Please note this is an old version of this entry, which may differ significantly from the current revision.
Neuromodulation, specifically spinal cord stimulation (SCS), has become a staple of chronic pain management for various conditions including failed back syndrome, chronic regional pain syndrome, refractory radiculopathy, and chronic post operative pain. Since its conceptualization, it has undergone several advances to increase safety and convenience for patients and implanting physicians.
- chronic pain
- spinal cord stimulation
- neuromodulation
- spinal cord injury
1. Introduction
Chronic pain was systemically classified in the International Classification of Diseases 11 (ICD-11) by the International Association for the Study of Pain (IASP) working group in collaboration with the World Health Organization (WHO) in 2019. They defined chronic pain as pain that persists or recurs for more than 3 months with further subcategorization into six other subgroups including its own disease entity [1]. In the United States alone, it has been found that 50.2 million adults (roughly 20.5% of the US population) report pain daily with significant limitations to social activities and activities of daily living and with associated statistically significant increased days missed from work compared to people without chronic pain (10.3 v. 2.8); this includes an estimated gross domestic product impact of approximately $296 billion dollars lost in productivity, annually [2].
The predominant pain locations in those that responded to this 2019 National Health Interview Survey were back pain, hand/shoulder/arm pain, and hips/knee/foot pain [2]. With the paradigm shift away from predominantly pharmacologic management, historically reliant upon narcotic management due to concerns from the opioid epidemic, opioid sparing management techniques have become popularized including therapy, non-narcotic pharmacologic management, and interventions [3]. A staple of interventional management of chronic pain has been dorsal column spinal cord stimulation (SCS).
Initially utilized primarily for management of pain conditions including failed back syndrome, refractory angina pectoris, peripheral vascular disease, and complex regional pain syndrome (CRPS), SCS has become a mainstay in chronic pain management [4]. Within the past decade, new indications for SCS including management of painful diabetic neuropathy and non-surgical low back pain have increased their utilization in clinical practice [5][6]. Here the researchers review the historical technologic nuances and recent advances in SCS devices (Figure 1).
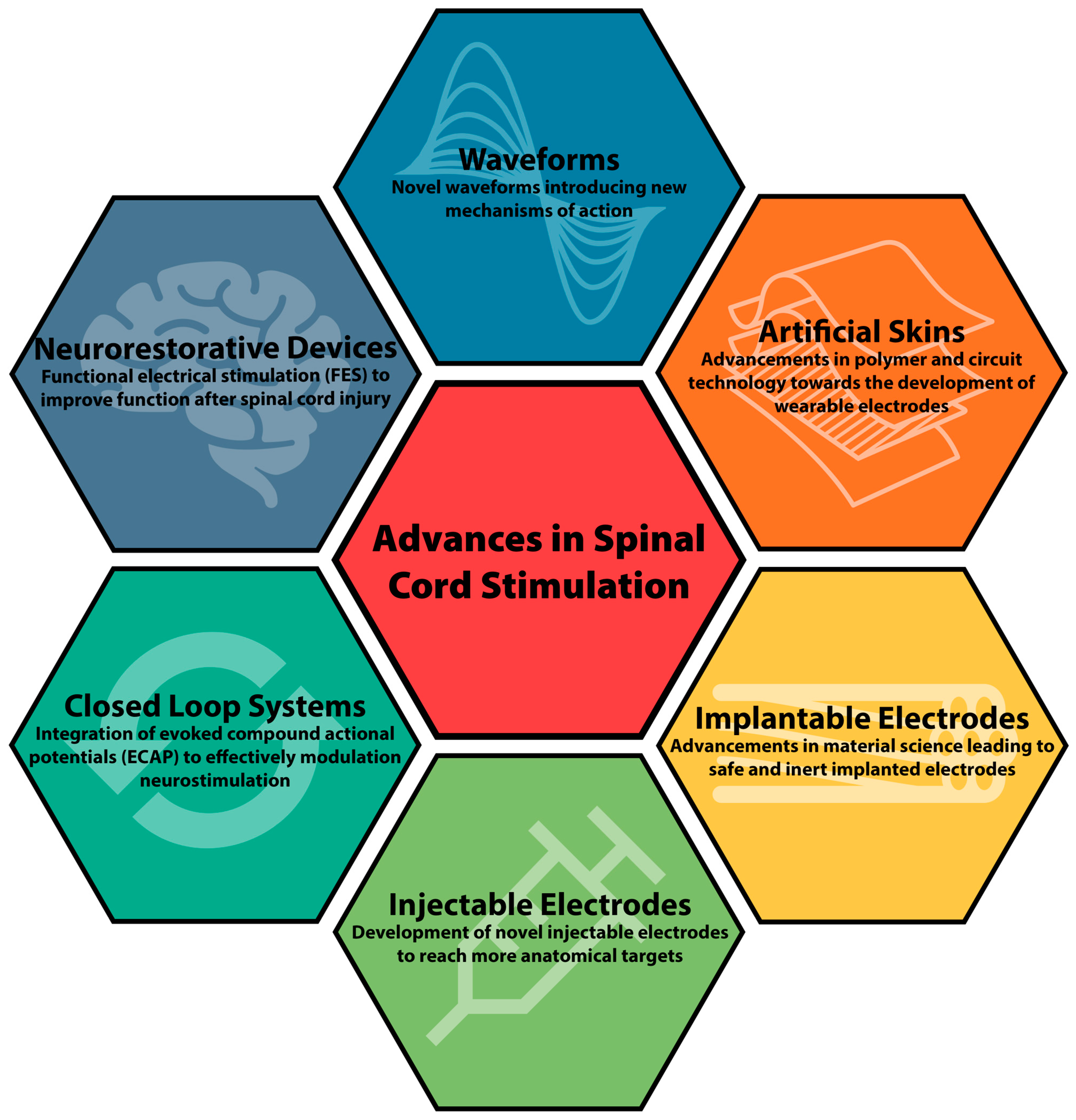
Figure 1. Schematic summary for advances in spinal cord stimulation.
2. History of Spinal Cord Stimulation
One of the earliest recorded forms of neuromodulation was by the ancient Romans in 15 A.D. to alleviate gout pain by incidental contact and subsequent electrical stimulation by torpedo fish (Figure 2) [7][8]. This early concept of transcutaneous electrical nerve stimulation (TENS) has evolved over time with the first modern machine for therapeutic electricity (“Electreat”) progressing to the modern day TENS unit pioneered by Dr. C. Norman Shealy [9]. Over time, advancements in the field of neuromodulation would result in the SCS systems that we have today.
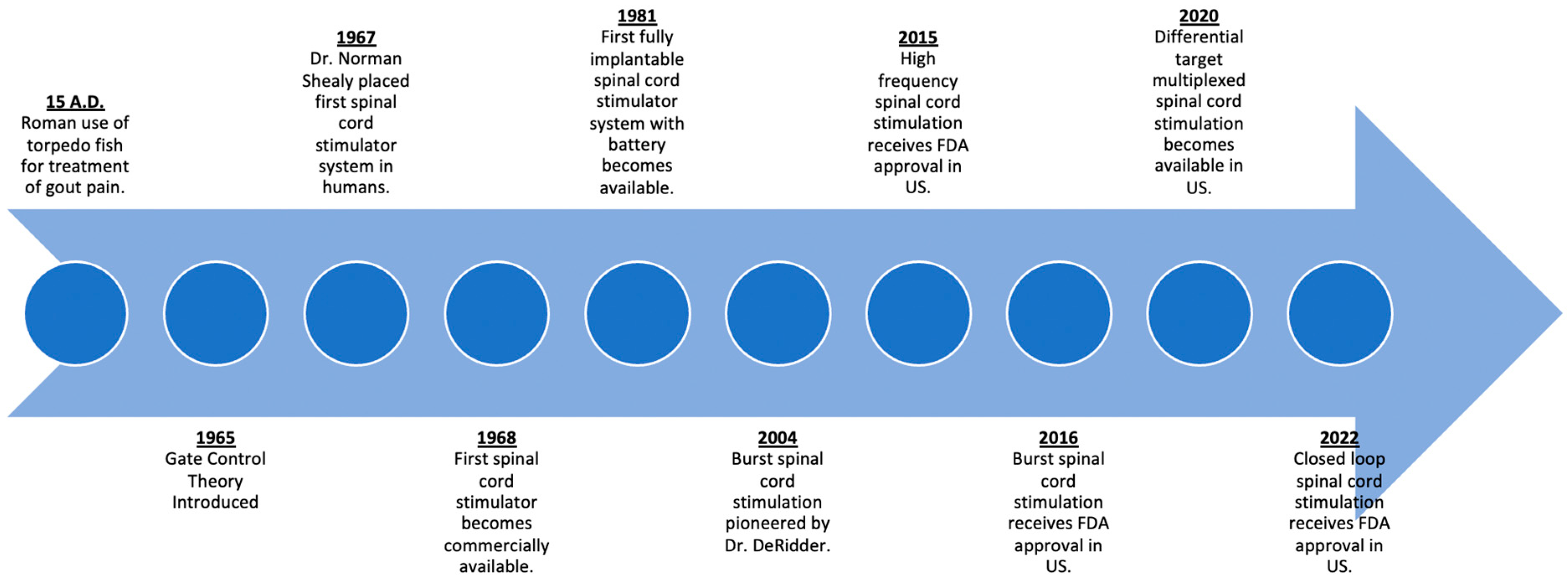
Figure 2. Timeline of advances in spinal cord stimulation.
The first commercially available SCS system was made by Medtronic in 1968, borrowing from their many developments and advances with cardiac pacemakers [8]. The following five decades would see advancements predominately in the hardware of these devices. Early systems consisted of an internal electrode that was surgically implanted via laminectomy. These internal electrodes were connected to a receiving antenna, which interfaced with an external generator and antenna via radiofrequency to generate the power needed for electrical stimulation [10]. By the 1980s, with the advent of lithium-based batteries, fully implantable systems were possible, with the implantable pulse generator (IPG) replacing the radiofrequency-based systems. Subsequently, percutaneously placed leads by needle placement was pioneered as an alternative means of lead placement without the need for a laminotomy [11].
The gate control theory of pain proposed by Malzack and Wall is the first proposed mechanism of action to explain SCS [12]. According to this theory, the transmission of pain signals depended on a balance of activity from larger A-beta fibers, smaller A-delta, and C fibers which modulated the transmission of pain by an inhibitory interneuron [12][13]. Dr. Norman Shealy also pioneered SCS by placing the first system in 1967. This initial configuration involved subdural electrodes placed at the dorsal columns at the level of T2-3. These electrodes were then connected to an external generator, where application of an electrical current 10 to 50 times a second relieved the patient’s chronic chest pain and abdominal pain [14]. It was believed that application of this electrical signal led to an activation of the larger A-beta fibers, leading to increased inhibitory interneuron activity and the closure of the “gate” to the transmission of pain signals. Further research has suggested that the mechanism for pain relief with conventional SCS is incompletely explained by the gate control theory; rather, there are numerous other spinal segmental and supraspinal mechanisms at play [15]. Current belief is that spinal cord stimulation differentially activates the dorsal horn of the dorsal column. Depending on the waveform and stimulation modality, differential activation of various rexed laminae, nerve cell types such as micro glial cells, or selective inhibitory pathway activation may account for the mechanism of action for this treatment modality [16][17][18].
3. Artificial Skins as Electrodes
Advancements in clinical medicine, informatics, and engineering have allowed for the emergence of wearable sensors and artificial skins. These skins or sensors may be able to monitor physiological changes that occur daily. Current applications for these range from the monitoring of clinical illness to the wellness and fitness arena [19]. Of interest is the utilization of these materials for use as spinal cord stimulator electrodes. There are a number of physical properties that skin is able to sense: pressure, strain, temperature, and light [20].
These artificial smart skins also have application in sensing bioelectrical signals, such as electromyography (EMG), electroencephalography (EEG), and electrocardiography (ECG). EEG/EMG is conventionally a rigid electrode combined with an electrolyte gel. Recent advancements by one group were able to show that electrodes can work as single conductive gels. This stretchable hydrogel was created by converting graphene oxide into conductive graphene through a reduction reaction. The performance of this hydrogel was comparable to that of commercially available electrodes [21].
Despite these advances, creating commercially viable artificial skin products presents a difficult challenge for material researchers. Currently in development are ultra-thin polymer foils that can conform to ambient and dynamic environments. These are organic transistors that contain an ultra-dense oxide gate dielectric that is a few nanometers thick and capable of repeated bending and stretching [22].
The fabrication of stretchable circuits with integrated transistors will allow for the next generation of skin electronics. Recently, a group was able to fabricate these intrinsically stretchable electronic polymers and able to achieve a skin-like character [23]. However, due to low gas permeability, most electronic skin devices do not allow for the secretion of sweat from the skin. This can lead to maceration and overhydration inhibiting the barrier function of skin [19]. The creation of a conductive and highly gas-permeable nano-mesh structure by one group may have overcome this issue [24]. Because of the increasing sophistication of materials used for smart skins, large-scale manufacturing will bring on a new set of challenges for the dissemination of this technology [19].
4. Implantable Electrode Material
Implanted electrodes is a robust field of interest in chronic pain management. Utilization of stimulator technology depends on effective electrode-cellular interface. These materials should be able to deliver stimulation to the target area without exceeding a cutoff threshold that could cause damage to the surrounding tissues [25]. Additionally, following implant, the body attempts to isolate the implant from the tissue. There are no side effects noted directly from SCS electrodes, though reports of allergies to spinal cord stimulator materials including electrodes and electrode malfunction have been reported [26][27]. Future materials aim to limit the foreign body response while still providing a durable, sensitive electrode [28].
One of the most important factors that influences the efficacy of stimulator function is impedance [25]. This factor is influenced by the types of materials used, the tissue-cellular interface, and the surface area of the electrode [29]. Increasing the size of the electrode can then allow for less impedance; however, this is often not feasible since limited space in the spine requires smaller electrodes that are resistant to bending and flexing [25][30].
Most commonly, substrates such as platinum/iridium, platinum, titanium, and gold are used for the stimulation and recording of electrodes. However, compared to bare electrodes, the addition of a coating allows for improved resolution [25]. Iridium oxide has been applied as a coating with hopes to improve neural stimulation [31]. The layers of iridium oxide offer improved injection capacity and lower impedance when compared to bare electrodes [30][31]. Despite the improved performance, the electrode structure is susceptible to degradation which could form cracks and leak particles into nearby tissues.
Despite a field that is largely dominated by metals, conductive polymers may offer improved electrical properties and reduced mechanical mismatch between electrodes and tissues [32]. Additionally, they are capable of housing and releasing drugs and bioactive materials [33]. These conductive polymers themselves are not conductive but work through a mechanism of doping. This means that a charge is introduced to the polymer chain by a dopant while preserving a neutral system [30][33]. The surface area of conductive polymers, due to nano structuring, is significantly increased, which allows for decreased impedance and improved capacitance [34].
5. Injectable Electrodes
Implanted devices utilized for recording and stimulation can aid patients suffering from a variety of neurological conditions. However, given the complexity and cost associated with these devices, some companies have looked for alternative methods to improve access for patients. One company has developed an injectable electrode (Injectrode®). This electrode is a flowable pre-polymer, and upon injection, cures to form a compliant neural electrode in vivo [35]. Given the ability to conform as needed, it may offer the ability to be utilized as an electrode for a variety of neuroanatomical targets.
The Injectrode® was initially prepared by mixing platinum curing silicone elastomer and silver metallic flakes [35]. This creates an electrode with a Young’s modulus less than 100 kPa. This offers an electrode that is much less stiff than the current neural interface wires. The reduction in mechanical mismatch may lead to reduced strain and stress on the device and surrounding anatomy [35][36]. This concept has been demonstrated with previous soft electrodes which have improved electrode acceptance and reduced inflammatory response [37].
More recently, one team attempted to compare afferent fiber recruitment utilizing the Injectrode® and DRG stimulation. This was achieved by exposing the L6 and L7 DRG in 4 cats. The DRG was then stimulated by either the Injectrode® or a stainless-steel electrode. The antidromic evoked compound action potentials (ECAP) in various nerves throughout the body were then recorded. The recruitment rates and charge-thresholds were then calculated. This study demonstrated that the Injectrode® recorded similar ECAP thresholds to that of the stainless-steel wire across all primary afferent neurons. Thus, the authors concluded that the Injectrode® can stimulate primary afferent neurons and may be applicable in the clinical setting [38]. Despite the significant success, further biocompatibility studies will need to be conducted. This is because the initial Injectrode® samples utilized silver, which has known toxic effects [39].
6. Closed-Loop Systems
The advent of closed-loop spinal cord stimulation was yet another major paradigm shift in regard to SCS strategies. While previous stimulation strategies operated via a fixed-output, open-loop manner, closed-loop stimulation allowed for the incorporation of real-time feedback. The linear electrode array design used in SCS systems allows for the evaluation of electrically evoked compound action potentials. A therapeutic stimulus from one of the electrodes leads to the generation of an ECAP, which then propagates both orthodromically and antidromically. The remainder of the unused electrodes can be utilized to measure the ECAP in both directions [40]. The difference between the charge generated at an electrode and the charge that is delivered to the spinal cord is directly proportional to the distance between the electrode and the target site. With the epidurally placed electrodes, this distance is primarily determined by the thickness of the dura and the dorsal CSF layer [41]. It is well established that postural changes and physiological movements (e.g., respirations, heartbeats) can result in minute changes in this distance, which can have a significant change in charge delivered per Coulomb’s law.
ECAPs are measurements of electrical response from the measured tissues of interest. In SCS, ECAPs are recordings of the total voltage change in the extracellular matrix that surrounds the bundles of axons and are measures of neural activation during SCS [42][43][44]. The ECAP electrodes are positioned at an optimal distance away from stimulating SCS electrodes in order to measure full potentials while minimizing artifact. Of note, artifacts are high-amplitude patterns that are caused by the SCS stimulation. Proper distancing between the stimulation and ECAP measurement electrodes allows time to differentiate between the artifact and ECAP because the artifact will present earlier than the ECAP itself. Short pulse widths, filtering, and template correlation are additional methods of reducing the artifact and sharpening the ECAP signals [42].
Each ECAP has a notable triphasic shape: a voltage peak (P1), negative deflection (N1), and second positive voltage peak (P2). The amplitude is the measured voltage difference between N1 and P2 [40][42]. ECAP with amplitude settings can be used to optimize spinal cord stimulator relief in open- and closed-loop systems. For example, when measured in the clinical setting, an optimal ECAP range for correlating clinical pain relief can be targeted and manually adjusted for during postural changes. Moreover, in closed-loop stimulator systems, ECAP feedback during distance changes between leads and target tissue can be used to automatically adjust stimulation levels, allowing for therapeutic compensation without manual manipulation. For optimal ECAP ranges, systems have suggested using the lowest amplitude that produces a perceptible paresthesia as the lower limit, while using the patient’s highest tolerable stimulation amplitude as the upper limit [42]. Thus, with the measurement of the generated ECAP and comparison to a target ECAP, SCS systems can utilize this real-time feedback to adjust current throughput accordingly to maximize the therapeutic effect [45].
7. Neurorestorative Devices
Closed-loop spinal cord stimulation has restorative implications for patients with spinal cord injury (SCI). Worldwide, SCI affects 10.4 to 83 per 1 million persons per year [46]. Disability from SCI is highly variable, ranging from mild weakness to tetraplegia with dependence on mechanical ventilation. Lifetime healthcare and living expenses are as high as $5.1 million per patient depending on age and the severity of injury [47]. This does not include indirect costs, including over $77,000 per year in lost wages and other benefits [47]. A patient’s degree of functional disability correlates with several negative outcomes: poor quality of life, reduced longevity, and mortality [48][49][50]. The relationship between functional disability and morbidity drives interest in true functional restoration for SCI patients.
Traditional medical treatment for SCI focuses on assistive equipment and physical therapy to optimize remaining motor function [46]. These help patients adapt to their level of disability but are limited in their ability to restore function. Over the past decade, functional electrical stimulation (FES) has emerged as an additional treatment modality. In FES, electrical stimulation is applied locally to a nerve or muscle to augment a specific weak motor activity [51]. Several devices exist, ranging from transcutaneous to fully implanted. However, these devices have significant limitations. They require triggering by the patient, either using a switch (e.g., the heel touching the ground when taking a step) or by voluntary activation of a nearby muscle [51]. FES also uses fixed stimulation output in response to triggering, not an adaptive response specific to the intended movement [51]. Tonic dorsal column spinal cord stimulation has also been used to augment strength in patients with incomplete SCI, but also suffers from the inability to finely adjust the desired motor response [52].
In contrast to FES, epidural electrical stimulation (EES) describes stimulation through electrodes implanted in the epidural space inside the spinal canal or placed transcutaneously over the spine. Scientific evidence has shown that EES of lumbosacral spinal circuitry can generate tonic and rhythmic patterns of motor activity in animals as well as humans diagnosed with complete SCI [53][54][55][56]. Moreover, EES in humans with complete loss of motor function after SCI enabled restoration of flexion and extension of leg movements, standing, and stepping following several months of training [57][58].
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering10020185
References
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27.
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2021, 163, e328–e332.
- Manchikanti, L.; Kaye, A.M.; Knezevic, N.N.; McAnally, H.; Slavin, K.; Trescot, A.M.; Blank, S.; Pampati, V.; Abdi, S.; Grider, J.S.; et al. Responsible, Safe, and Effective Prescription of Opioids for Chronic Non-Cancer Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2017, 20, S3–S92.
- Lee, A.W.; Pilitsis, J.G. Spinal cord stimulation: Indications and outcomes. Neurosurg. Focus 2006, 21, E3.
- Petersen, E.A.; Stauss, T.; Scowcroft, J.; Brooks, E.; White, J.; Sills, S.; Amirdelfan, K.; Guirguis, M.; Xu, J.; Yu, C.; et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients with Painful Diabetic Neuropathy: A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 687–698.
- Al-Kaisy, A.; Van Buyten, J.P.; Kapural, L.; Amirdelfan, K.; Gliner, B.; Caraway, D.; Subbaroyan, J.; Edgar, D.; Rotte, A. 10 kHz spinal cord stimulation for the treatment of non-surgical refractory back pain: Subanalysis of pooled data from two prospective studies. Anaesthesia 2020, 75, 775–784.
- Gildenberg, P.L. Evolution of spinal cord surgery for pain. Clin. Neurosurg. 2006, 53, 11–17.
- Nahm, F.S. From the torpedo fish to the spinal cord stimulator. Korean J. Pain 2020, 33, 97–98.
- Teoli, D.; An, J. Transcutaneous Electrical Nerve Stimulation; StatPearls: Treasure Island, FL, USA, 2022.
- Gildenberg, P.L. History of Electrical Neuromodulation for Chronic Pain. Pain Med. 2006, 7, S7–S13.
- Deer, T.R.; Lamer, T.J.; Pope, J.E.; Falowski, S.M.; Provenzano, D.A.; Slavin, K.; Golovac, S.; Arle, J.; Rosenow, J.M.; Williams, K.; et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Safety Guidelines for the Reduction of Severe Neurological Injury. Neuromodulation 2017, 20, 15–30.
- Melzack, R.; Wall, P. Pain mechanisms: A new theory. Science 1965, 150, 971–979.
- Wall, P.D. Presynaptic Control of Impulses at the First Central Synapse in the Cutaneous Pathway. Prog. Brain Res. 1964, 12, 92–118.
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth. Analg. 1967, 46, 489–491.
- Caylor, J.; Reddy, R.; Yin, S.; Cui, C.; Huang, M.; Huang, C.; Rao, R.; Baker, D.G.; Simmons, A.; Souza, D.; et al. Spinal cord stimulation in chronic pain: Evidence and theory for mechanisms of action. Bioelectron. Med. 2019, 5, 12.
- Lee, K.; Lee, D.; Kagan, Z.; Wang, D.; Bradley, K. Differential Modulation of Dorsal Horn Neurons by Various Spinal Cord Stimulation Strategies. Biomedicines 2021, 9, 568.
- Chakravarthy, K.; Fishman, M.A.; Zuidema, X.; Hunter, C.W.; Levy, R. Mechanism of Action in Burst Spinal Cord Stimulation: Review and Recent Advances. Pain Med. 2019, 20 (Suppl. S1), S13–S22.
- Vallejo, R.; Bradley, K.; Kapural, L. Spinal Cord Stimulation in Chronic Pain: Mode of Action. Spine 2017, 42 (Suppl. S14), S53–S60.
- Someya, T.; Amagai, M. Toward a new generation of smart skins. Nat. Biotechnol. 2019, 37, 382–388.
- Nie, B.; Liu, S.; Qu, Q.; Zhang, Y.; Zhao, M.; Liu, J. Bio-inspired flexible electronics for smart E-skin. Acta Biomater. 2022, 139, 280–295.
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 1601916.
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwödiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458–463.
- Wang, S.; Xu, J.; Wang, W.; Wang, G.-J.N.; Rastak, R.; Molina-Lopez, F.; Chung, J.W.; Niu, S.; Feig, V.R.; Lopez, J.; et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 2018, 555, 83–88.
- Miyamoto, A.; Lee, S.; Cooray, N.F.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913.
- Jalili, R.; Kanneganti, A.; Romero-Ortega, M.I.; Wallace, G.G. Implantable electrodes. Curr. Opin. Electrochem. 2017, 3, 68–74.
- Ochani, T.D.; Almirante, J.; Siddiqui, A.; Kaplan, R. Allergic Reaction to Spinal Cord Stimulator. Clin. J. Pain 2000, 16, 178–180.
- Simopoulos, T.; Sharma, S.; Aner, M.; Gill, J.S. The Long-Term Durability of Multilumen Concentric Percutaneous Spinal Cord Stimulator Leads. Pain Pract. 2018, 18, 845–849.
- Durand, D.M.; Ghovanloo, M.; Krames, E. Time to address the problems at the neural interface. J. Neural Eng. 2014, 11, 020201.
- Miller, S.; Matharu, M.S. The Use of Electroceuticals and Neuromodulation in the Treatment of Migraine and Other Headaches. In Electroceuticals: Advances in Electrostimulation Therapies; Majid, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–33.
- Luo, Y.; Sun, S.; Xie, S. Spinal cord stimulators: Implanted electrode materials, power transfer mechanism, and their applications. In Proceedings of the International Conference on Optoelectronic Materials and Devices (ICOD 2021), Guangzhou, China, 10–12 December 2021; Volume 1216412.
- Cogan, S.; Troyk, P.; Ehrlich, J.; Plante, T. In Vitro Comparison of the Charge-Injection Limits of Activated Iridium Oxide (AIROF) and Platinum-Iridium Microelectrodes. IEEE Trans. Biomed. Eng. 2005, 52, 1612–1614.
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e17–e35.
- Mandal, H.S.; Kastee, J.S.; McHail, D.G.; Rubinson, J.F.; Pancrazio, J.J.; Dumas, T.C. Improved Poly(3,4-Ethylenedioxythiophene) (PEDOT) for Neural Stimulation. Neuromodulation 2015, 18, 657–663.
- Wallace, G.G.; Moulton, S.E.; Kapsa, R.M.I.; Higgins, M.J. Organic Conducting Polymers. In Organic Bionics; Wiley-VCH: Weinheim, Germany, 2012; pp. 81–112.
- Trevathan, J.K.; Baumgart, I.W.; Nicolai, E.N.; Gosink, B.A.; Asp, A.J.; Settell, M.; Polaconda, S.R.; Malerick, K.D.; Brodnick, S.K.; Zeng, W.; et al. An Injectable Neural Stimulation Electrode Made from an In-Body Curing Polymer/Metal Composite. Adv. Healthc. Mater. 2019, 8, e1900892.
- Sridharan, A.; Nguyen, J.; Capadona, J.; Muthuswamy, J. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo. J. Neural Eng. 2015, 12, 036002.
- Sohal, H.S.; Clowry, G.J.; Jackson, A.; O’Neill, A.; Baker, S.N. Mechanical Flexibility Reduces the Foreign Body Response to Long-Term Implanted Microelectrodes in Rabbit Cortex. PLoS ONE 2016, 11, e0165606.
- Dalrymple, A.N.; Ting, J.E.; Bose, R.; Trevathan, J.K.; Nieuwoudt, S.; Lempka, S.F.; Franke, M.; Ludwig, K.A.; Shoffstall, A.J.; Fisher, L.E.; et al. Stimulation of the dorsal root ganglion using an Injectrode®. J. Neural Eng. 2021, 18, 056068.
- Drake, P.L.; Hazelwood, K.J. Exposure-Related Health Effects of Silver and Silver Compounds: A Review. Ann. Occup. Hyg. 2005, 49, 575–585.
- Parker, J.L.; Karantonis, D.M.; Single, P.S.; Obradovic, M.; Cousins, M.J. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain 2012, 153, 593–601.
- He, J.; Barolat, G.; Holsheimer, J.; Struijk, J. Perception threshold and electrode position for spinal cord stimulation. Pain 1994, 59, 55–63.
- Vallejo, R.; Chakravarthy, K.; Will, A.; Trutnau, K.; Dinsmoor, D. A New Direction for Closed-Loop Spinal Cord Stimulation: Combining Contemporary Therapy Paradigms with Evoked Compound Action Potential Sensing. J. Pain Res. 2021, 14, 3909–3918.
- Brooker, C.; Russo, M.; Cousins, M.; Taylor, N.; Holford, L.; Martin, R.; Boesel, T.; Sullivan, R.; Hanson, E.; Gmel, G.; et al. ECAP-Controlled Closed-Loop Spinal Cord Stimulation Efficacy and Opioid Reduction Over 24-Months: Final Results of the Prospective, Multicenter, Open-Label Avalon Study. Pain Pract. 2021, 21, 680–691.
- Levy, R.; Deer, T.; Poree, L.; Rosen, S.; Kapural, L.; Amirdelfan, K.; Soliday, N.; Leitner, A.; Mekhail, N. Multicenter, Randomized, Double-Blind Study Protocol Using Human Spinal Cord Recording Comparing Safety, Efficacy, and Neurophysiological Responses Between Patients Being Treated with Evoked Compound Action Potential–Controlled Closed-Loop Spinal Cord Stimulation or Open-Loop Spinal Cord Stimulation (the Evoke Study). Neuromodulation Technol. Neural Interface 2019, 22, 317–326.
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M.; et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): A double-blind, randomised, controlled trial. Lancet Neurol. 2020, 19, 123–134.
- Karsy, M.; Hawryluk, G. Modern Medical Management of Spinal Cord Injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 65.
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury. Facts and Figures at a Glance. 2017. Available online: https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%202020.pdf (accessed on 19 October 2022).
- Cao, Y.; Massaro, J.F.; Krause, J.S.; Chen, Y.; Devivo, M.J. Suicide Mortality After Spinal Cord Injury in the United States: Injury Cohorts Analysis. Arch. Phys. Med. Rehabilitation 2014, 95, 230–235.
- Strauss, D.J.; DeVivo, M.J.; Paculdo, D.R.; Shavelle, R.M. Trends in Life Expectancy After Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2006, 87, 1079–1085.
- Chamberlain, J.D.; Meier, S.; Mader, L.; von Groote, P.M.; Brinkhof, M.W. Mortality and Longevity after a Spinal Cord Injury: Systematic Review and Meta-Analysis. Neuroepidemiology 2015, 44, 182–198.
- Marquez-Chin, C.; Popovic, M.R. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: A review. Biomed. Eng. Online 2020, 19, 34.
- Duffell, L.D.; Donaldson, N.D.N. A Comparison of FES and SCS for Neuroplastic Recovery After SCI: Historical Perspectives and Future Directions. Front. Neurol. 2020, 11, 607.
- Capogrosso, M.; Milekovic, T.; Borton, D.; Wagner, F.; Moraud, E.M.; Mignardot, J.-B.; Buse, N.; Gandar, J.; Barraud, Q.; Xing, D.; et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016, 539, 284–288.
- Wenger, N.; Moraud, E.M.; Gandar, J.; Musienko, P.; Capogrosso, M.; Baud, L.; Le Goff, C.G.; Barraud, Q.; Pavlova, N.; Dominici, N.; et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat. Med. 2016, 22, 138–145.
- Wenger, N.; Moraud, E.M.; Raspopovic, S.; Bonizzato, M.; DiGiovanna, J.; Musienko, P.; Morari, M.; Micera, S.; Courtine, G. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci. Transl. Med. 2014, 6, 255ra133.
- Dimitrijevic, M.R.; Gerasimenko, Y.; Pinter, M.M. Evidence for a spinal central pattern generator in humans. Ann. N. Y. Acad. Sci. 1998, 860, 360–376.
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682.
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250.
This entry is offline, you can click here to edit this entry!
 Encyclopedia
Encyclopedia
