zank, J.; Mehlin, M.; Fritz, P. Electrochemical co-deposition of indium and gallium for chalcopyrite solar cells. Thin Solid Films 1996, 286, 259–263.
- Guimard, D.; Kurdi, J.; Lincot, D.; Grand, P.; Bodereau, N. Efficient CIGS solar cells prepared by electrodeposition. In Proceedings of the 3rd World Conference on Photovoltaic Energy Conversion, Osaka, Japan, 11–18 May 2003.
- Ganchev, M.; Kois, J.; Kaelin, M.; Tiwari, A. Preparation of Cu(In,Ga)Se2 layers by selenization of electrodeposited Cu–In–Ga precursors. Thin Solid Films 2006, 511–512, 325–327.
- Dale, P.; Anura, P.; Zoppt, G.; Ian, F.; Scilla, R. Deposition and Characterization of Copper Chalcopyrite Based Solar Cells using Electrochemical Techniques. ECS Meet. Abstr. 2007, 6, 535.
- Feng, K.; Ao, J.; Sun, G.; He, Q.; Sun, Y. Properties of CuInxGa1−xSe2 thin films grown from electrodeposited precursors with different levels of selenium content. Curr. Appl. Phys. 2010, 10, 886–888.
- Hilda, R.; Hasan, T.; Siti, Z.; Nandang, M. Electrodeposition Technique to Fabrication CIGS using Pure Selenium and SeO2 as Selenium Source. Mal. J. Fund. Appl. Sci. 2022, 18, 367–373.
- Chen, S.; Wang, S.; Kuo, S.; Juang, J.; Lee, P. A Comprehensive Study of One-Step Selenization Process for Cu(In1−xGax)Se2 Thin Film Solar Cells. Nanoscale Res. Lett. 2017, 12, 208.
- Song, X.; Xu, J.; Li, M.; Lin, W.; Luo, X. A review on development prospect of CZTS based thin film solar cells. Int. J. Photoenergy 2014, 2014, 613173.
- Nandur, A.; White, B. Growth of Cu2ZnSnS4 (CZTS) by Pulsed Laser Deposition for Thin Film Photovoltaic Absorber Material. In Proceedings of the APS March Meeting 2014, Denver, CO, USA, 3–7 March 2014; Available online: https://ui.adsabs.harvard.edu/abs/2014APS..MARF24003N/abstract (accessed on 30 November 2022).
- Tiwari, D.; Tapas, K.; Ray, A.; Dutt, T. Cu2ZnSnS4 thin films by simple replacement reaction route for solar photovoltaic application. Thin Solid Films 2014, 551, 42–45.
- Wei, W.; Shen, H.; Yao, H.; Tang, Z.; Lu, Y. Effect of sulfurization temperature on the property of Cu2ZnSnS4 thin film by eco-friendly nanoparticle ink method. Appl. Phys. A 2017, 123, 599.
- Ennaoui, A.; Hock, R.; Jost, S.; Kirbs, A.; Weber, A.; Lux, M. Cu2ZnSnS4 thin film solar cells from electroplated precursors: Novel low-cost perspective. Thin Solid Films 2009, 517, 2511–2514.
- Tsai, H.; Chen, Y.; Wang, Y.; Chen, C.; Thomas, R.; Hsu, C.; Chueh, Y. Facile Growth of Cu2ZnSnS4Thin-Film by One-Step Pulsed Hybrid Electrophoretic and Electroplating Deposition. Sci. Rep. 2016, 6, 19102.
- Li, Z.; Wang, S.; Ma, X.; Min, Y.; Liu, T.; Liu, S. A simple structure of Cu2ZnSnS4/CdS solar cells prepared by sputtering. Phys. B Condens. Matter 2017, 526, 80–83.
- Sandip, M.; Ramphal, S.; Dimitra, S.; Elias, S.; Huse, N. Enhancement in the efficiency of crystalline Cu2ZnSnS4 thin film solar cell by using various buffer layers. Superlattices Microstruct. 2017, 109, 240–248.
- Scragg, J.; Tomas, K.; Timo, W.; Tove, E.; Margareta, K. Effects of Back Contact Instability on Cu2ZnSnS4 Devices and Processes. Chem. Mater. 2013, 25, 3162–3171.
- Khalil, M.; Nobili, L.; Magagnin, L.; Donne, A.; Mereu, A.; Hart, L. CZTS thin flm solar cells on fexible Molybdenum foil by electrodeposition-annealing route. J. Appl. Electrochem. 2021, 51, 209–218.
- Rongrong, C.; Fan, J.; Li, H.; Liu, C.; Mai, Y. Efficiency enhancement of Cu2ZnSnS4 solar cells via surface treatment engineering. R. Soc. Open Sci. 2018, 5, 171163.
- Jian, C.; Yan, C.; Wei, L.; Ning, S.; Liu, F.; Huang, S. Cu2ZnSnS4 thin film solar cell fabricated by magnetron sputtering and sulfurization. MRS Online Proc. Libr. 2014, 1638, 901.
- Cho, J.; Agus, I.; Park, S.; Kim, W.; Yoon, S.; Min, K. Synthesis of Cu2ZnSnS4 Thin Films by a Precursor Solution Paste forThin Film Solar Cell Applications. ACS Appl. Mater. Interfaces 2013, 5, 4162–4165.
- Vigil, O.; Maykel, C.; Jimenez, D.; Aguilar, M. Electrical properties of sprayed Cu2ZnSnS4 thin films and its relation with secondary phase formation and solar cell performance. Sol. Energy Mater. Sol. Cells 2015, 132, 557–562.
- Yusuf, Y.; Eka, C.; Gema, R.; Harbi, S.; Harito, C. All-Solution-Non-Vacuum Fabrication Process of CZTS Solar Cell using ZTO as Non-Toxic Buffer Layer. Int. J. Nanoelectron. Mater. 2020, 13, 307–314.
- Hu, J.; Tong, W.; Muhammad, I.; Umar, F.; Shuo, C. Pulsed laser deposited and sulfurized Cu2ZnSnS4 thin film for efficient solar cell. Sol. Energy Mater. Sol. Cells 2021, 233, 111383.
- Tatsuo, F.; Hiroshi, N.; Shin, T.; Ito, T.; Ryoji, A. Fabrication of Cu2ZnSnS4 thin film solar cells by sulfurization. R&D Rev. Toyota CRDL 2013, 44, 1–18.
- Hyun, Y.; Ji, H.; Joshi, B.; Ra, Y.; Sam, Y.; Ho, K.; Scott, J. CuInSe2 (CIS) Thin Film Solar Cells by Electrostatic Spray Deposition. J. Electrochem. Soc. 2012, 159, H444–H449.
- Vahid, A.; Brian, W.; Matthew, G.; Reid, K. Spray-deposited CuInSe2 nanocrystal photovoltaics. Energy Environ. Sci. 2010, 3, 1600–1606.
- Meadows, H.; Regesch, D.; Sendler, J.; Misra, S.; Gerliz, V.; Dale, P. CuInSe2 semiconductor formation by laser annealing. Thin Solid Films 2015, 582, 23–26.
- Sejin, A.; Tae, H.; Ara, C.; Jae, H.; Shin, K.; Yoon, K.; Park, H. CuInSe2 Thin-Film Solar Cells with 7.72% Efficiency Prepared via Direct Coating of a Metal Salts/Alcohol-Based Precursor Solution. ChemSusChem 2012, 5, 1773–1777.
- Ara, C.; Ahn, S.; Yoon, H.; Gwak, J.; Ahn, K.; Shin, K. Non-vacuum processed CuInSe2 thin films fabricated with a hybrid ink. Sol. Energy Mater. Sol. Cells 2013, 109, 17–25.
- Saidi, H.; Durand, B.; Lazzari, J.; Boujmil, M. Elaboration and characterization of CuInSe2 thin films using one step electrodeposition method on silicon substrate for photovoltaic application. Mater. Res. Express 2018, 5, 016414.
- Mandati, S.; Misra, P.; Boosagulla, D. Economic pulse electrodeposition for flexible CuInSe2 solar cells. Mater. Renew Sustain. Energy 2020, 9, 19.
- Kar, M.; Hugh, W.; Hugh, H. Chemical liquid deposition of CuInSe2 and CuIn(S,Se)2 films for solar cells. Thin Solid Films 2012, 520, 5431–5437.
- Se, A.; Kim, C.; Yun, H.; Gwak, J.; Jeong, S.; Ryu, B. CuInSe2 (CIS) Thin Film Solar Cells by Direct Coating and Selenization of Solution Precursors. J. Phys. Chem. C 2010, 114, 8108–8113.
- Huang, W.; Lyu, L.; Huang, M.; Chang, S.; Tseng, C.; Tuan, H. Solvothermal Synthesis of Zincblende and Wurtzite CuInS2 Nanocrystals and Their Photovoltaic Application. Langmuir 2012, 28, 8496–8501.
- Wangwei, C.; Qi, J.; Dong, C.; Chen, J.; Shen, Z.; He, Y.; Yang, S. Solution-Processed in Situ Growth of CuInS2 Nanoparticle Films for Efficient Planar Heterojunction Solar Cells with a Dual Nature of Charge Generation. ACS Appl. Energy Mater. 2019, 2, 5231–5242.
- Scheer, R.; Walter, T.; Schock, W.; Lewerenz, H. CuInS2 based thin film solar cells with 10.2% efficiency. Appl. Phys. Lett. 1993, 63, 3294.
- Weil, B.; Yi, C.; Connor, T. CuInS2 Solar Cells by Air-Stable Ink Rolling. J. Am. Chem. Soc. 2010, 132, 6642–6643.
- Mehdi, D.; Ershad, P.; Tehrani, A.; Nima, T. A novel low-temperature growth of uniform CuInS2 thin films and their application in selenization/sulfurization-free CuInS2 solar cells. Mater. Today Commun. 2021, 26, 102050.
- Hina, P.; Khan, S.; Ahmed, N.; Jamil, Q. Synthesis and characterization of CuInS2 nanostructures and their role in solar cell applications. Mater. Chem. Phys. 2022, 290, 126602.
- Woo, J.; Park, S.; Kim, W.; Kim, M. Fabrication of nanocrystal ink-based superstrate-type CuInS2 thin film solar cells. Nanotechnology 2012, 23, 265401.
- Tang, M.; Tian, Q.; Hu, X.; Peng, Y.; Xue, Y.; Yang, J.; Hu, J. In situ preparation of CuInS2 films on a flexible copper foil and their application in thin film solar cells. CrystEngComm 2012, 14, 1825–1832.
- Lakhe, M.; Nandu, B. Characterization of electrochemically deposited CuInTe2 thin films for solar cell applications. Sol. Energy Mater. Sol. Cells 2014, 123, 122–129.
- Manorama, L.; Nandu, B.; Mahapatra, S. Development of CuInTe2 thin film solar cells by electrochemical route with low temperature (80 °C) heat treatment procedure. Mater. Sci. Eng. B 2016, 204, 20–26.
- Jing, G.; Pei, Y.; Zhou, Z.; Zhou, W.; Kou, D.; Wu, S. Solution-Processed Cu2ZnSn(S,Se)4 Thin-Film Solar Cells Using Elemental Cu, Zn, Sn, S, and Se Powders as Source. Nanoscale Res. Lett. 2015, 10, 335.
- Gee, Y.; Ju, R.; Jo, W.; Son, D.; Kim, D.; Kang, J. Nanoscale observation of surface potential and carrier transport in Cu2ZnSn(S,Se)4 thin films grown by sputtering-based two-step process. Nanoscale Res. Lett. 2014, 9, 10.
- Chate, P.; Hankare, P.; Sathe, D. Characterization of cadmium selenide films for photovoltaic applications. J. Alloys Compd. 2010, 505, 140–143.
- Kanghua, L.; Lin, X.; Song, B.; Yang, X.; Chen, C.; Tang, J. Rapid thermal evaporation for cadmium selenide thin-film solar cells. Front. Optoelectron. 2021, 14, 482–490.
- Wang, K.; Cheng, J.; Yang, X. Enhanced Photovoltaic Properties in Sb2S3 Planar Heterojunction Solar Cell with a Fast Selenylation Approach. Nanoscale Res. Lett. 2018, 13, 270.
- Hsiao, Y.; Lu, C.; Ji, L. Characterization of photovoltaics with In2S3 nanoflakes/p-Si heterojunction. Nanoscale Res. Lett. 2014, 9, 32.
- Mostafa, S.; Atef, S.; Ahmed, I.; Said, E. Synthesis, Characterization and Performance of Cu2SnS3 for Solar Cell Application. Int. J. Sci. Eng. Res. 2015, 6, 1447–1453.
- Chaudhari, J.; Joshi, U. Fabrication of high quality Cu2SnS3 thin film solar cell with 1.12% power conversion efficiency obtain by low cost environment friendly sol-gel technique. Mater. Res. Express 2018, 5, 036203.
- Prasert, S.; Katy, H.; Kim, S.; Heo, J.; Helen, H.; Tonio, B. Enhancing the efficiency of SnS solar cells bia band off set engineering with a zinc oxysulfide buffer layer. Appl. Phys. Lett. 2013, 110, 10539.
- Vinaya, K.; Shin, Y.; Kim, J. Photovoltaic behavior of the room temperature grown RF-Sputtered SnS thin films. Opt. Mater. 2019, 88, 594–600.
- Tien, D.; Van, T.; Manh, H.; Kumar, V.; Raj, M. Synthesis of Ag-embedded SnS films by the RF method for photovoltaic applications. Surf. Interfaces 2021, 25, 101151.
- Joel, V.; Joao, O.; Jacek, J.; Della, E.; Chesman, S. Solution-Processed CuSbS2 Thin Films and Superstrate Solar Cells with CdS/In2S3 Buffer Layers. ACS Appl. Energy Mater. 2020, 3, 7885–7895.
- Qinmiao, C.; Dou, X.; Li, Z.; Chen, J.; Zhou, F.; Zhuang, S. Study on the photovoltaic property of Cu4SnS4 synthesized by mechanochemical process. Optik 2014, 125, 3217–3220.
- Mohsen, C.; Farid, J.; Ramin, Y. Optical, electrical, and photovoltaic properties of PbS thin films by anionic and cationic dopants. Appl. Phys. A Mater. Sci. Process. 2017, 123, 390.
- Rex, S.; Deva, A.; Shkir, M.; AlFaify, S.; Valanarasu, S. Fabrication and characterization of lead sulfide (PbS) thin film based heterostructure (FTO/CdS/PbS/Ag) solar cell by nebulizer spray method. Mater. Res. Express 2019, 6, 056416.
- Ho, S.M. Studies of Power Conversion Efficiency and Optical Properties of Ni3Pb2S2 Thin Films. Makara J. Sci. 2017, 21, 3.
- Kisan, C.; Guar, L.; Pradip, D.; Sanadi, R.; Kamble, G. Photovoltaic Application Study of Zinc Telluride Thin Films Grown by Chemical Bath Deposition Method. Adv. Mater. Phys. Chem. 2021, 11, 131–144.
This entry is offline, you can click here to edit this entry!
