Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Emergency Medicine
Critically ill patients are at risk of skin wounds, which reduce their quality of life, complicate their pharmacological regimens, and prolong their hospital stays in intensive care units (ICUs), while also increasing overall mortality and morbidity rates. Cold atmospheric plasma (CAP) has been proposed as a viable option for many biological and medical applications, given its capacity to reduce wound bacterial contamination and promote wound healing.
- cold atmospheric plasma
- wound
- critical care
- infection
1. Background
Intensive care units (ICUs) are specialized hospital facilities where critically ill patients with high mortality and morbidity risks are treated.
ICU patients may have skin injuries due to trauma or accidents, such as burn wounds, or due to other causes. They can also be the outcome of primary diseases or their complications, as well as clinical circumstances, along with complex therapy regimens [1].
Trauma patients admitted to intensive care units may present different types of skin injuries, ranging from abrasions and excoriations to penetrating wounds, puncture wounds, cutting wounds, blunt-force trauma wounds, lacerated wounds, and lacerated-contused wounds [2], all possibly due to different causes, such as traffic accidents, stab wounds, falls, etc.
Trauma accounts for 4.3 million annual deaths globally, with road traffic injuries prevailing among adolescents and young adults, while falls predominate among the elderly [3].
Burn injuries are the most common and destructive type of trauma caused by thermal, chemical, electrical, or radiation contact; they are classified based on their depth (first-degree, superficial burns; second-degree, partial-thickness burns; and third-degree, full-thickness burns) and the percentage of total body area affected [4].
According to the WHO, 11 million burns of all types occur worldwide each year, of which 180,000 fatal [5]. Patients with severe burns (>20% of the total body surface area) will require ICU hospitalization to reduce the risk of mortality [6].
Another significant challenge for these types of trauma patients in ICUs is health care-associated infections (HCAIs), which are infections acquired while seeking treatment for medical or surgical conditions and are the most common adverse event during care delivery [7].
HCAIs are a severe issue for patient safety, and their consequences might include longer hospitalizations, long-term disabilities, increased antimicrobial agent resistance, a significant additional financial burden for the health system, high expenditures for individuals and their families, and a mortality increase [8][9]. In Europe, HCAIs cause 16 million extra hospital days and almost 37,000 fatalities, with an annual cost of 7 billion euros [10].
With an incidence ranging from 3.3% to 52.9% [11], critically ill patients, particularly those with HCAIs, are at high risk of developing pressure injuries; the causes are related to prolonged hospitalization, immobility, deep sedation, the use of vasoactive drugs, hypotension, anasarca, organ dysfunction, and altered nutritional status [12].
Pressure sores are areas of localized skin damage and underlying tissues, caused by pressure, friction, and cutting. Their predisposing factors are related to the patient’s characteristics (hypo-trophy, malnutrition, diabetes, hypo-perfusion, skin constantly exposed to moisture, altered sensory perception, limited mobility, and age) [13], as well as factors directly related to the care procedures adopted during hospitalization (prolonged bed rest, drug side-effects, and surgical operations) [14].
Pressure sores are a clinically significant issue. Their occurrence can impair functional recovery and lead to infectious challenges, as they create a breeding ground for bacterial super infections that can be localized or escalate to generalized sepsis, lengthening hospitalizations [15] as well as increasing morbidity and mortality [16].
These patients also require the use of modern monitoring and treatment techniques, both invasive and noninvasive, which can result in varying degrees of skin damage. Complications caused by medical devices are related to their capacity to induce infections [17] and also issues related to their implementation [18]. Wounds caused by infectious agents are the most common in intensive care settings and occur during severe diseases. Bed rest, moisture, and obesity all contribute to inflammatory dermatitis in the major skin folds, with stratum corneum damage promoting the entrance of germs such as Candida albicans or beta-hemolytic Streptococcus bacteria [19].
Management of skin wounds in critically ill patients is crucial, as these wounds reduce quality of life, complicate treatment regimens, lengthen ICU stays, and increase mortality and morbidity [20][21].
Cold atmospheric plasma (CAP) has been proposed as an option for many different biological and medical applications as a result of its ability to reduce the bacterial count within a wound and promote wound healing.
Plasma is a form of ionized gas that has a high concentration of charged particles (OH−, H2O+, and electrons), reactive chemicals (oxygen free radicals, or “ROS,” and reactive nitrogen species, or “RNS”), excited molecules, and UV photons (UVB, UVC) [22]. The physical-chemical properties of plasma are affected by a variety of factors, including the kind of gas or mixture of gases employed and the applied energy, pressure, and atmosphere [23].
The use of physical plasma enables two approaches: the use of plasma-based or plasma-integrated procedures to treat surfaces/materials/devices in order to apply particular dressings and the direct application of physical plasma in the human or animal body in order to use its therapeutic benefits [23]. Its application is reported in a large and growing number of articles that consider it a very promising therapy [24][25].
2. Characteristics of Cold Atmospheric Plasma
In physics, plasma is referred to as the fourth state of matter (after the solid, liquid, and gas phases), or ionized gas, depending on its temperature, and it can be divided into hot plasma and cold plasma. Plasma can be distinguished into standard (“thermal”) plasma, at 4000–5000 K, and low-temperature (“cold” or “non-thermal”) plasma, at 30–50 °C, which generates oxygen and nitrogen free radicals with positive and/or negative ions [26].
Since high temperatures can cause thermal damage to organisms, the medical field must consider a type of plasma at atmospheric pressure with its temperature close to room temperature [27]. To be considered “plasma,” a gas must meet certain criteria, including being molecularly neutral (or near-neutral), having a Debye shield (charged particles capable of counteracting an electrostatic field within a Debye), and having a plasma frequency, defined as the natural oscillation frequency that determines particle movement, causing the gas to return to its neutral state [28].
The main components of CAP include ions, electrons, metastables, photons, and electromagnetic fields. After a reaction with environmental air, CAP forms a hierarchical group of reactive oxygen and nitrogen species (RONS) that promote increased skin tissue microcirculation, increased monocyte stimulation, increased cell migration, and stimulation of the keratinocytes and fibroblasts primarily involved in wound healing.
Plasma can be applied directly or indirectly. In the former case, cell lines receive plasma discharges in vitro and animal or human tissue in vivo; in the latter case, a plasma-activated solution is used [28]. To improve CAP efficiency, helium (He), argon (Ar), nitrogen (N2), oxygen (O2), artificial air, and two or more mixtures of these gases can be used to generate CAP [29].
Energy is required to produce and maintain the plasma. Several devices have been developed in the biomedical field that use electrical energy. Some methods used to produce CAP include: dielectric barrier discharge (DBD), atmospheric-pressure plasma jets (APPJ), plasma needles, and plasma pencils [30].
3. Wound Area Reduction
Wound healing is a complex process involving four distinct phases: hemostasis, inflammation, skin proliferation, and remodeling [31]. Wounds can typically be classified as acute and chronic wounds. Acute wounds include abrasions, scalds, burns, or postoperative incisions; chronic wounds do not heal in an orderly manner, frequently remaining in the inflammatory phase for too long and sometimes being complicated by systemic diseases, age, and repeated trauma, such as diabetic ulcers, venous ulcers, arterial ulcers, and pressure sores [32].
CAP may promote wound healing through its antiseptic effects, stimulating the proliferation and migration of skin cells by activating or inhibiting integrin receptors on the cell surface or through its pro-angiogenic effect [33]. It also appears to act by triggering the production of nitric oxide (NO), which promotes cell migration and the assembly of endothelial cells into vessel-like structures useful for wound neo-vascularization [34].
Treatment with CAP can be adapted according to the different stages of wound healing; argon plasma was found to be better in promoting coagulation, while helium plasma was more effective in healing [35].
Amini et al. [36] demonstrated that treatment with CAP modifies the persistence levels of inflammatory cytokines and growth factors including IL-1, IL-8, TGF-β, TNF-α, and INF-γ, promoting healing through a more rapid initiation of the proliferative phase. In addition, CAP generates reactive oxygen species (ROS) and nitrogen species (RNS), which can increase the synthesis of pro-angiogenic factors, consequently promoting wound healing [37] (Figure 1 and Figure 2).
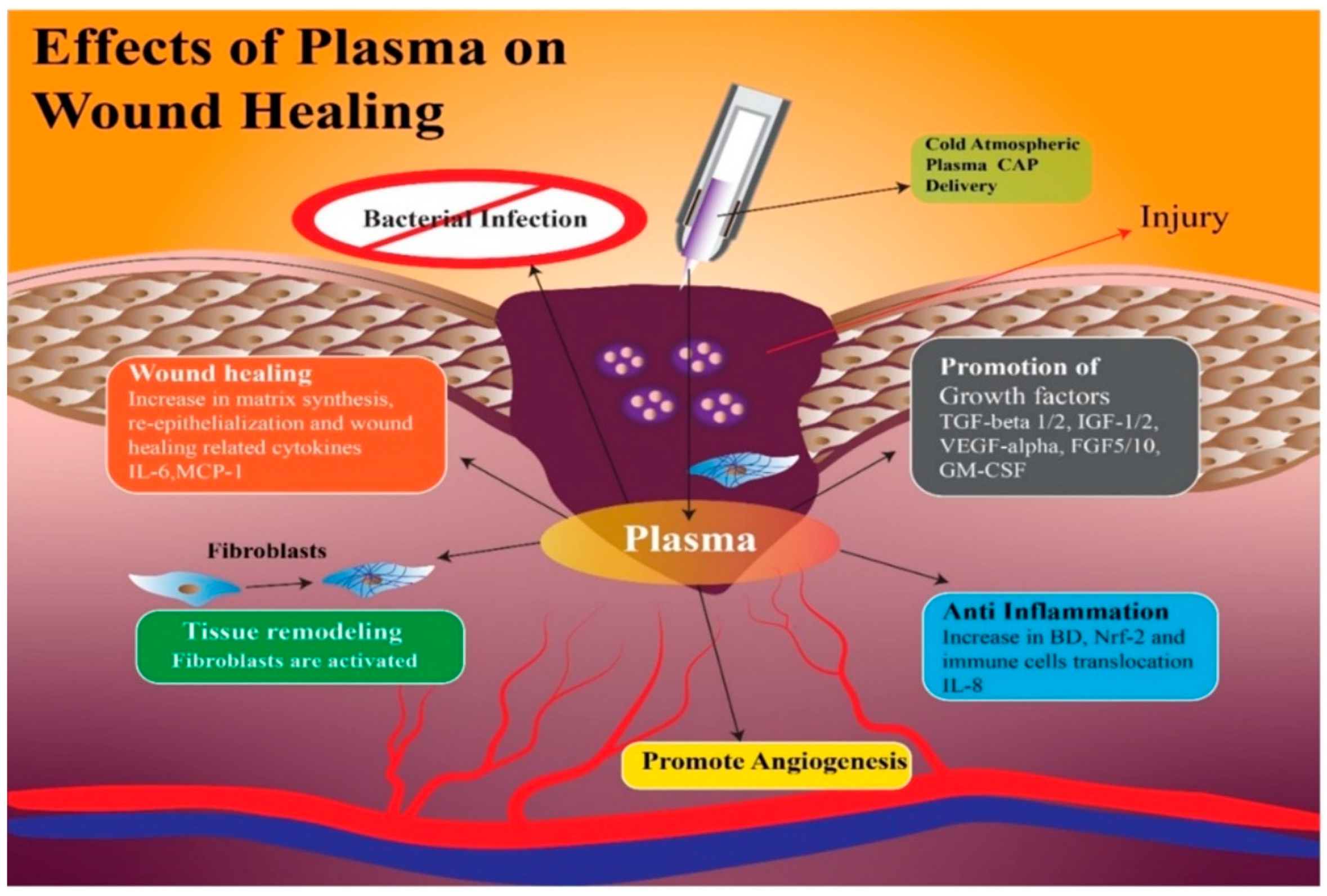
Figure 1. With the application of CAP on the wound, there is an increase in growth factors such as Tumor Growth Factor-β 1 and 2; Insulin Growth Factor 1 and 2; Vascular-Endothelial Growth Factor-α; Granulocyte-Macrophage Colony-Stimulating Factor, which facilitate angiogenesis with the anti-inflammatory effect of interleukin-8. Finally, the wound heals with re-epithelialization and synthesis of a new matrix, thanks to the fibroblasts and cytokines, Membrane Cofactor Protein-1 and Interleukin-6.
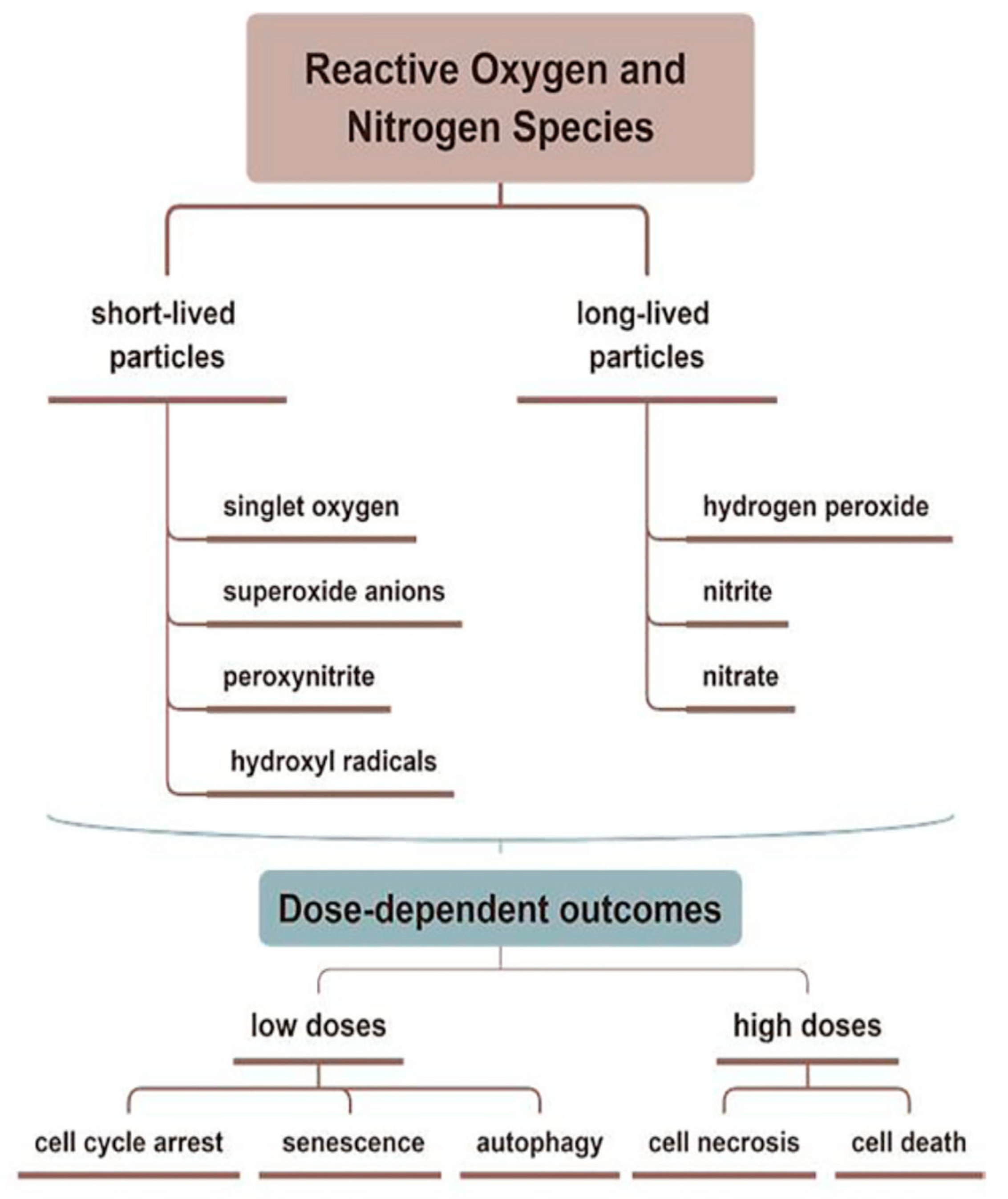
Figure 2. Reactive oxygen and reactive nitrogen species (ROS and RNS) related to CAP are divided into short- and long-lived molecules, which play different roles in the treating skin diseases. CAP promotes wound healing through antiseptic and pro-angiogenic effects, stimulating the proliferation and migration of skin cells by activating/inhibiting integrin receptors.
The duration of CAP therapy should be carefully monitored during wound treatment, as overdosage could cause necrosis or apoptosis, invalidating the wound healing process [38].
Nervous system trauma and neurodegeneration often result in permanent functional deficits due to the limited regenerative capacity of the brain and spinal cord. It is known that the central nervous system (CNS) has a limited ability to regenerate following an injury or neurodegenerative disease [39], with a major impact on quality of life for patients. Kativar et al. [40] concluded that astrocytes, glial cells that support neurons through protein secretion of and neurotrophic factors, can respond to properly calibrated nanosecond-pulse dielectric discharge plasma treatment, which can directly promote their growth and improve their ability to enhance neuronal regeneration after an injury. It is interesting to note the importance of the therapeutic dose on responses: low intensities (≤10 mJ) caused no measurable changes, while high intensities (≥90 mJ) generally resulted in widespread cell death. Intermediate intensities (10–50 mJ) elicited a physiological response, resulting in improved cell regeneration (Figure 1 and Figure 3).
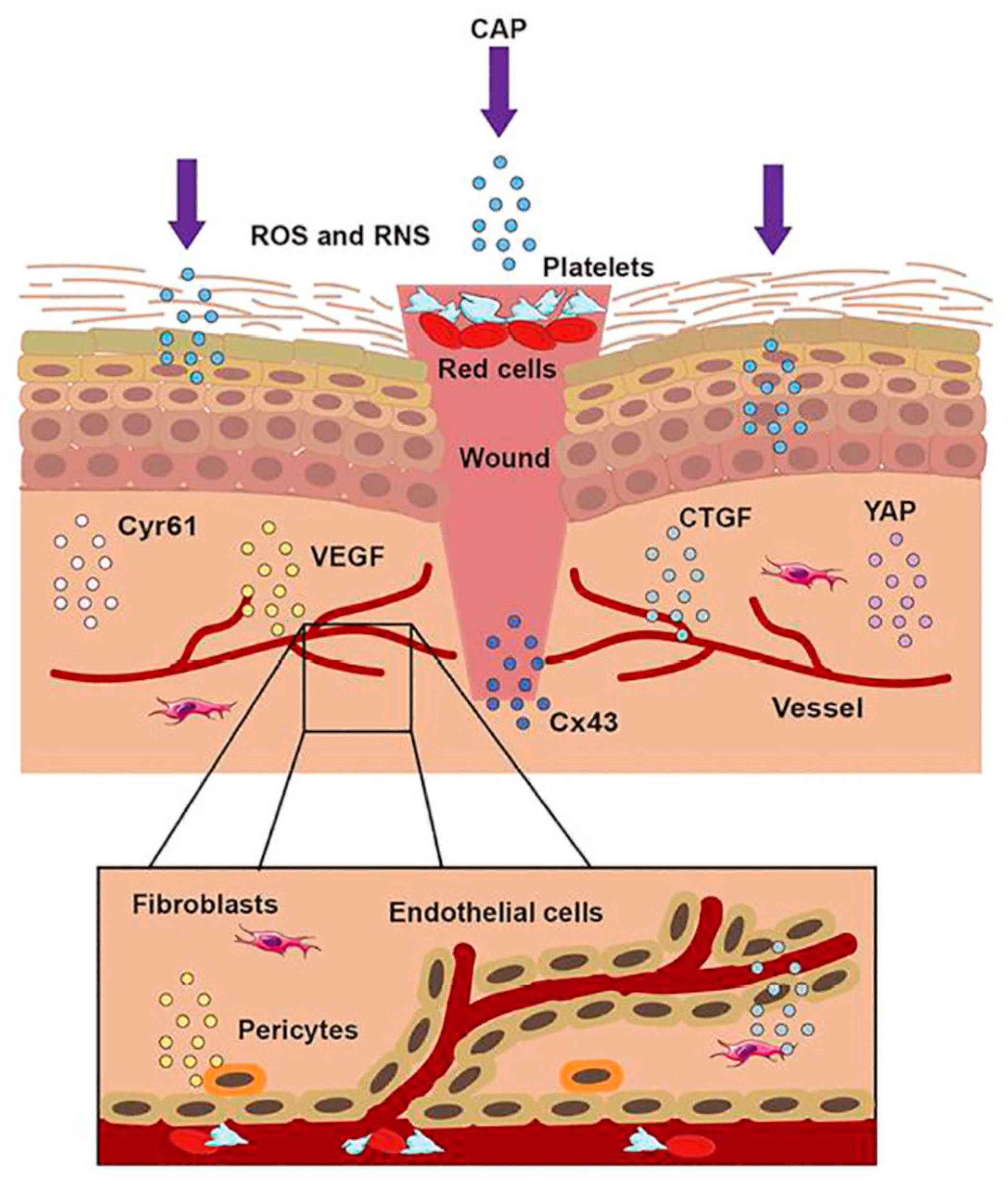
Figure 3. The treatment of CAP on a wound. When the skin was injured, the first step was to form a blood scab to protect the wound. CAP could accomplish wound healing through short-lived and long-lived ROS and RNS. CAP could promote the formation of new blood vessels, strengthen the release of Connective Tissue Growth Factor (CTGF) and Vascular Endothelial Growth Factor (VEGF), activate the Yes-Associated Protein (YAP) pathway, and upregulate the expression of Connexin 43 (Cx43) and Cysteine-rich angiogenic inducer 61 (Cyr61).
4. Bacterial Load Reduction
CAP’s bactericidal activity has received 20 years of attention and investigation [39]. The potential uses of CAP for infection control in clinical settings have grown significantly during the last two decades. The ability of CAP to effectively eradicate bacterial biofilms has been demonstrated by several bacterial studies [40][41][42][43]. This is what inspired the concept of utilizing CAP to lower the bacterial wound burden and so improve healing [44]. Early published studies employed jet plasma using argon as the carrier gas. In 2010, a prospective study used argon plasma treatment for 5 min via a CAP device called MicroPlaSter alpha, which resulted in a very significant reduction in bacterial load, compared to standard wound care alone [44]. In fact, after the application of CAP, a mean reduction of 1.10 log10 was observed in the intervention group and a reduction of 0.41 log10 in the control group. Although the difference in the mean reduction between the two arms of the study is significant, an intervention that achieves a bacterial reduction of 1 log10 would hardly be considered relevant as “bactericidal” or “antimicrobial.” In fact, general agreement indicates that the effective bactericidal action of a device refers to a reduction of at least 3 log10 in the number of viable bacterial cells tested [45][46]. A following study used a second-generation device known as the MicroPlaSter beta, which has a flexible four-joint therapy arm which enables treatment of difficult-to-reach areas. A patient with Hailey-Hailey illness (also known as benign chronic pemphigus) and secondary infections with Candida albicans and Proteus mirabilis saw rapid clinical recovery. Another study [47] clearly demonstrates that a short CAP treatment of 2 min is sufficient to achieve significant reduction in the bacterial load on chronic infected wounds in vivo. Efficacy and tolerability were demonstrated in both generations of devices (MicroPlaSter alpha and MicroPlaSter beta). These studies treated venous, arterial, diabetic, and traumatic ulcers, and reduced bacterial infection was observed regardless of bacterial type.
In 2021, Abbasi et al. [48] examined the effect of CAP on P. aeruginosa isolated from burn infections in vitro and in vivo. The research results showed no bacterial growth, as well as wound healing in mice. The study concluded that CAP decreased the expression of the alp gene that is one of the virulence factors of P. aeruginosa (Figure 4).
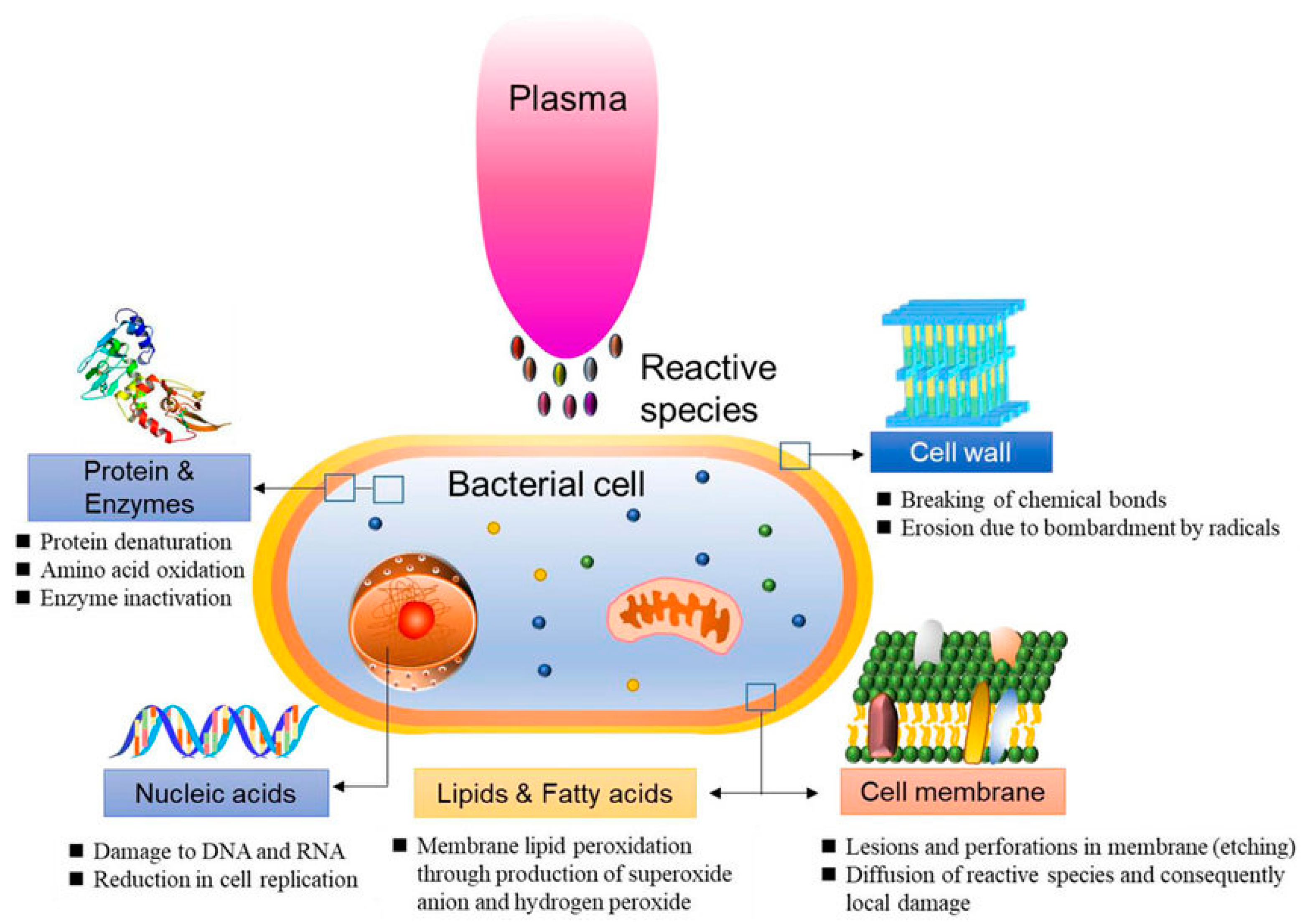
Figure 4. Schematic representation of bacterial reduction induced by CAP.
Some studies have described a hybrid treatment for wound healing: CAP therapy combined with antibiotic treatment.
Nguyen et al. [49] applied cold plasma in treating patients with severe COVID-19 who had skin injuries such as burns, pressure ulcers, shingles, and contact or atopic dermatitis. Before the plasma application, all the skin injuries were treated with antibiotics and albumin infusions. After 14 days of cold plasma irradiation, 14/20 patients had complete epithelialization. Previously, when cold plasma irradiation was not applied, these lesions took longer to epithelialize or ulcerated even further, with more exudation despite the use of antibiotics.
In a randomized clinical trial, Stratmann et al. [50] applied CAP therapy on patients with diabetic foot ulcers. All wounds were treated with systemic antibiotics during the study and were Wagner Armstrong Grade 1B or 2B. Eligible wounds were randomized to receive either a placebo or CAP. In this randomized clinical trial, CAP therapy emerged as an efficient treatment in terms of wound surface reduction and wound closure time.
This entry is adapted from the peer-reviewed paper 10.3390/jpm13050736
References
- Zanza, C.; Romenskaya, T.; Thangathurai, D.; Ojetti, V.; Saviano, A.; Abenavoli, L.; Robba, C.; Cammarota, G.; Franceschi, F.; Piccioni, A.; et al. Microbiome in Critical Care: An Unconventional and Unknown Ally. Curr. Med. Chem. 2022, 29, 3179–3188.
- Miller, P.; Smith, I.M.; White, D.M. Wound Management in the ICU. In Interventional Critical Care; Taylor, D.A., Sherry, S.P., Sing, R.F., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 401–409.
- Soni, K.D.; Bansal, V.; Arora, H.; Verma, S.; Wärnberg, M.G.; Roy, N. The State of Global Trauma and Acute Care Surgery/Surgical Critical Care. Crit. Care Clin. 2022, 38, 695–706.
- Klausen, M.; Heydorn, A.; Ragas, P.; Lambertsen, L.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Biofilm Formation by Pseudomonas Aeruginosa Wild Type, Flagella and Type IV Pili Mutants: Roles of Bacterial Motility in the Formation of the Flat P. Aeruginosa Biofilm. Mol. Microbiol. 2003, 48, 1511–1524.
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.-P. Recent Trends in Burn Epidemiology Worldwide: A Systematic Review. Burns 2017, 43, 249–257.
- Meskini, M.; Esmaeili, D. The Study of Formulated Zoush Ointment against Wound Infection and Gene Expression of Virulence Factors Pseudomonas Aeruginosa. BMC Complement. Altern. Med. 2018, 18, 185.
- Bates, D.W.; Larizgoitia, I.; Prasopa-Plaizier, N.; Jha, A.K.; Research Priority Setting Working Group of the WHO World Alliance for Patient Safety. Global Priorities for Patient Safety Research. BMJ 2009, 338, b1775.
- Allegranzi, B.; Bagheri Nejad, S.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of Endemic Health-Care-Associated Infection in Developing Countries: Systematic Review and Meta-Analysis. Lancet 2011, 377, 228–241.
- Burke, J.P. Infection Control—A Problem for Patient Safety. N. Engl. J. Med. 2003, 348, 651–656.
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Blank, H.-P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M.; et al. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016, 13, e1002150.
- Stegensek Mejía, E.M.; Jiménez Mendoza, A.; Romero Gálvez, L.E.; Aparicio Aguilar, A. Úlceras por presión en diversos servicios de un hospital de segundo nivel de atención. Enferm. Univ. 2015, 12, 173–181.
- Edsberg, L.E.; Langemo, D.; Baharestani, M.M.; Posthauer, M.E.; Goldberg, M. Unavoidable Pressure Injury: State of the Science and Consensus Outcomes. J. Wound Ostomy Cont. Nurs. Off. Publ. Wound Ostomy Cont. Nurses Soc. 2014, 41, 313–334.
- Allman, R.M.; Goode, P.S.; Patrick, M.M.; Burst, N.; Bartolucci, A.A. Pressure Ulcer Risk Factors among Hospitalized Patients with Activity Limitation. JAMA 1995, 273, 865–870.
- Bereded, D.T.; Salih, M.H.; Abebe, A.E. Prevalence and Risk Factors of Pressure Ulcer in Hospitalized Adult Patients; a Single Center Study from Ethiopia. BMC Res. Notes 2018, 11, 847.
- Graves, N.; Birrell, F.; Whitby, M. Effect of Pressure Ulcers on Length of Hospital Stay. Infect. Control Hosp. Epidemiol. 2005, 26, 293–297.
- Manzano, F.; Pérez-Pérez, A.M.; Martínez-Ruiz, S.; Garrido-Colmenero, C.; Roldan, D.; Jiménez-Quintana, M.D.M.; Sánchez-Cantalejo, E.; Colmenero, M. Hospital-Acquired Pressure Ulcers and Risk of Hospital Mortality in Intensive Care Patients on Mechanical Ventilation. J. Eval. Clin. Pract. 2014, 20, 362–368.
- Chaves, F.; Garnacho-Montero, J.; Del Pozo, J.L.; Bouza, E.; Capdevila, J.A.; de Cueto, M.; Domínguez, M.Á.; Esteban, J.; Fernández-Hidalgo, N.; Fernández Sampedro, M.; et al. Diagnosis and Treatment of Catheter-Related Bloodstream Infection: Clinical Guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology and (SEIMC) and the Spanish Society of Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med. Intensiv. 2018, 42, 5–36.
- Smit, J.M.; Raadsen, R.; Blans, M.J.; Petjak, M.; Van de Ven, P.M.; Tuinman, P.R. Bedside Ultrasound to Detect Central Venous Catheter Misplacement and Associated Iatrogenic Complications: A Systematic Review and Meta-Analysis. Crit. Care 2018, 22, 65.
- Fischer, M.; William, T.; Wohlrab, J. Skin Diseases in Intensive Care Medicine. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2009, 7, 108–115.
- Akiki, R.K.; Anand, R.S.; Borrelli, M.; Sarkar, I.N.; Liu, P.Y.; Chen, E.S. Predicting Open Wound Mortality in the ICU Using Machine Learning. J. Emerg. Crit. Care Med. 2021, 5, 13.
- Cox, J. Predictors of Pressure Ulcers in Adult Critical Care Patients. Am. J. Crit. Care Off. Publ. Am. Assoc. Crit. Care Nurses 2011, 20, 364–375.
- Niedźwiedź, I.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The State of Research on Antimicrobial Activity of Cold Plasma. Pol. J. Microbiol. 2019, 68, 153–164.
- VON Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.-D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026.
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxidative Med. Cell. Longev. 2019, 2019, e3873928.
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of Cold-Atmospheric Plasma in Oncology: A Concise Systematic Review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918786475.
- Martusevich, A.K.; Surovegina, A.V.; Bocharin, I.V.; Nazarov, V.V.; Minenko, I.A.; Artamonov, M.Y. Cold Argon Athmospheric Plasma for Biomedicine: Biological Effects, Applications and Possibilities. Antioxidant 2022, 11, 1262.
- Friedman, P.C. Cold Atmospheric Pressure (Physical) Plasma in Dermatology: Where Are We Today? Int. J. Dermatol. 2020, 59, 1171–1184.
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932.
- Nguyen, D.B.; Lee, W.G. Effects of Ambient Gas on Cold Atmospheric Plasma Discharge in the Decomposition of Trifluoromethane. RSC Adv. 2016, 6, 26505–26513.
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of Production and Application in Dentistry and Oncology. Med. Gas Res. 2013, 3, 21.
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321.
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542.
- Haertel, B.; von Woedtke, T.; Weltmann, K.-D.; Lindequist, U. Non-Thermal Atmospheric-Pressure Plasma Possible Application in Wound Healing. Biomol. Ther. 2014, 22, 477–490.
- Duchesne, C.; Banzet, S.; Lataillade, J.-J.; Rousseau, A.; Frescaline, N. Cold Atmospheric Plasma Modulates Endothelial Nitric Oxide Synthase Signalling and Enhances Burn Wound Neovascularisation. J. Pathol. 2019, 249, 368–380.
- García-Alcantara, E.; López-Callejas, R.; Morales-Ramírez, P.R.; Peña-Eguiluz, R.; Fajardo-Muñoz, R.; Mercado-Cabrera, A.; Barocio, S.R.; Valencia-Alvarado, R.; Rodríguez-Méndez, B.G.; Muñoz-Castro, A.E.; et al. Accelerated Mice Skin Acute Wound Healing in Vivo by Combined Treatment of Argon and Helium Plasma Needle. Arch. Med. Res. 2013, 44, 169–177.
- Amini, M.R.; Sheikh Hosseini, M.; Fatollah, S.; Mirpour, S.; Ghoranneviss, M.; Larijani, B.; Mohajeri-Tehrani, M.R.; Khorramizadeh, M.R. Beneficial Effects of Cold Atmospheric Plasma on Inflammatory Phase of Diabetic Foot Ulcers; a Randomized Clinical Trial. J. Diabetes Metab. Disord. 2020, 19, 895–905.
- Xu, Z.; Shen, J.; Zhang, Z.; Ma, J.; Ma, R.; Zhao, Y.; Sun, Q.; Qian, S.; Zhang, H.; Ding, L.; et al. Inactivation Effects of Non-Thermal Atmospheric-Pressure Helium Plasma Jet on Staphylococcus Aureus Biofilms. Plasma Process. Polym. 2015, 12, 827–835.
- Xu, G.-M.; Shi, X.-M.; Cai, J.-F.; Chen, S.-L.; Li, P.; Yao, C.-W.; Chang, Z.-S.; Zhang, G.-J. Dual Effects of Atmospheric Pressure Plasma Jet on Skin Wound Healing of Mice. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2015, 23, 878–884.
- Yiu, G.; He, Z. Glial Inhibition of CNS Axon Regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627.
- Katiyar, K.S.; Lin, A.; Fridman, A.; Keating, C.E.; Cullen, D.K.; Miller, V. Non-Thermal Plasma Accelerates Astrocyte Regrowth and Neurite Regeneration Following Physical Trauma In Vitro. Appl. Sci. 2019, 9, 3747.
- Longhitano, Y.; Zanza, C.; Thangathurai, D.; Taurone, S.; Kozel, D.; Racca, F.; Audo, A.; Ravera, E.; Migneco, A.; Piccioni, A.; et al. Gut Alterations in Septic Patients: A Biochemical Literature Review. Rev. Recent Clin. Trials 2020, 15, 289–297.
- Cotter, J.J.; Maguire, P.; Soberon, F.; Daniels, S.; O’Gara, J.P.; Casey, E. Disinfection of Meticillin-Resistant Staphylococcus Aureus and Staphylococcus Epidermidis Biofilms Using a Remote Non-Thermal Gas Plasma. J. Hosp. Infect. 2011, 78, 204–207.
- Maisch, T.; Shimizu, T.; Li, Y.-F.; Heinlin, J.; Karrer, S.; Morfill, G.; Zimmermann, J.L. Decolonisation of MRSA, S. Aureus and E. Coli by Cold-Atmospheric Plasma Using a Porcine Skin Model In Vitro. PLoS ONE 2012, 7, e34610.
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A First Prospective Randomized Controlled Trial to Decrease Bacterial Load Using Cold Atmospheric Argon Plasma on Chronic Wounds in Patients. Br. J. Dermatol. 2010, 163, 78–82.
- Gallant-Behm, C.L.; Yin, H.Q.; Liu, S.; Heggers, J.P.; Langford, R.E.; Olson, M.E.; Hart, D.A.; Burrell, R.E. Comparison of in Vitro Disc Diffusion and Time Kill-Kinetic Assays for the Evaluation of Antimicrobial Wound Dressing Efficacy. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2005, 13, 412–421.
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The Prevalence of Biofilms in Chronic Wounds: A Systematic Review and Meta-Analysis of Published Data. J. Wound Care 2017, 26, 20–25.
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.-U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and Safe Use of 2° Min Cold Atmospheric Argon Plasma in Chronic Wounds: Results of a Randomized Controlled Trial. Br. J. Dermatol. 2012, 167, 404–410.
- Abbasi, E.; Mehrabadi, J.F.; Nourani, M.; Namini, Y.N.; Mohammadi, S.; Esmaeili, D.; Abbasi, A. Evaluation of Cold Atmospheric-Pressure Plasma against Burn Wound Infections and Gene Silencing. Iran. J. Microbiol. 2021, 13, 544–552.
- Nguyen, T.X.; Nguyen, D.H.; Ho-Man, T.P.; Bui, V.D.A.; Phan, P.N. Cold Plasmamed Beam as a Supporting Treatment of Soft Tissue Injuries in Severe COVID-19 Patients: A Preliminary Report. Med. Devices Auckl. N. Z. 2022, 15, 277–283.
- Stratmann, B.; Costea, T.-C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs. Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411.
This entry is offline, you can click here to edit this entry!
