Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Graphene-based nanomaterials have attracted significant interest as potential adsorbents for Pb(II) preconcentration and removal due to their high specific surface area, exceptional porosities, numerous adsorption sites and functionalization ease. Particularly, incorporation of magnetic particles with graphene adsorbents offers an effective approach to overcome the separation problems after a lead enrichment step.
- graphene-based nanomaterials
- Pb(II) ions
- adsorption
1. Introduction
Among the heavy metals, lead is extremely toxicant, affecting multiple body systems, and is particularly harmful to young children. Exposure to lead can result in significant health issues, such as damage to brain, kidney, liver, bones, blood and the nervous system [1][2]. The main contamination sources of this element are anthropogenic, such as chemical and battery manufacturing, plastic and printing industries, smelting and mining operations. Due to its non-biodegradable nature and continuous use, lead content accumulates in the environment. Keeping in view the severe toxicity of lead ions, the Environmental Protection Agency (EPA) has established 1.0 mg/L as the maximum permissible concentration of Pb in industrial wastewater and 0.015 mg/L in drinking water, while the World Health Organization (WHO) has set a maximum guideline value of 0.01 mg/L. The European Commission (EC) has proposed lead limits down to 0.005 mg/L [3]. Thus, the development of new analytical procedures for the accurate and sensitive determination of lead is of great importance.
Despite the high sensitivity provided by the atomic and mass spectrometric techniques in trace analysis, a preconcentration step is very often needed to improve their limits of detection further. Additionally, matrix components which cause spectral interference can be removed during this step. Solid phase extraction (SPE) is commonly used as a preconcentration technique owing to its simple procedure, high preconcentration factor and minimal regeneration costs [4][5]. The application of alternative approaches to conventional SPE column-packing such as solid phase microextraction (SPME) [6][7] and dispersive micro solid phase extraction (DMSPE) [8][9] have been also evaluated with carbon-derived materials in the sample preparation step. In recent years, analyte-loaded nanoparticles with magnetic properties were introduced that can be easily isolated from a sample solution by applying an external magnetic field [10][11].
Industrial effluents can contain dissolved lead as well as its various compounds, such as lead salts, oxides, and sulfides. Lead accumulation over time can cause severe ecological problems in water reservoirs due to their toxicity and biocumulation in food chains [12]. It is also a potential health hazard for humans and plants as lead has the tendency to be absorbed through the skin, respiratory and digestive systems. Thus, the need for efficient methods of removing lead ions from wastewater before release into the environment is a matter of concern [13][14][15]. Various treatment technologies have been employed for the removal of lead, such as chemical precipitation, adsorption, ion exchange, chemical oxidation, membrane-based filtration, electrochemical treatment. The selection of the most suitable treatment for lead-contaminated wastewater depends on its initial concentration, pH, environmental impacts as well as economic parameters compared to other technologies. Generally, most of the recent studies have focused on adsorption due to easy operation, high sorption capacity and improved selectivity for specific metal ions. Other techniques have some limitations such as generation of a large amount of sludge, low efficiency, high energy consumption and costly disposal.
Over the last decade, various nanomaterials have been widely applied as powerful adsorbents for trace metal preconcentration and removal of environmental contaminants as they exhibit large surface area, fast adsorption capability and easy functionalization or coatings [16][17][18][19][20]. Among them, graphene derivatives such as graphene oxide and reduced graphene oxide have been integrated in various sample preparation steps, improving the detection sensitivity and selectivity [21][22][23][24]. As these nanomaterials utilise mostly one type of interaction, nanocomposites can combine the properties of carbon materials with metal and metal oxide nanoparticles or chelating polymers [25][26][27]. This allows for further improvement in efficiency of extraction as a result of the synergistic effects resulting from interactions between different materials. Nanocomposities exhibit intrinsic surface reactivity and can strongly chemisorb several substances, with applications in a variety of fields. Their properties can be easily modulated by controlling the methods of their production and additionally the versatile synergistic interactions.
2. Graphene and Its Derivatives
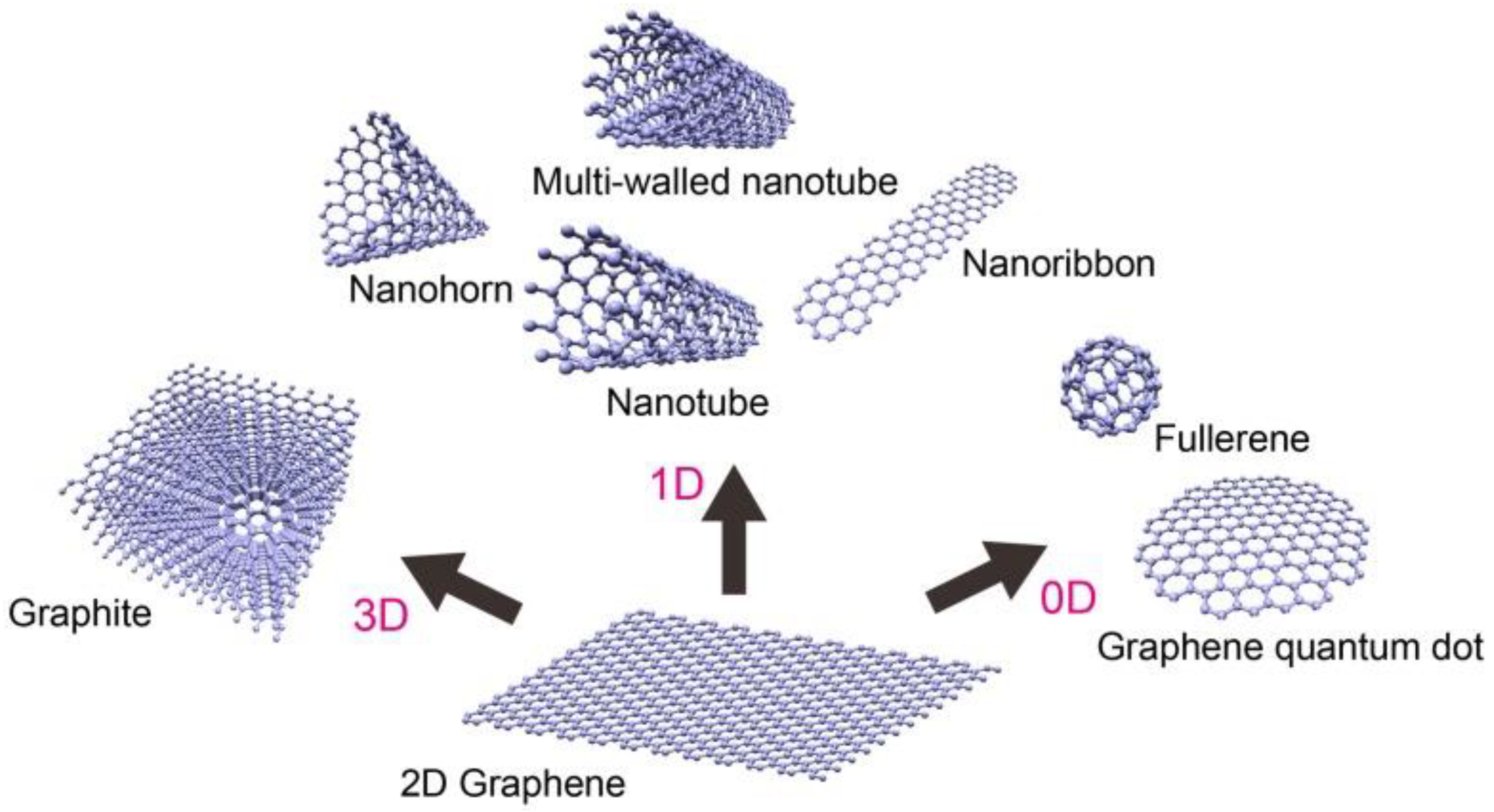
In recent years, carbon-based nanomaterials, such as graphene (G), graphene oxide (GO) and reduced graphene oxide (rGO), have become one of the research hotspots. They exhibit high surface area, unique structural regularity, chemical inertness, electrical conductivity, thermal and mechanical stability. Graphene is considered the fundamental structure of all carbon allotropes and much research has been done in the field of analytical extraction processes involving graphene and its derivatives as the main material [28][29]. Graphene is a single-atom-thick sheet of sp2-hybridized carbon atoms arranged in a planar honeycomb structure. It can be stacked to form graphite and rolled to form carbon nanotubes (Figure 1) [29].

Figure 1. Formation of carbon-based nanomaterials [29].
3. Adsorption Parameters
The important parameters which affect the efficiency of adsorption include pH value of the solution, surface charge of sorbent and its amount, extraction time, temperature as well as concentration and volume of eluent. In acidic medium, the functional groups are protonated and competition occurs in adsorption between H+ and Pb(II) ions. With increasing pH value, sorption of metal ions is enhanced. At pH < 6 the predominant lead species is Pb2+, while in the range of pH 7–10 Pb(OH)+ and Pb(OH)2 mainly occur [28]. Thus, in this pH range of solution, besides sorption on the nanoparticles, Pb(OH)2 may have a significant participation. On the other hand, as the pH of a solution increases, more hydroxyl and carboxylic acid groups are ionised; thus, the surface charge of carbon-based materials becomes more negative.
Sometimes the presence of variety of metal ions, which are usually found in natural samples, can have a competitive effect on the sorption process of Pb(II) and cause a decrease in its adsorption efficiency. For this reason, high adsorbent capacity as well as selectivity is beneficial. Competitive adsorption experiments using GO showed that the affinity of divalent metal ions decreased in the order: Pb(II) > Cu(II) ≫ Cd(II) > Zn(II) [30]. The dispersibility of GO in water changes remarkably after adsorption of metal ions and the tendency to agglomerate and precipitate was observed.
Usually, the traditional “one variable on time” approach was used to find optimum sample pH, sorbent amount, extraction time and volume of eluent for a Pb(II) extraction process. The chemometric techniques based on factorial designs and response surface methodology were additionally applied in order to identify the relationship between independent variables.
4. Isotherm, Thermodynamic and Kinetic Studies
Adsorption capacity on the studied graphene-based nanoparticles was increased by increasing the equilibrium Pb(II) concentration until saturation of the adsorbent. Mostly Freundlich and Langmuir isotherm models were used to explain the mechanism of Pb(II) uptake, the nature of active sites on their surface and to evaluate the maximum adsorption capacity values. The Freundlich model mainly describes a multilayer and heterogeneous process, while the Langmuir model specifies a monolayer adsorption process on a homogeneous surface with all the adsorption sites of equal affinity. The other employed isotherm models were Dubin–Redushkewich and Temkin models. Considering the obtained regression coefficient (R2) of the adsorption isotherms, in most cases the calculated results showed that the Langmuir model gave a better fitting in comparison to to the other three isotherms. The equation for this model is as: q = qm • KL ce/(1 + KLce), where q is the amount of metal ions adsorbed per gram of sorbent, ce denotes the equilibrium concentration in solution, qm is the monolayer theoretical saturation capacity and KL represents the Langmuir constant that relates to the affinity of binding sites. The values of qm and KL were calculated from the intercept and slope of the linear plot of 1/q against 1/ce. The adsorption capacity is an important factor because it determines how much sorbent is required for quantitative separation or preconcentration of the analyte from a given solution.
Thermodynamic parameters, ΔG°, ΔH° and ΔS°, are indicators of the possible type and mechanism of the adsorption process. The positive value of enthalpy (ΔH°) specifies that the adsorption process is endothermic in nature, while a positive ΔS° value shows an increase in the randomness at the solid-surface interface. Furthermore, the Gibss free energy (ΔG°) can be evaluated according to the equation: ΔG° = ΔH° − TΔS°. The mostly negative reported ΔG° value demonstrated the spontaneous nature of the adsorption process at room temperature. For example, Foroughi et al. reported enthalpy changes (ΔH°) of −21.62 to −26.65 kJ/mol, entropy changes (ΔS°) of 0.1259 kJ/mol·K and Gibbs free energy (ΔG°) of 15.90 kJ/mol for adsorption of Pb(II) onto GO@chitosn@Fe3O4-EDTA nanocomposite in the temperature range of 298–338 K [31]. These results revealed an endothermic and spontaneous adsorption process.
In order to compare the kinetics of lead ion removal in a quantitative way, the experimental data were fitted to the kinetic models. Pseudo-first and pseudo-second order models are the most often employed approaches. The rate constants k1 and k2 were evaluated from the most common linearized forms of these models:
ln(q0 − qt) = ln(qe) − k1t pseudo-first order
t/qt = 1/k2qe2 + t/qe pseudo-second order
The vast majority of published papers reported better fitting to pseudo-second-order model, suggesting a chemisorption proces. However, some papers criticize the use of the lineralized form of that model [32][33][34].
5. Preconcentration of Pb(II) for Analytical Applications
The composition of the analyzed sample is often very complex and the concentration of Pb(II) ions is low. The matrix components could cause several interferences during recording of the analytical signals, and thus, obtaining an accurate and reliable result is very difficult. Using a preliminary preconcentration step in the analytical procedure, the interference components are removed with simultaneous improvement of the method sensitivity. Choosing selective sorbent for this purpose requires consideration of the sample matrix and technique for the final detection, while using adequate experimental conditions such as sorbent mass, volume of loading sample and eluent higher enrichment factors is important. The eluent volume has a significant effect on obtaining the highest analytical signal. Low volume would not permit quantitative desorption, while a large volume dilutes the analyte and consequently the value of the enrichment factor (EF, defined as the ratio of sample volume to eluent volume) is decreased.
In comparison to conventional sorbent materials, graphene-based nanoparticles possess great properties, such as high surface area, enhanced mechanical and chemical stability, and possibility of functionalisation (e.g., hydroxylation, carboxylation, amidation, thiolation, silinization and polymer grafting) to increase their selectivity. Thus, they have found several applications in analytical extraction processes [27][35][36]. In the classical SPE technique, GO and rGO can be packed in minicolumns, cartridges and pipette-tips or implemented in polymeric membranes.
Sorption of Pb(II) on an unmodified GO surface mainly involves the electrostatic attraction between the opposite charges and surface complexation. However, its recovery using dispersive solid-phase extraction in the pH range of 4–6 was only about 60% [37]. With rGO, under the same conditions, the metal removal increased to 90%. Graphene oxide, synthesized by the well-known modified Hummer method using graphite from waste dry cell battery, showed in the batch experiments 98.9% removal of Pb from its 10 mg/L solution at pH 4. The maximum adsorption capacity was calculated to be 55.80 mg/g [38], while Guerrero-Fajardo et al. reported impressive adsorption capacity for Pb(II) of 987.3 mg/g for GO prepared from graphite sheets [33]. An even higher value (1119 mg/g) was reported earlier for GO prepared through the oxidation of graphite using potassium dichromate [39].
The addition of magnetic properties to the GO derivatives facilitates their collection from a sample solution. Such a procedure can be successfully used for extraction purposes, even from difficult to handle samples, without high speed centrifugation and filtration. Soylak et al. reported at least 95% recovery of lead ions at pH 6 (from a solution containing 10 µg of Pb in 100 mL) on magnetic graphite oxide at 2 min of time with the vortex mixing [40]. The maximum sorption capacity of 9.9 mg/g was obtained and the limit of detection was 28 µg/L using flame atomic absorption spectrometry (FAAS) for detection. Due to the corrosive power of 3 mol/L HNO3 in 10% acetone used as the eluent, that sorbent was replaced after 10 cycles of sorption/desorption processes.
Several compounds, including well-known reagents and the new materials, have been reported for the functionalization of GO surface that enables more interactions between these functional groups and Pb(II). It has been proved several times that bonding different chemical linkers to graphene or its coating improves the sorption efficiency and selectivity. Thus, a key parameter for development of the new and innovative sorbents for selective preconcentration of lead ions is modification of the carbon surface.
The attachment of dimethylglyoxime ligand to GO@Fe3O4 adsorbent, using 3-chloropropyl-trimethoxy silane (CPTMS) as a spacer, resulted in higher extraction efficiency and selectivity for Pb(II) in comparison to the direct binding of this ligand to the surface of the nanocomposite [41]. Additionally, smaller particle size with higher ratio of the surface to volume were synthesized. Relatively low EF value (23) and sorption capacity (45.05 mg/g) values were obtained with sonication for 9 min contact time. Sorbent was found to be reusable for at least 14 sequential cycles and was applied for quantification of trace amounts of lead in water, hair and nail samples.
Ionic liquids (ILs) have recently been widely used for the development of various types of adsorbents, because they can interact with several molecules through different interactions, such as hydrogen bonding, π-π, electrostatic, dispersive and dipolar interactions [42][43]. Moreover, their properties and adsorptive selectivity can be matched by adjusting the structure of their constituent anion (inorganic or organic) and large asymmetric cation. Fe3O4@GO nanospheres were dispersed in ionic liquid 1-butyl-3-methylimidazolium tetra- fluoroborate (BmimBF4) to form a ferrofluid for extraction of Pb(II) complexed with 1,5-bis(di-2-pyridil) methylene thio-carbonyl hydrazine [44]. The ferrofluid and a sample were kept in contact for 5 min and after separation using the magnet, elution was performed with 1 mL of 5% HNO3 solution. This was applied in magnetic DMSPE and graphite furnace AAS for determination of Pb(II) in seawater samples in the concentration range of 0.04–0.025 µg/L with LOD of 0.008 µg/L. Similar Fe3O4@GO/BmimBF4 nanocomposite have been proposed by Rofouei et al. but the Pb(II) ions were earlier complexed with 1-(2-pyridylazo)-2-naphthol [45].
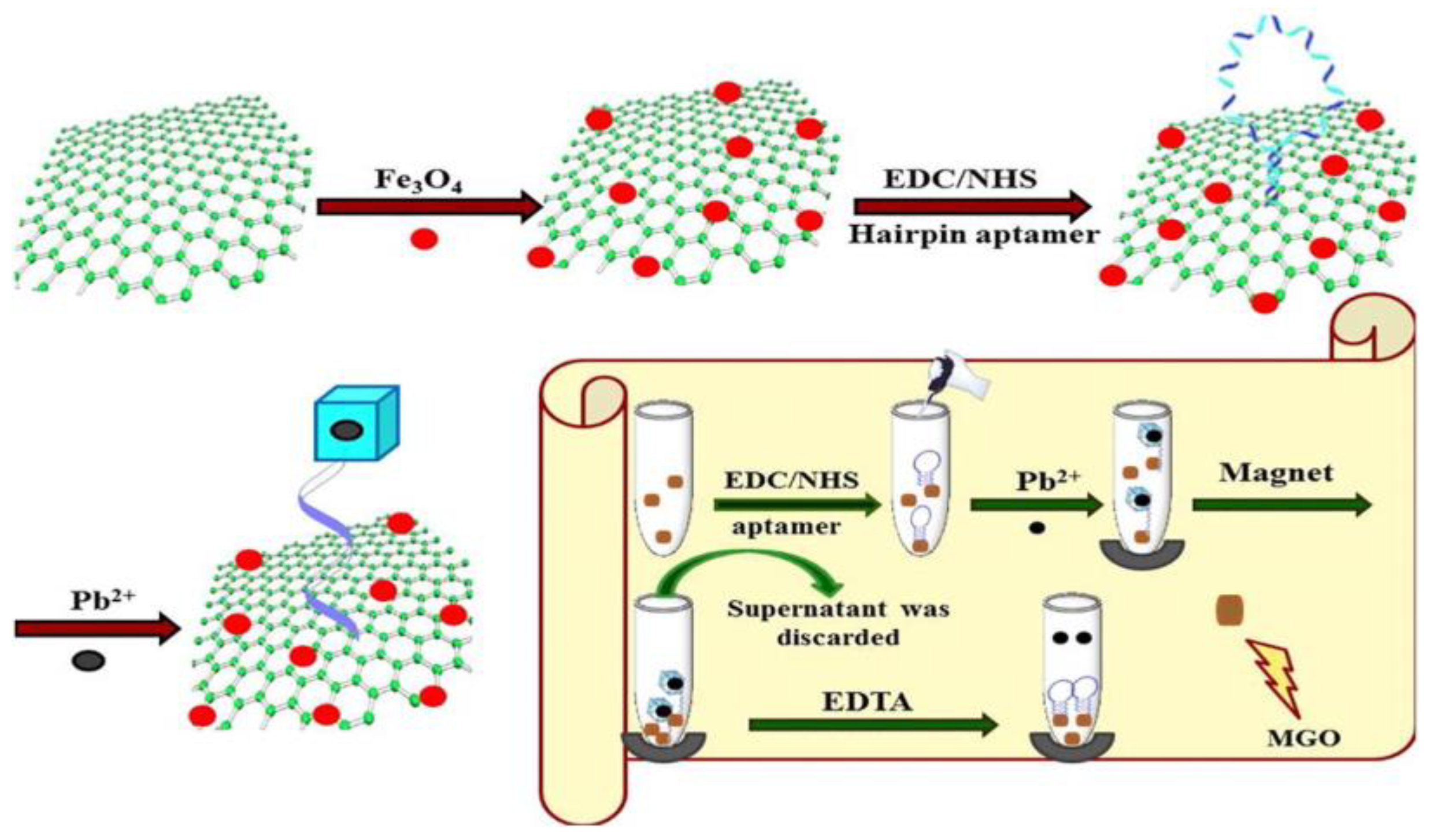
In recent years, biosorption processes using various natural materials has attracted attention and several studies have reported the development of aptamer-based biosensors or their combination with different nanomaterials for applications in food and environmental analysis [46]. Shamsipur et al. [47] proposed the new magnetic biosorbent obtained by covalent immobilization of aptamer as affinity probe on a Fe3O4@GO surface (Figure 2). It was applied for preconcentration of Pb(II) based on hairpin oligonucleotides forming G-quadruplex structure in the presence of target ions. An enrichment factor of 50 was obtained for blood and urine samples using 500 µL of 0.4 mol/L EDTA as an eluent. The proposed method was characterized by high selectivity, a low detection limit of 0.05 µg/L and wide linearity of the calibration curve over the range of 0.3–867 µg/L.

Figure 2. Scheme for synthesis of Fe3O4@GO nanocomposite with hairpin aptamer and the procedure for preconcentration of Pb(II) using this biosorbent [47].
The metal-organic frameworks (MOFs) constructed by metal ions or their clusters and organic ligands through coordination bonds are considered as a favorable platform for adsorption of Pb(II) due to large surface areas, permanent porosity, multi-functionalization, changeable structures and open metal sites [48]. The combination of MOFs with magnetic graphene-based materials improved the overall structural properties and introduced magnetic separation. The nanocomposite with Zr(IV) ions (denoted as UiO66-NH2) and Fe3O4@GO nanoparticles exhibited the maximum adsorption capacity at pH 6 for Pb(II) at 344.8 mg/g with 60 min contact time [49]. This high adsorption was mainly attributed to the complexation with the amino groups from UiO66-NH2 and oxygen containing groups (mainly hydroxyl and carboxyl) by GO. That nanocomposite selectively adsorbed Pb(II) in the presence of other metal ions such as Na(I), K(I), Al(III), Ni(II), Cu(II) and Zn(II) with an EF value of 40. It showed satisfactory recovery of lead ions from different environmental samples.
6. Removal of Pb(II) from Wastewaters
The removing of toxic lead ions from industrial and mining waste effluents has become a key concern [14][15][50][51]. Its presence in the environment may cause long-term health risks to humans and ecosystem. Among various treatments for removal of Pb(II), adsorption methods using synthetic and natural adsorbents offer relatively low costs of operation, materials, and waste discharge. Some disadvantages can be limited adsorption capacity of most applied materials and the need for their more frequent replacement. Thus, adsorption is best used for wastes with moderate to low concentrations of lead.
Various parameters such as temperature, contact time, lead ion concentration, the chemical structure of the adsorbent, its amount, and the presence of other pollutants influence the adsorption process for lead removal. However, the type and chemical structure of an adsorbent and its adsorption capacity are the most critical factors in successful performance.
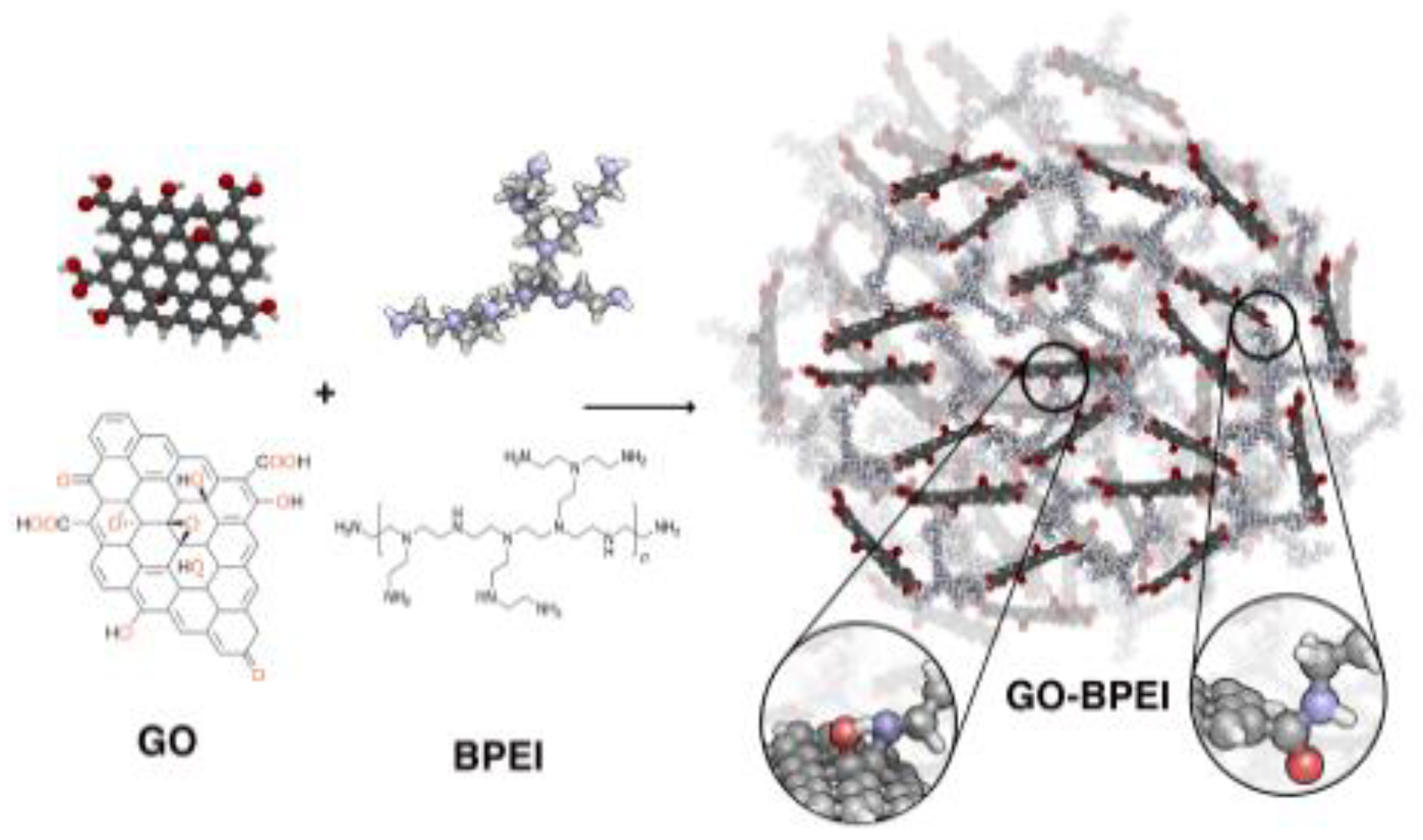
Graphene oxide functionalized with polyethylenimine (GO-BPEI) in the form of highly porous foams has been proposed for removal of Pb(II) from waters at a large scale [52]. A schematic representation of its synthesis is presented in Figure 3. This nanocomposite exhibits an unusually large adsorption capacity as 3390 mg/g at pH 5, but the kinetics of the adsorption process are very slow; adsorption equilibrium was obtained after 400 min. The GO-BPEI foam saturated with lead ions can be regenerated upon treatment with nitric acid. However, a 20% decrease in the adsorption capacity was observed after ten cycles, probably caused by hydrolysis of amide bonds in the adsorbent structure. The performance of the proposed adsorbent has been checked only for tap water with the presence of alkaline metal ions (up to 75 mg/L) as a matrix but not for real wastewater samples.

Figure 3. Schematic representation of the GO- branched polyethylenimine synthesis [52].
Piperazine-modified magnetic graphene oxide (Pip@MGO) nanocomposite also exhibits high adsorption capacity for lead ions, taking advantage of the coordinating capability of piperazine for metal ions and also the high surface area of graphene oxide [53]. At pH 6 it is equal to 558.2 mg/g. In order to evaluate the application of the proposed method in removing lead ions from real samples, river and seawater samples as well as petrochemical wastewater were spiked by known concentrations of Pb(II) at 5 and 10 mg/L concentration level and the adsorption process was performed using only 7 mg of Pip@MGO adsorbent. The recoveries were in the range of 93–99% but the removal efficiency of lead ions was reduced to less than 90% after four consecutive adsorption-regeneration cycles.
The incorporation of melamine into Zr-MOFs was successfully performed by the oven-promoted method [54]. It has a sphere-like morphology and a diameter of about 50 nm. The adsorption capacity for Pb(II) was increased to 205 mg/g under the conditions of pH 5 and 40 °C in comparison to the unmodified MOFs (122 mg/g). The coordination interaction between the amino groups and lead ions was confirmed as the adsorption mechanism. Lu et al. proposed a hydrothermal method to modify an Fe-based MOF with graphene oxide, forming a sandwich structure [55].
This entry is adapted from the peer-reviewed paper 10.3390/ma16031078
References
- Debnath, B.; Singh, W.S.; Manna, K. Sources and toxicological effects of lead on human health. Indian J. Med. Spec. 2019, 10, 66–71.
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420.
- Tsaridou, C.; Karabelas, A.J. Drinking water standards and their implementation—A critical assessment. Water 2021, 13, 2918.
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC Trends Anal. Chem. 2018, 108, 347–369.
- Li, Y.K.; Yang, T.; Chen, M.L.; Wang, J.H. Recent advances in nanomaterials for analysis of trace heavy metals. Crit. Rev. Anal. Chem. 2021, 51, 353–372.
- Reyes-Garces, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in solid phase microextraction and perspective on future directions. Anal. Chem. 2019, 90, 302–360.
- Treviño, M.J.S.; Zarazùa, S.; Płotka-Wasylka, J. Nanosorbents as materials for extraction processes of environmental contaminants and others. Molecules 2022, 27, 1067.
- Chisvert, A.; Cárdenas, S.; Lucena, R. Dispersive micro-solid phase extraction. TrAC Trends Anal. Chem. 2019, 112, 226–233.
- Bagheri, A.R.; Aramesh, N.; Gong, Z.; Cerda, V.; Lee, H.K. Two-dimensional materials as a platform in extraction methods: A review. TrAC Trends Anal. Chem. 2022, 152, 116606.
- Öztürk, E.; Dalgıç, G.D.; Büyükpınar, Ç.; Bakırdere, S. Magnetic nanoparticles based solid phase extraction methods for the determination of trace elements. Crit. Rev. Anal. Chem. 2020, 52, 231–249.
- Corps Ricardo, A.I.; Abujaber, F.; Guzmán Bernardo, F.J.; Martin-Doimeadios, R.C.R.; Rios, A. Magnetic solid phase extraction as a valuable tools for elemental speciation analysis. Trends Environ. Anal. Chem. 2020, 27, e00997.
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179.
- Chowdhury, I.R.; Chowdhury, S.; Mazumder, M.A.J.; Al-Ahmet, A. Removal of lead ions (Pb2+) from water and wastewater: A review on the low-cost adsorbents. Appl. Water Sci. 2022, 12, 185.
- Arbabi, M.; Hemati, S.; Amiri, M. Removal of lead ions from industrial wastewater: A review of removal methods. Int. J. Epidemiol. Res. 2015, 2, 105–109.
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36.
- Khan, W.A.; Arain, M.B.; Soylak, M. Nanomaterials-based solid phase extraction and solid phase microextraction for heavy metals food toxicity. Food Chem. Toxicol. 2020, 145, 111704.
- Nouri, N.; Khorram, P.; Duman, O.; Sibel, T.; Hassan, S. Overview of nanosorbents used in solid phase extraction techniques for the monitoring of emerging organic contaminants in water and wastewater samples. Trends Environ. Anal. Chem. 2020, 25, e00081.
- Gusain, R.; Kumar, N.; Sinha Ray, S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111.
- Vesali-Naseh, M.; Vesali Naseh, M.R.; Ameri, P. Adsorption of Pb (II) ions from aqueous solutions using carbon nanotubes: A systematic review. J. Clean. Prod. 2021, 291, 125917.
- Pyrzynska, K. Nanomaterials in speciation analysis of metals and metalloids. Talanta 2020, 212, 120784.
- de Toffoli, A.L.; Maciel, E.V.S.; Fumes, B.H.; Lanças, F.M. The role of graphene-based sorbents in modern sample preparation techniques. J. Sep. Sci. 2018, 41, 288–302.
- Manousi, N.; Rosenberg, E.; Deliyanni, E.A.; Zachariadis, G.A. Sample preparation using graphene-oxide-derived nanomaterials for the extraction of metals. Molecules 2020, 25, 2411.
- Kumar, M.; Chung, J.S.; Hur, S.H. Graphene composites for lead ions removal from aqueous solutions. Appl. Sci. 2019, 9, 2925.
- Grajek, H.; Jonik, J.; Witkiewicz, Z.; Wawer, T.; Purchała, M. Applications of graphene and its derivatives in chemical analysis. Crit. Rev. Anal. Chem. 2020, 50, 445–471.
- Navrotskaya, A.G.; Aleksandrova, D.D.; Krivoshapkina, M.; Sillanpää, M.; Krivoshapkin, P.V. Hybrid materials based on carbon nanotubes and nanofilters for environmental analysis. Front. Chem. 2020, 8, 546.
- Díez-Pascual, A.M. Carbon-based polymer nanocomposites for high-performance applications. Polymers 2020, 12, 872.
- Pena-Pereira, F.; Romero, V.; de la Calle, I.; Lavilla, I.; Bendicho, C. Graphene-based nanocomposites in analytical extraction processes. TrAC Trends Anal. Chem. 2021, 142, 116303.
- Ali, I.; Basheer, A.A.; Mbianda, X.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180.31.
- Nakano, H.; Tetsuka, H.; Spencer, M.J.S.; Morishita, T. Chemical modification of group IV graphene analogs. Sci. Technol. Adv. Mater. 2018, 19, 76–100.
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689.
- Foroughi, M.; Azqhandi, M.H.A. A biological-based adsorbent for a non-biodegradable pollutant: Modeling and optimization of Pb(II) remediation using GO-CS-Fe3O4-EDTA nanocomposite. J. Mol. Liq. 2020, 318, 114077.
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.Z. Asdorption kinetic modeling using pseudo-first order and pseudo-second ordar rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032.
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2007, 120, 88–116.
- Robati, D. Pseudo-second-order kinetics equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotubes. J. Nanostruc. Chem. 2013, 3, 55.
- Jing, W.; Wang, J.; Kuipers, B.; Bi, W.; Chen, D.D.Y. Recent application of graphene and grapgene-based materials as sorbents in trace analysis. TrAC Trends Anal. Chem. 2021, 137, 116212.
- Li, Y.; Lan, S.; Zhu, T. Recent advances of graphene-based sorptive materials in extraction: A review. TrAC Trends Anal. Chem. 2021, 142, 116319.
- Manousi, N.; Deliyanni, E.; Zachariadis, G. Multi-element determination of toxic and nutrient elements by ICP-AES after dispersive solid-phase extraction with modified graphene oxide. Appl. Sci. 2020, 10, 8722.
- Azam, G.; Kabir, H.; Shaikh, A.A.; Ahmed, S.; Mahmud, M.; Yasmin, S. A rapid and efficient adsorptive removal of lead from water using graphene oxide prepared from waste dry cell battery. J. Water Process. Eng. 2022, 46, 102597.
- Guerrero -Fajardo, C.; Giraldo, L.; Moreno-Piraján, J. Preparation and characterization of graphene oxide for Pb(II) and Zn(II) ions adsorption from aqueous solution: Experimental, thermodynamic and kinetic study. Nanomaterials 2020, 10, 1022.
- Soylak, M.; Acar, D.; Yilmaz, E.; El-Khodary, S.A.; Morsy, M.; Ibrahim, M. Magnetic graphene oxide as an efficient adsorbent for the separation and preconcentration of Cu(II), Pb(II), and Cd(II) from environmental samples. J. AOAC Int. 2017, 100, 1544–1550.
- Barabi, S.; Seidi, M.; Manouchehri, R.; Alizadeh, M. Lead analysis by μSPE/FF-AAS: A comparative study based on dimethylglyoxime functionalized silica-coated magnetic iron/graphene oxides. Anal. Biochem. 2022, 653, 11473.
- Wang, N.; Cui, B. An overview of ionic liquid-based adsorbents in food analysis. TrAC Trends Anal. Chem. 2022, 146, 116496.
- Chatzimitakos, T.; Stalikas, C. Carbon-based nanomaterials functionalized with ionic liquids for microextraction in sample preparation. Separations 2017, 4, 14.
- Montoro-Leal, J.P.; García-Mesa, C.; Siles Cordero, M.T.; López Guerrero, M.M.; Alonso, E.V. Magnetic dispersive solid phase extraction for simultaneous enrichment of cadmium and lead in environmental water samples. Microchem. J. 2020, 155, 104796.
- Rofouei, M.; Jamshidi, S.; Seidi, S.S.; Saleh, A. A bucky gel consisting of Fe3O4 nanoparticles, graphene oxide and ionic liquid as an effcient sorbent for extraction of heavy metal ions from water prior to their determination by ICP-OES. Microchim. Acta 2017, 184, 3425–3432.
- McConnell, E.M.; Nguyen, J.; Li, Y. Aptamer-based biosensor for environmental monitoring. Front. Chem. 2020, 8, 434.
- Shamsipur, M.; Farzin, L.; Tabrizi, M.A.; Sheibani, S. Functionalized Fe3O4/graphene oxide nanocomposites with hairpin aptamers for the separation and preconcentration of trace Pb2+ from biological samples prior to determination by ICP MS. Mater. Sci. Eng. C 2017, 77, 459–469.
- Chen, Y.; Bai, X.; Ye, Z. Recent progress in heavy metal ion decontamination based on metal–organic Framework. Nanomaterials 2020, 10, 1481.
- Wang, Y.; Lin, K.; Liu, Y.; Deng, X. Nanocomposities of functionalized metal-organic frameworks and magnetic graphene oxide for selective adsorption and efficient determination of lead(II). J. Solid State Chem. 2022, 313, 123300.
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmetal remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620.
- Aigbe, U.O.; Osibote, O.A. Carbon derived nanomaterials for the sorption of heavy metals from aqueous solution: A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100578.
- Pakulski, D.; Czepa, W.; Witomska, S.; Aliprandi, A.; Pawluć, P.; Patroniak, V.; Ciesielski, A.; Samori, P. Graphene oxide-branched polyethylenimine foams for efficient removal of toxic cations from water. J. Mater. Chem. A 2018, 6, 9384–9390.
- Alboghbeish, M.; Larki, A.; Saghanezhad, S.J. Effective removal of Pb(II) ions using piperazine-modified magnetic graphene oxide nanocomposite; optimization by response surface methodology. Sci. Rep. 2022, 12, 9658.
- Yin, N.; Wang, K.; Xia, Y.; Li, Z. Novel melamine modified metal-organic frameworks for remarkably high removal of heavy metal Pb(II). Desalination 2018, 430, 120–127.
- Lu, M.; Li, L.; Shen, S.; Chen, D.; Han, W. Highly effcient removal of Pb2+ by a sandwich structure of metal-organic framework/GO composite with enhanced stability. New J. Chem. 2019, 43, 1032–1037.
This entry is offline, you can click here to edit this entry!
