Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Dynamic regulation of myosin filaments is a crucial factor in the ability of airway smooth muscle (ASM) to adapt to a wide length range. Increased stability or robustness of myosin filaments may play a role in the pathophysiology of asthmatic airways. Biochemical techniques for the purification of myosin and associated regulatory proteins could help elucidate potential alterations in myosin filament properties of asthmatic ASM.
- airway smooth muscle
- asthma
- myosin filaments
- myosin regulatory proteins
1. Introduction
In hollow organs that undergo large volume changes, the smooth muscle lining the walls readily modifies its contractile function in order to suit the changing geometry. A striking property of smooth muscle myosin is its ability to be assembled and disassembled rapidly in an intact, functioning smooth muscle cell. This evanescence of myosin filament allows the muscle to swiftly rearrange its contractile units and adapt to large changes in muscle cell length [1], thereby maintaining optimal physiological functions [2]. Evanescence of myosin filaments has been proposed as one of the key mechanisms responsible for the proper functions of airway smooth muscle (ASM) and its alteration responsible for the pathological features in some airway diseases [3]. It has been suggested that overly stable myosin filaments (i.e., lack of evanescence) may contribute to the pathophysiology of asthma [4][5]. An increased Rho kinase protein content reported in asthmatic airways [6] may lead to increased stability of myosin filaments, resulting in the lack of response of asthmatic airways to the bronchodilatory and bronchoprotective effect of deep inspiration [7][8][9][10][11].
Smooth muscle myosin molecules in solution are known to self-assemble into filaments, and the stability of the filaments can be tested in vitro [12]. One critical piece of missing information is the structural stability of myosin filaments from asthmatic ASM compared with that of non-asthmatic ASM. To better understand the dynamics of myosin, it is useful to establish an effective methodology for isolating functional smooth muscle myosin from the airways.
While the properties of smooth muscle myosin in vitro are largely modified by the ionic conditions [13] and pH [14], they are well regulated in vivo by the cellular milieu, resulting in the characteristic malleability of the filaments in comparison to that of skeletal muscle. It was originally shown that the solubilized smooth muscle myosin molecule may adopt one of two possible configurations: the folded one (a loop) being more soluble, or the extended (straight) one, less soluble, aggregating to dimers and tetramers [15][16]. Such tetramers are associated with coiled-coil rods, forming what is considered in vivo the myosin filament-building units [17].
In skeletal muscle, contraction is primarily regulated by the troponin regulatory complex, which includes a Ca2+ binding subunit. By contrast, there is no troponin in smooth muscle [18]. Instead, its analogous protein calmodulin (CaM), which has four Ca2+ binding sites, can respond to changing intracellular Ca2+ concentration by binding to Ca2+. The resulting Ca2+- CaM complex activates the myosin light chain kinase (MLCK). MLCK, in turn, phosphorylates the 20 kDa regulatory myosin light chain (ReLC) to initiate a contraction [19][20][21][22][23]. Relaxation requires dephosphorylation of the ReLC by myosin light chain phosphatase (MLCP). Although other modulatory proteins such as telokin (TL) and structural proteins such as filamin or caldesmon are associated with myosin filaments, Sobieszek and colleagues [24][25] concluded that the regulatory complex is composed of CaM, MLCK, and MLCP and that this regulatory complex is bound to (and is co-purified with) the myosin filaments. Another group of researchers has also pointed out that MLCK and CaM co-purified with smooth muscle myosin due to their tight association with myosin rather than actin [26]. Depending on buffer composition, various degrees of MLCK can be copurified and/or removed from myosin [27]. The employment of a reliable procedure for the purification of smooth muscle myosin and the study of the regulatory complex is of primary importance to the understanding of normal physiological functions and possible dysfunctions of ASM.
2. Evolvement of the Sobieszek–Bremel Purification Approach for Smooth Muscle Myosin (from Gizzard to Airway Smooth Muscle)
Smooth muscle contains myosin and actin, both tethered with their regulatory proteins and enzymes. In order to purify it, myosin must be separated from all other components. To find the best way to separate out or purify myosin while preserving its biological function and characteristics is the basis of the evolvement of the Sobieszek–Bremel purification approach over the years.
Sparrow and colleagues [28] were the first to develop a method to extract actomyosin that maintained some sensitivity to Ca2+. However, the extracted actomyosin exhibited relatively low ATPase activity. A remarkable advancement in the approach and procedures of myosin purification was achieved by Sobieszek and Bremel [18]. These authors obtained purified myosin from a crude actomyosin by precipitation at a high MgCl2 concentration followed by precipitation in ammonium sulphate (am.sulf.). The essence of this approach is to create the condition for myosin and actin to be both in filamentous form and dissociated. This is accomplished by extensive fragmentation (mincing) of the smooth muscle tissue followed by thorough homogenization at low ionic strength and removal of the non-contractile proteins by repetitive washing and centrifugation. Crude actomyosin is then extracted from the resulting myofibril-like preparation (MYF) in the presence of ATP and calcium-chelating agents (EDTA and EGTA). The ATPase activity of the crude actomyosin is relatively high and Ca2+ sensitive, representing a suitable source for myosin purification, namely a simple precipitation at high MgCl2 concentration [22].
In a follow-up study, it was determined that divalent cations alone were effective in causing actomyosin precipitation overnight; the obtained actomyosin was 4–5 times more sensitive to Ca2+ than that obtained with am.sulf. precipitation, although the high Mg2+ concentration appeared to have determined a reduced actin-activated ATPase activity [29]. In a study using pig stomachs, the actomyosin obtained using 2 mM Ca2+ precipitation showed high ATPase activity, but very low sensitivity to Ca2+ [19]. The authors tested various modifications to the original Sobieszek–Bremel method in pig stomachs but did not detect substantial improvements. Tested modifications included total precipitation of the crude actomyosin with am.sulf. (55–60% saturation), precipitation with Ca2+ or Mg2+ together with am.sulf. (60% saturation), and extraction at intermediate or high ionic strength (0.12 and 0.5 M KCl, respectively).
In a further modification of the method described in a study using turkey gizzard, Bis Wash (BW) buffer was used in place of the “washing buffer” of the original recipe to obtain MYF, followed by low ionic strength actomyosin extraction solution (LAMES) to obtain crude actomyosin. Actin was then removed from crude actomyosin to yield pure myosin [23]. This protocol was successfully applied to bovine tracheal smooth muscle [12]. The general scheme of the purification process is depicted in Figure 1. Modification and improvements from the original method have been described in the subsequent reviews by Small and Sobieszek [20] and Sobieszek [23]. The scheme in Figure 1 of the present report is an extension of previously published schemes to include the latest observations and improvements, specifically for bovine tracheal smooth muscle. During purifications of myosin from gizzard muscle and pig stomach, researchers had no difficulty in collecting the assembled filaments after dialysis against BW solution, provided that the dialysis was sufficiently long and included two or three changes in the BW. Dialysis in the cold room was terminated when precipitation became visible at the top part of the dialyzing bag; despite some “loss” of myosin in the supernatant, the amounts of purified myosin were always large. However, this was not the case for the MYF from bovine tracheal smooth muscle. In the case of the airways, the amount of starting material is limited, thus requiring further improvement of the protocol to minimize the loss of myosin in the process of purification.
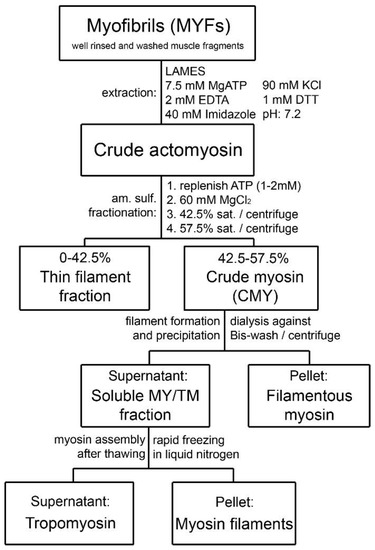
Figure 1. Schematic diagram of the purification steps developed for bovine tracheal smooth muscle myosin starting from isolated myofibrils. The approach was originally developed for gizzard muscle [18].
There are important technical steps in the Sobieszek protein purification method for smooth muscle myosin that must be taken into consideration. When starting from the whole muscle, it is important to dissect smooth muscle free of connective tissue and fat, so that the preparation contains as little as possible contamination of non-muscle tissue. The presence of large amounts of connective tissue in the muscle preparation makes myosin extraction difficult. The presence of filamentous actin can also significantly modify the physical and biochemical properties of myosin preparation [23]. Therefore, the purity of the starting tissue is of great importance to ensure the quality and quantity of extracted myosin.
After dissection, the muscle can either be used fresh or frozen before the homogenization step. If used fresh, the muscle should be minced extensively, since, as noted in the original publication for this procedure [18], the mincing step ultimately determines the size of the fibril bundles. Freezing the dissected muscle in liquid nitrogen (LN2) [12] allows for pulverization of the muscle tissue, which has the advantage of achieving better contact of the tissue with the homogenization solution as well as optimal preservation of the biochemical properties of myosin. Whether to use minced or frozen tissue also depends on the amount of preparation. For minimal portions (grams) of muscle, the use of frozen and then pulverized tissue yields better results; for large amounts of muscle, mincing fresh tissue may be a more appropriate way to facilitate homogenization. A mincer equipped with a special 1.25 mm-hole plate is a great option for large amounts of tissue when considering the losses of material involved. After mincing, the researchers recommend using the Sorvall type omni-mixer homogenizer, because of its very effective cutting edges of the blades, and a tight glass–glass Dounce-type homogenizer. Two or more cycles of homogenization, centrifugation, and resuspension produce the MYF-like preparation from which crude actomyosin is extracted.
3. Characterization of the Purified Myosin from Bovine Tracheal Smooth Muscle
3.1. Composition of Purified Bovine Tracheal Smooth Muscle Actomyosin
Although large volumes of extraction buffer and several repeated extractions in succession are required to completely extract crude actomyosin from smooth muscle MYF, a single extraction, with the extraction buffer volume at five times that of the muscle myofibril, yields a considerable portion of the actomyosin in high concentration (up to 25 mg/mL). The predominant or readily identified species in the extracted smooth muscle crude actomyosin are, besides myosin with its regulatory (Re) and essential (Es) light chains (LCs), actin and the actin-binding proteins tropomyosin (TM), α-actinin, and filamin. Other proteins in the crude actomyosin extract need further purification steps to be identified as bands on the gel. These proteins include the regulatory MLCK and its activator CaM, as well as MLCP. A high-molecular-weight protein normally not entering the gradient gels can be also recognized during myosin purification of the bovine tracheal smooth muscle. This most likely corresponds to the smitin discovered by Kim and Keller [30]. The role of smitin in smooth muscle is not currently understood, although it can be hypothesized, based on its large titin-like size, that it interacts with myosin and may be involved in the myosin filament assembly. Researchers suggest that the observed smearing or reduced penetration of the myosin heavy chains into the top stacking gel containing urea could be due to the presence of smitin in the purified myosin.
3.2. Regulatory Light Chain (ReLC) Phosphorylation of Purified Myosin
Regulatory light chain phosphorylation of the purified myosin can be estimated from UG PAGE first introduced by Sobieszek and Jertschin [31], or determined exactly by 32P incorporation from radioactive ATP. For exact determination, the phosphorylation reaction is initiated in the presence of 0.1 mM CaCl2 by the addition of an ATP solution in which γ32P-ATP is only present at a negligible concentration in comparison to “cold”, unlabeled ATP. The mixed suspension is quenched in a solution of 8.5 M urea and 40 mM 4-(hydroxymercuril) benzoic acid with vortex. The quenched mixture is spotted on 2 × 4 cm pieces of Whatman 3MM chromatographic paper and processed for radioactivity counting. The pieces are then dropped into a 10% TCA solution and extensively washed with several changes of water to remove the non-incorporated radioactive ATP. The advantage of this determination is in the use of water as a convenient “scintillant” (Cherenkov radiation!) for the counts per minute instead of the flammable organic scintillant commonly used for radioactivity determination [32]. Another advantage of using radioactive ATP is the relatively short decay time of the radioactive 32P, since it only requires a few months of storage for the radioactive waste to be safely disposed of as a non-radioactive one.
In the case of the parallel 32P incorporation and UG PAGE determination of the ReLC phosphorylation, the reaction is terminated by the addition of 8.5 M urea as previously described [12]. The ReLC has a high phosphorylation rate, therefore it can only be determined by this method at 0°C (on ice) when the phosphorylation rate is reduced by 10-fold in comparison to that at 37 °C. At 0 °C, the phosphorylation reaches a maximum within 30–60 s from the starting of the phosphorylation reaction, which then slowly declines as a result of dephosphorylation by the presence of MLCP. Dephosphorylation is enhanced by the depletion of ATP as well as by the residual ATPase activity of the myosin alone [12]. The essential light chains (EsLCs) can be recognized on UG PAGE gels to migrate as a doublet because of their different charges.
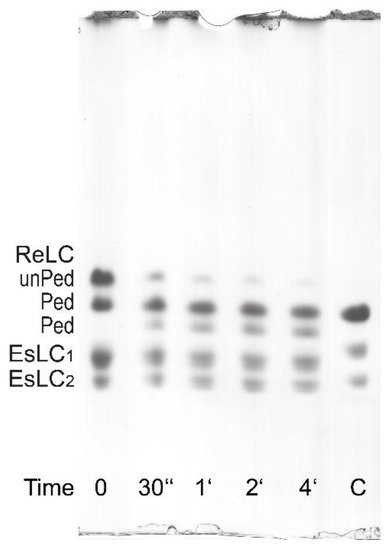
Figure 7. Phosphorylation assay of the purified bovine tracheal smooth muscle myosin ReLC on UG PAGE. As indicated by the labels on the left, the top bands correspond to unphosphorylated ReLC (unPed); the second and the third bands correspond to phosphorylated ReLC (Ped). The lower doublets correspond to pairs of the EsLCs and are present in all lanes. The phosphorylation assay was performed at 0 °C (on ice) by adding MgATP in the presence of 0.1 mM CaCl2. The decreasing intensity of the top bands and the increasing intensity of the third bands show the presence of the endogenous CaM/MLCK complex. For comparison, a phosphorylated turkey gizzard myosin (lane C) is included.
It is also possible to evaluate myosin phosphorylation from standard (non-radioactive) ATPase activity assay (AA) with phosphate determination. In this case, after the termination of the reaction with 10% TCA solution, the normally discarded and denatured small protein pellet can be resuspended in water and pelleted again to be dissolved in the urea/2-mercaptoethanol solution and processed as for the UG PAGE [33]. As shown in Figure 7, the ReLC is phosphorylated by the endogenous CaM/MLCK complex. The second band in Figure 7 can be interpreted as ReLC being phosphorylated at the first and/or second sites, while the third band can be interpreted as phosphorylation occurring at the third and/or fourth sites based on the notion of ordered or sequential phosphorylation described by Persechini and Hartshorne [34].
3.3. Formation of Filamentous Myosin by Dialysis
Purified myosin molecule retains its secondary and tertiary structure. Visualized under an atomic force microscope, non-phosphorylated myosin molecules in a solution of high ionic strength (500 mM) clearly show the head and tail regions [12]. Myosin filaments form when the ionic strength is lowered, especially when ReLC phosphorylation is not inhibited. The filaments can be formed by dialyzing a solution of myosin monomers (1.3 mg/mL) at a high ionic strength relaxing solution (composition in mM: 5 EGTA, 1 Mg2+, 5 MgATP, and 1- PIPES, sufficient KCl to produce 500 mM ionic strength, pH adjusted to 7.0 at 25 °C) against a buffer containing 2 mM MgCl2 over 17 h at 4 °C during which the ionic strength is decreased linearly and gradually from 500 to 88 mM.
Alternatively, purified myosin at a concentration of 2 μg/mL can readily form filaments in activating solution at low ionic strength (80 mM) (composition in mM: same as the above relaxing solution with the addition of 100 nM microcystin, 2 mM CaCl2, and 5 mM ATP to induce ReLC phosphorylation). The formation of filaments confirms that the C-terminal end of the coiled–coil domain, i.e., the critical 28 residues responsible for myosin filament formation [35] is completely intact. The co-purified or native-like CaM/MLCK complex facilitates the phosphorylation of the ReLC, which promotes myosin filament assembly [36] and increases filament stability, such that the filaments do not disassemble even in the presence of ATP [14]. When visualized under an atomic microscope or an electronic microscope, myosin filaments formed in relaxing solution appear to have larger diameters than those formed in activating solution, though filaments formed in activating solution appear in greater numbers [12]. Furthermore, the filaments formed in activating solution are much more resilient than filaments assembled in relaxing solution when perturbed with ultrasonic agitations, thus confirming that when ReLC is phosphorylated the filament stability is increased [12].
This entry is adapted from the peer-reviewed paper 10.3390/cells12030514
References
- Pratusevich, V.R.; Seow, C.Y.; Ford, L.E. Plasticity in canine ASM. J. Gen. Physiol. 1995, 105, 73–94.
- Kuo, K.-H.; Herrera, A.M.; Wang, L.; Paré, P.D.; Ford, L.E.; Stephens, N.L.; Seow, C.Y. Structure-function correlation in airway smooth muscle adapted to different lengths. Am. J. Physiol. Physiol. 2003, 285, C384–C390.
- Wang, L.; Chitano, P.; Seow, C.Y. Filament evanescence of myosin II and smooth muscle function. J. Gen. Physiol. 2021, 153, e202012781.
- Lan, B.; Deng, L.; Donovan, G.M.; Chin, L.Y.M.; Syyong, H.T.; Wang, L.; Zhang, J.; Pascoe, C.; Norris, B.A.; Liu, J.C.-Y.; et al. Force maintenance and myosin filament assembly regulated by Rho-kinase in airway smooth muscle. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L1–L10.
- Wang, L.; Chitano, P.; Seow, C.Y. Mechanopharmacology of Rho-kinase antagonism in airway smooth muscle and potential new therapy for asthma. Pharmacol. Res. 2020, 159, 104995.
- Wang, L.; Chitano, P.; Paré, P.D.; Seow, C.Y. Upregulation of smooth muscle Rho-kinase protein expression in human asthma. Eur. Respir. J. 2019, 55, 1901785.
- Kapsali, T.; Permutt, S.; Laube, B.; Scichilone, N.; Togias, A. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J. Appl. Physiol. 2000, 89, 711–720.
- Nadel, J.A.; Tierney, D.F. Effect of a previous deep inspiration on airway resistance in man. J. Appl. Physiol. 1961, 16, 717–719.
- Scichilone, N.; Kapsali, T.; Permutt, S.; Togias, A. Deep Inspiration-induced Bronchoprotection Is Stronger than Bronchodilation. Am. J. Respir. Crit. Care Med. 2000, 162, 910–916.
- Scichilone, N.; Pyrgos, G.; Kapsali, T.; Anderlind, C.; Brown, R.; Permutt, S.; Togias, A. Airways Hyperresponsiveness and the Effects of Lung Inflation. Int. Arch. Allergy Immunol. 2001, 124, 262–266.
- Skloot, G.; Togias, A. Bronchodilation and Bronchoprotection by Deep Inspiration and Their Relationship to Bronchial Hyperresponsiveness. Clin. Rev. Allergy Immunol. 2003, 24, 55–72.
- Ip, K.; Sobieszek, A.; Solomon, D.; Jiao, Y.; Paré, P.; Seow, C. Physical Integrity of Smooth Muscle Myosin Filaments is Enhanced by Phosphorylation of the Regulatory Myosin Light Chain. Cell. Physiol. Biochem. 2007, 20, 649–658.
- Rovner, A.S.; Fagnant, P.M.; Lowey, S.; Trybus, K.M. The carboxylterminal isoforms of smooth muscle myosin heavy chain determine thick filament assembly properties. J. Cell Biol. 2002, 156, 113–124.
- Kendrick-Jones, J.; Cande, W.Z.; Tooth, P.J.; Smith, R.C.; Scholey, J.M. Studies on the effect of phosphorylation of the 20,000Mr light chain of vertebrate smooth muscle myosin. J. Mol. Biol. 1983, 165, 139–162.
- Suzuki, H.; Onishi, H.; Takahashi, K.; Watanabe, S. Structure and Function of Chicken Gizzard Myosin1. J. Biochem. 1978, 84, 1529–1542.
- Trybus, K.M.; Huiatt, T.W.; Lowey, S. A bent monomeric conformation of myosin from smooth muscle. Proc. Natl. Acad. Sci. USA 1982, 79, 6151–6155.
- Sobieszek, I.J.; Sobieszek, A. Myosin assembly of smooth muscle: From ribbons and side polarity to a row polar helical model. J. Muscle Res. Cell Motil. 2022, 43, 113–133.
- Sobieszek, A.; Bremel, R.D. Preparation and Properties of Vertebrate Smooth-Muscle Myofibrils and Actomyosin. JBIC J. Biol. Inorg. Chem. 1975, 55, 49–60.
- Small, J.V.; Sobieszek, A. Ca-Regulation of Mammalian Smooth Muscle Actomyosin via a Kinase-Phosphatase-Dependent Phosphorylation and Dephosphorylation of the 20000-Mr Light Chain of Myosin. JBIC J. Biol. Inorg. Chem. 1977, 76, 521–530.
- Small, J.V.; Sobieszek, A. The Contractile Apparatus of Smooth Muscle. Int. Rev. Cytol. 1980, 64, 241–306.
- Sobieszek, A. Vertebrate smooth muscle myosin Enzymatic and structural properties. In The Biochemis-try of Smooth Muscle; Stephens, N.L., Ed.; University Park Press: Baltimore, MD, USA, 1977; pp. 413–443.
- Sobieszek, A. Ca-Linked Phosphorylation of a Light Chain of Vertebrate Smooth-Muscle Myosin. JBIC J. Biol. Inorg. Chem. 1977, 73, 477–483.
- Sobieszek, A. Smooth Muscle Myosin: Molecule Conformation, Filament Assembly and Associated Regulatory Enzymes. In Airways Smooth Muscle: Biochemical Control of Contraction and Relaxation; Respiratory Pharmacology and Pharmacotherapy; Raeburn, D., Giembycz, M.A., Eds.; Birkhäuser: Basel, Switzerland, 1994.
- Sobieszek, A.; Babiychuk, E.B.; Ortner, B.; Borkowski, J. Purification and Characterization of a Kinase-associated, Myofibrillar Smooth Muscle Myosin Light Chain Phosphatase Possessing a Calmodulin-targeting Subunit. J. Biol. Chem. 1997, 272, 7027–7033.
- Sobieszek, A.; Borkowski, J.; Babiychuk, V.S. Purification and Characterization of a Smooth Muscle Myosin Light Chain Kinase-Phosphatase Complex. J. Biol. Chem. 1997, 272, 7034–7041.
- Hong, F.; Haldeman, B.D.; John, O.A.; Brewer, P.D.; Wu, Y.-Y.; Ni, S.; Wilson, D.P.; Walsh, M.P.; Baker, J.E.; Cremo, C.R. Characterization of Tightly Associated Smooth Muscle Myosin–Myosin Light-Chain Kinase–Calmodulin Complexes. J. Mol. Biol. 2009, 390, 879–892.
- Sobieszek, A. Vectorial activation of smooth muscle myosin filaments and its modulation by telokin. Can. J. Physiol. Pharmacol. 2005, 83, 899–912.
- Sparrow, M.P.; Maxwell, L.C.; Ruegg, J.C.; Bohr, D.F. Preparation and properties of a calcium ion-sensitive actomyosin from arteries. Am. J. Physiol. Content 1970, 219, 1366–1372.
- Sobieszek, A.; Small, J. Myosin-linked calcium regulation in vertebrate smooth muscle. J. Mol. Biol. 1976, 102, 75–92.
- Kim, K.; Keller, T.C. Smitin, a novel smooth muscle titin–like protein, interacts with myosin filaments in vivo and in vitro. J. Cell Biol. 2002, 156, 101–112.
- Sobieszek, A.; Jertschin, P. Urea-glycerol-acrylamide gel electrophoresis of acidic low molecular weight muscle proteins: Rapid determination of myosin light chain phosphorylation in myosin, actomyosin and whole muscle samples. Electrophoresis 1986, 7, 417–425.
- Sobieszek, A. Smooth muscle myosin as a calmodulin binding protein. Affinity increase on filament assembly. J. Muscle Res. Cell Motil. 1990, 11, 114–124.
- Sobieszek, A.; Matusovsky, O.; Permyakova, T.V.; Sarg, B.; Lindner, H.; Shelud’Ko, N. Phosphorylation of myorod (catchin) by kinases tightly associated to molluscan and vertebrate smooth muscle myosins. Arch. Biochem. Biophys. 2006, 454, 197–205.
- Persechini, A.; Hartshorne, D.J. Ordered phosphorylation of the two 20,000 molecular weight light chains of smooth muscle myosin. Biochemistry 1983, 22, 470–476.
- Ikebe, M.; Komatsu, S.; Woodhead, J.L.; Mabuchi, K.; Ikebe, R.; Saito, J.; Craig, R.; Higashihara, M. The Tip of the Coiled-coil Rod Determines the Filament Formation of Smooth Muscle and Nonmuscle Myosin. J. Biol. Chem. 2001, 276, 30293–30300.
- Sobieszek, A. Self-assembly of smooth muscle myosin filaments: Adaptation of filament length by telokin and Mg·ATP. Eur. Biophys. J. 2022, 51, 449–463.
This entry is offline, you can click here to edit this entry!
