Isorhamnetin glycosides (IGs) are a class of essential flavonoids derived from dietary and medicinal plants such as Opuntia ficus-indica, Hippophae rhamnoides, and Ginkgo biloba.
- isorhamnetin glycosides
- phytonutrients
- health-promoting effects
- sources
1. Introduction
2. Structure of IGs
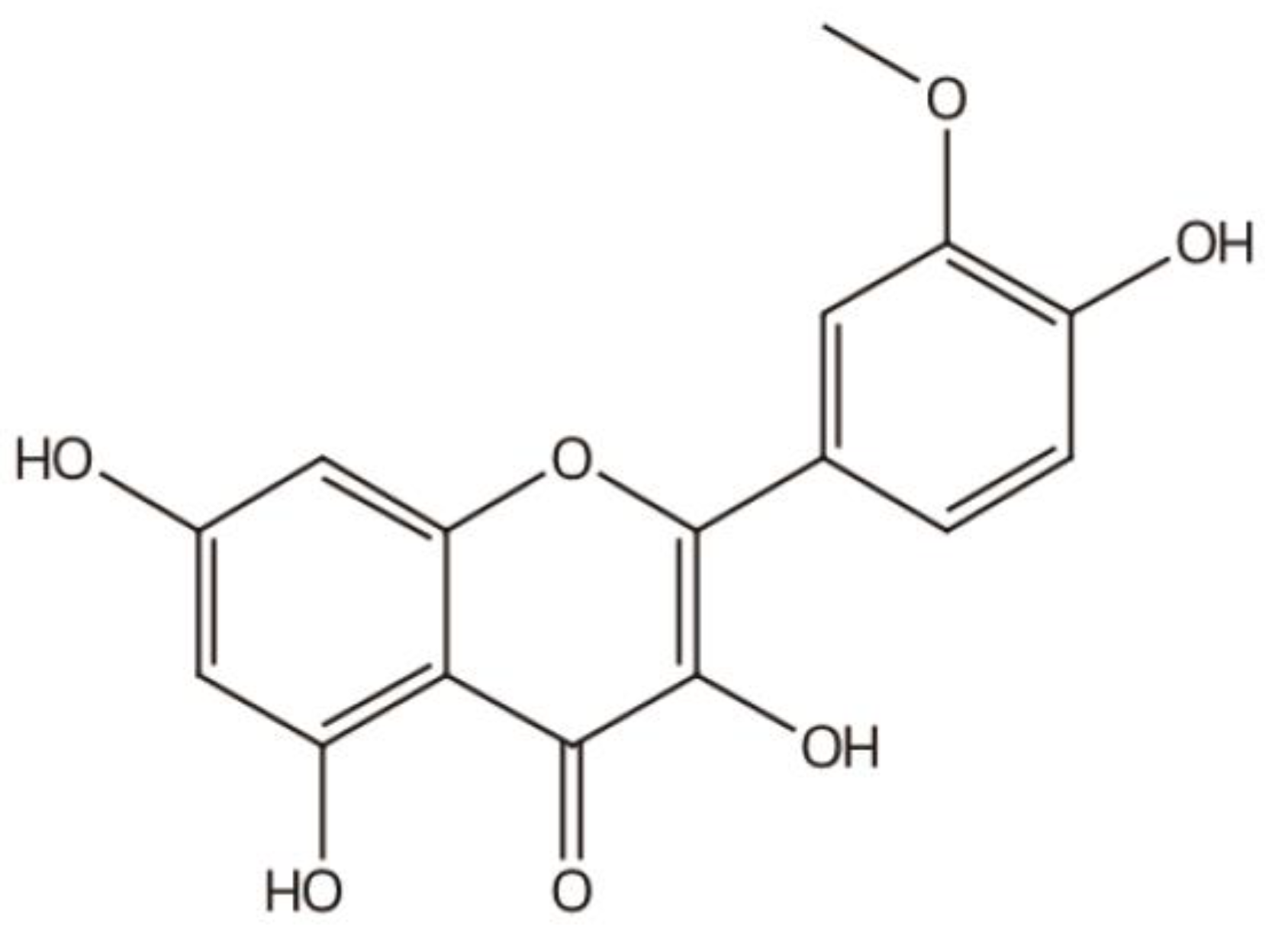
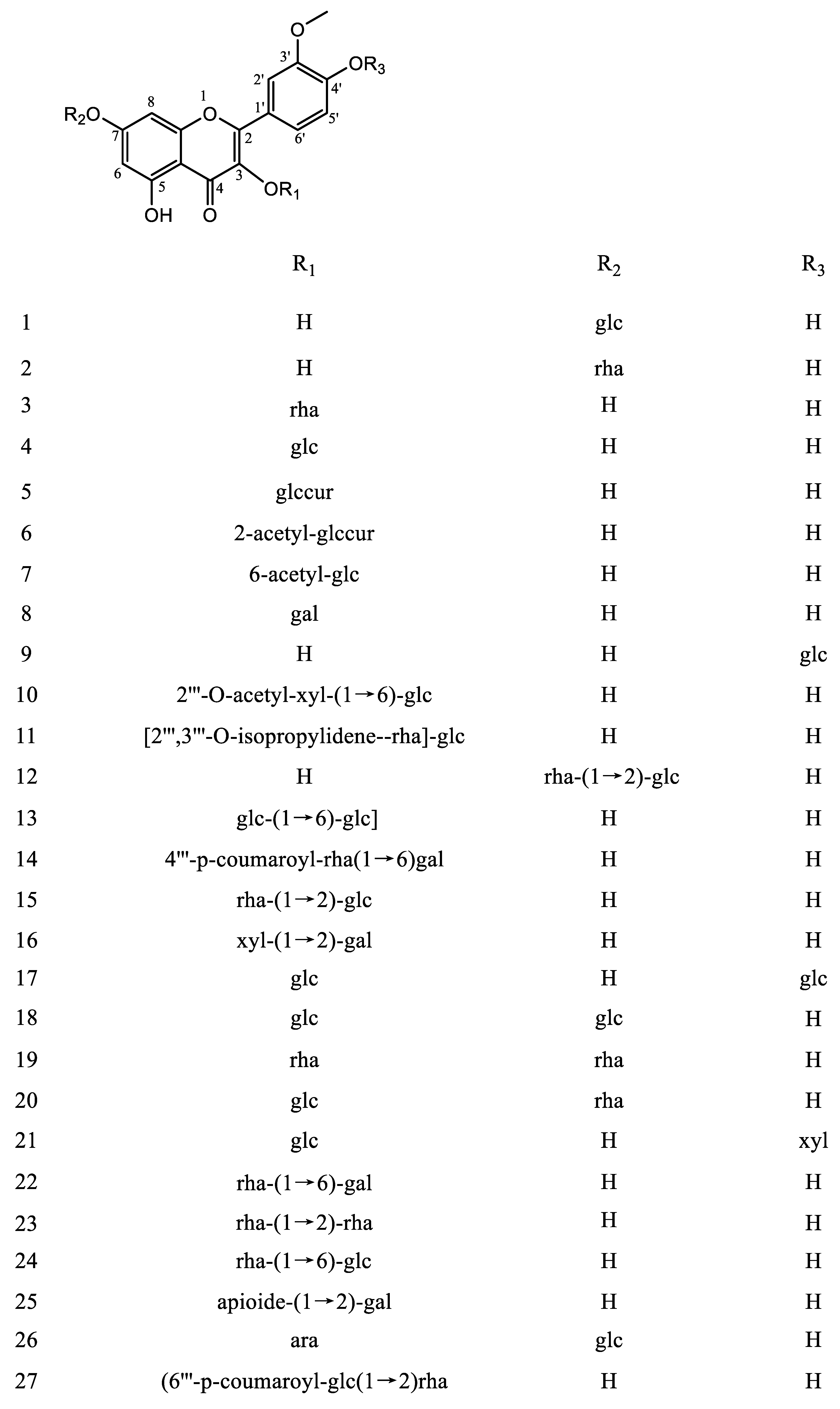
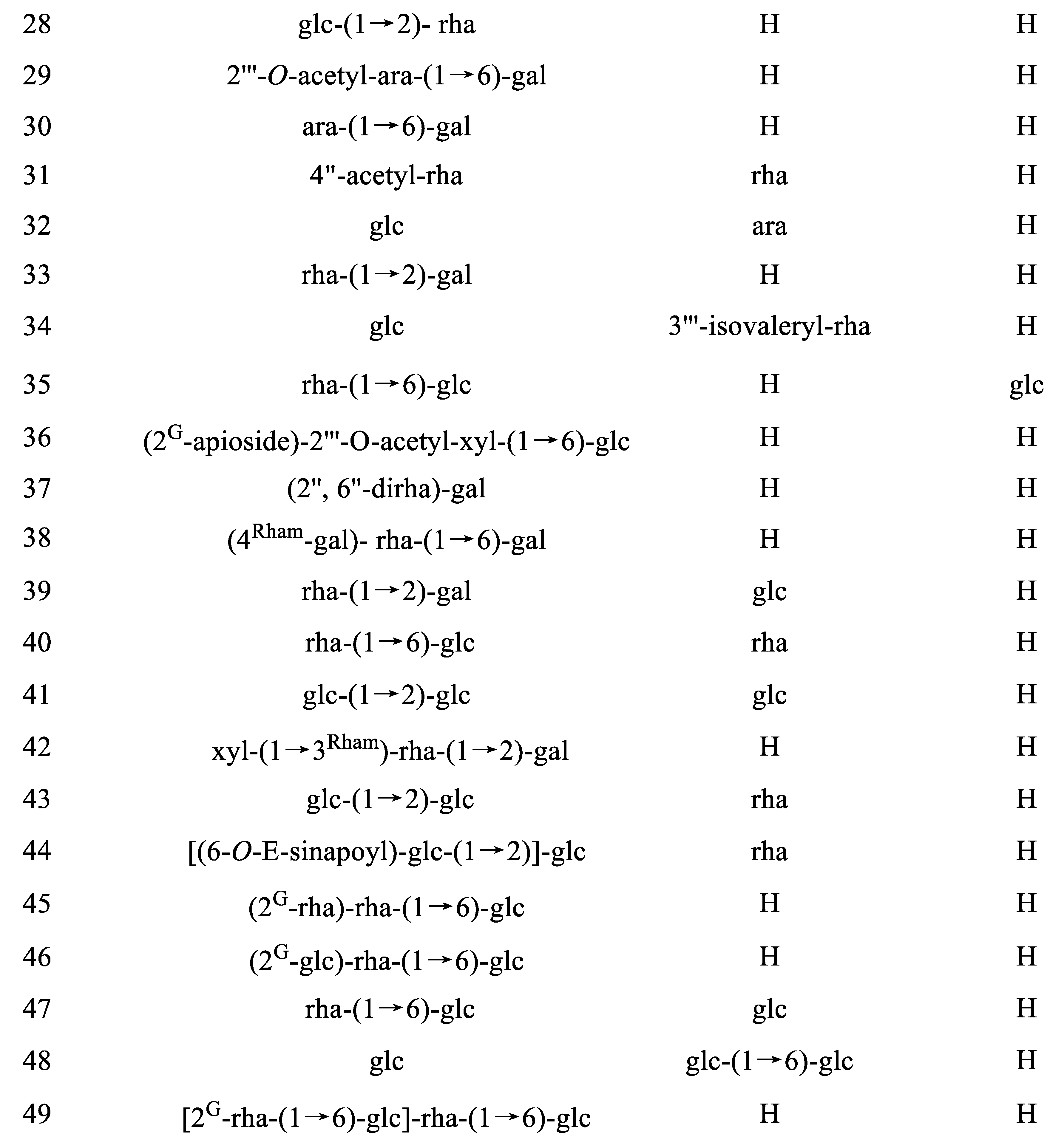
|
No. |
Name |
Trivial Name |
Source |
Ref. |
|---|---|---|---|---|
|
Monoglycosides |
||||
|
1 |
Isorhamnetin-7-O-β-d-glucoside |
Brassicin |
Centaurea cyanus Centaurea kotschyi var. kotschyi Cnicus wallichi Russowia Sogdiana Tagetes lucida (Asteraceae) Sedum sarmentosum Bunge Nitraria tangutorum Bolor |
[29] [30] [31] [32] [33] [34] [22] |
|
2 |
Isorhamnetin-7-O-α-l-rhamnoside |
Carduncellus eriocephalus Nitraria tangutorum Bolor Atriplex centralasiatica Laportea bulbifera Wedd. V. galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert Raphanus raphanistrum L. Caragana intermedia |
[35] [22] [36] [21] [37] [38] [39] |
|
|
3 |
Isorhamnetin-3-O-α-l-rhamnoside |
Laportea bulbifera Wedd. |
[21] |
|
|
4 |
Isorhamnentin-3-O-β-d-glucoside |
Astragalus centralpinus Solidago canadensis L. Hippophae rhamnoids Sambucus nigra L. Calendula officinalis |
[40] [28] [20] [41] [42] |
|
|
5 |
Isorhamnetin-3-O-β-d-glucuronide |
Arnica montana Persicaria thunbergii Senecio giganteus Polygonum aviculare L. Senecio argunensis Turcz. |
[43] [44] [45] [46] [47] |
|
|
6 |
Isorhamnetin-3-O-β-d-(2-acetyl-glucuronide) |
Polygonum aviculare L. |
[46] |
|
|
7 |
Isorhamnetin-3-O-β-d (6-acetyl-glucoside) |
Solidago canadensis L. |
[28] |
|
|
8 |
Isorhamnetin-3-O-β-d-galactoside |
Senecio argunensis Turcz. |
[47] |
|
|
9 |
Isorhamnetin-4′-O-β-d glucoside |
Allium cepa L. |
[23] |
|
|
Diglycosides |
||||
|
10 |
Isorhamnetin-3-O-[2‴-O-acetyl−β-d-xyloside-(1→6)-β-d-glucoside] |
Gymnocarpos decander |
[27] |
|
|
11 |
Isorhamnetin-3-O-[2‴,3‴-O-isopropylidene-α-l-rhamnoside]—(1→6)-β-d-glucoside |
Tetraena aegyptia |
[48] |
|
|
12 |
Isorhamnetin-7-O-α-l-rhamnoside-(1→2)-β-d-glucoside |
Isorhamnetin-7-O-β-neohesperidoside |
Cleome droserifolia |
[12] |
|
13 |
Isorhamnetin-7-O-β-d-glucoside-(1→6)-β-d-glucoside |
Astragaloside or Isorhamnetin-7-O-gentiobioside |
Astragalus altaicus |
[49] |
|
14 |
Isorhamnetin-3-O-β-(4‴-p-coumaroyl-α-rhamnosy]—(1→6)-galactoside) |
Aerva javanica |
[50] |
|
|
15 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→2)-β-d-glucoside |
Isorhamnetin-3-O-β-neohesperidoside |
Hippophae rhamnoids Typha augustifolia L. Calendula officinalis |
[20] [51] [42] |
|
16 |
Isorhamnetin-3-O-β-d-xylosidel-(1→2)-β-d-galactoside |
Prunus padus L. |
[52] |
|
|
17 |
Isorhamnetin-3,4′-O-β-d-diglucoside |
Allium ascalonicum Lepidium apetalum willd |
[24] [53] |
|
|
18 |
Isorhamnetin-3,7-O-β-d-diglucoside |
Sedum sarmentosum Bunge Carduncellus eriocephalus |
[34] [35] |
|
|
19 |
Isorhamnetin-3,7-O-α-l-dirhamnoside |
Laportea bulbifera Wedd. |
[21] |
|
|
20 |
Isorhamnetin-3-O-β-d-glucoside-7-O-α-l-rhamnoside |
Brassidine |
Sinapis arvensis Atriplex centralasiatica Hippophae rhamnoids |
[54] [36] [20] |
|
21 |
Isorhamnetin-3-O-β-d-glucoside-4′-O-β-d-xyloside |
Diplotaxis harra (Forssk.) Boiss |
[26] |
|
|
22 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-galactoside |
Isorhamnetin-3-O-robinobioside |
Nitraria retusa |
[55] |
|
23 |
Isorhamnetin-3-O-α-rhamnoside-(1→2)-rhamnoside |
Laportea bulbifera Wedd. |
[21] |
|
|
24 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside |
Narcissin Isorhamnetin-3-O-rutinoside |
V. galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert opuntia ficus-indica Hippophae rhamnoids Ginkgo biloba Sambucus nigra L. Calendula officinalis |
[37] [18] [20] [41] [42] |
|
25 |
Isorhamnetin-3-O-β-d-apioide (1→2)-β-d-galactoside |
V. galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert |
[37] |
|
|
26 |
Isorhamnetin-3-O-α-l-arabinoside-7-O-β-d-glucoside |
Callianthemum taipaicum Narcissus pseudonarcissus |
[57] [58] |
|
|
27 |
Isorhamnetin-3-O-β-d- (6‴-p-coumaroyl-α-glucoside-(1→2)-rhamnoside) |
Ginkgo biloba |
[56] |
|
|
28 |
Isorhamnetin-3-O-β-d-glucoside-(1→2)-α-l-rhamnoside |
Ginkgo biloba |
[56] |
|
|
29 |
Isorhamnetin-3-O-[2‴-O-acetyl−α-l-arabinoside-(1→6)-β-d-galactoside] |
Trillium tschonoskii Maxim. Trillium apetalon Makino. and T. kamtschaticum Pallas. |
[59] [60] |
|
|
30 |
Isorhamnetin-3-O−α-l-arabinoside-(1→6)-β-d-galactoside |
Trillium apetalon Makino. and T. kamtschaticum Pallas. |
[60] |
|
|
31 |
Isorhamnetin-3-O-α-(4″-acetyl-rhamnoside)-7-O-α-rhamnoside |
Cleome droserifolia |
[12] |
|
|
32 |
Isorhamnetin-3-O-β-d-glucoside-7-O-α-l-arabinoside |
Eschscholtzia mexicana Greene |
[61] |
|
|
33 |
Isorhamnetin-3-O-α-l-rhamnoside(1→2)]-β-d-galactoside |
Glycine max (L.) Merr. |
[62] |
|
|
34 |
Isorhamnetin-3-O-β-glucoside-7-O-α-(3″′-isovaleryl)-rhamnoside |
Lepidium apetalum |
[53] |
|
|
Triglycosides |
||||
|
35 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-4′-O-β-d-glucoside |
Isorhamnetin-3-rutinoside-4′-glucoside |
Mercurialis annua |
[26] |
|
36 |
Isorhamnetin-3-O-(2G-β-d-apiofuranosyl) [2‴-O-acetyl−β-d-xyloside-(1→6)-β-d-glucoside] |
Gymnocarpos decander |
[27] |
|
|
37 |
Isorhamnetin-3-O-(2″,6″-O-α-l-dirhamnoside)-β-d-galactoside |
Alangium premnifolium Lysimachia fortunei |
[63] [64] |
|
|
38 |
Isorhamnetin-3-O-(4Rham-β-d-galactosyl)-α-l-rhamnoside-(1→6)-β-d-galactoside] |
Isorhamnetin-3-O-4Rham-galactosyl-robinobioside |
Nitraria retusa |
|
|
39 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→2)-β-d-galactoside-7-O-β-d-glucoside |
Blackstonia perfoliata |
[66] |
|
|
40 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-7-O-α-l-rhamnoside |
Isorhamnetin-3-rutinoside-7-rhamnoside |
Cassia italica Hippophae rhamnoides |
[67] [68] |
|
41 |
Isorhamnetin-3-O-β-glucoside-(1→2)-β-d-glucoside-7-β-d-glucoside |
Brassicoside or Isorhamnetin-3-O-sophoroside-7-O-β-d-glucoside |
Brassica napus |
[54] |
|
42 |
Isorhamnetin-3-O-β-d-xyloside-(1→3Rham)-α-l-rhamnoside-(1→6)-β-d-galactoside |
Isorhamnetin 3-xylosyl-robinobioside |
Nitraria retusa |
[55] |
|
43 |
Isorhamnetin-3-O-β-glucoside-(1→2)-β-d-glucoside-7-O-α-l-rhamnoside |
Isorhamnetin-3-O-sophoroside-7-O-rhamnoside |
Hippophae rhamnoids |
[20] |
|
44 |
Isorhamnetin-3-O-[(6-O-E-sinapoyl)-β-d-glucoside-(1 → 2)]-β-d-glucoside-7-O-α-l-rhamnoside |
Hippophae rhamnoids |
[20] |
|
|
45 |
Isorhamnetin-3-O-(2G-α-l-rhamnoside)-α-l-rhamnoside-(1→6)-β-d-glucoside |
Typhaneoside |
Typha augustifolia L. Calendula officinalis |
[51] [42] |
|
46 |
Isorhamnetin-3-O-(2G-β-d-glucoside)-α-l-rhamnoside-(1→6)-β-d-glucoside |
Boldo Folium |
[69] |
|
|
47 |
Isorhammetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-7-O-β-d-glucoside |
Isorhammetin-3-rutinoside-7-glucoside |
Hippophae rhamnoids Mercurialis annua |
[20] [26] |
|
48 |
Isorhamnetin-3-O-β-d-glucoside-7-O-β-d-glucoside-(1→6)-β-d-glucoside |
Isorhamnetin-3-O-glucoside-7-O-gentiobioside |
Lepidium apetalum willd |
[53] |
|
Tetraglycosides |
||||
|
49 |
Isorhamnetin-3-O-[2G-α-l-rhamnoside-(1→6)-β-d-glucoside]-α-l-rhamnoside-(1→6)-β-d-glucoside |
Boldo Folium |
[69] |
|
3. Sources of IGs

4. IG Identification and Quantification Methods
4.1. Spectral Techniques and Mass Spectrometry
4.2. Chromatographic Techniques
5. The Health-Promoting Effects of IGs
5.1. Antioxidant Activity
5.2. Anti-Inflammatory Activity
5.3. Anti-Cancer Activity
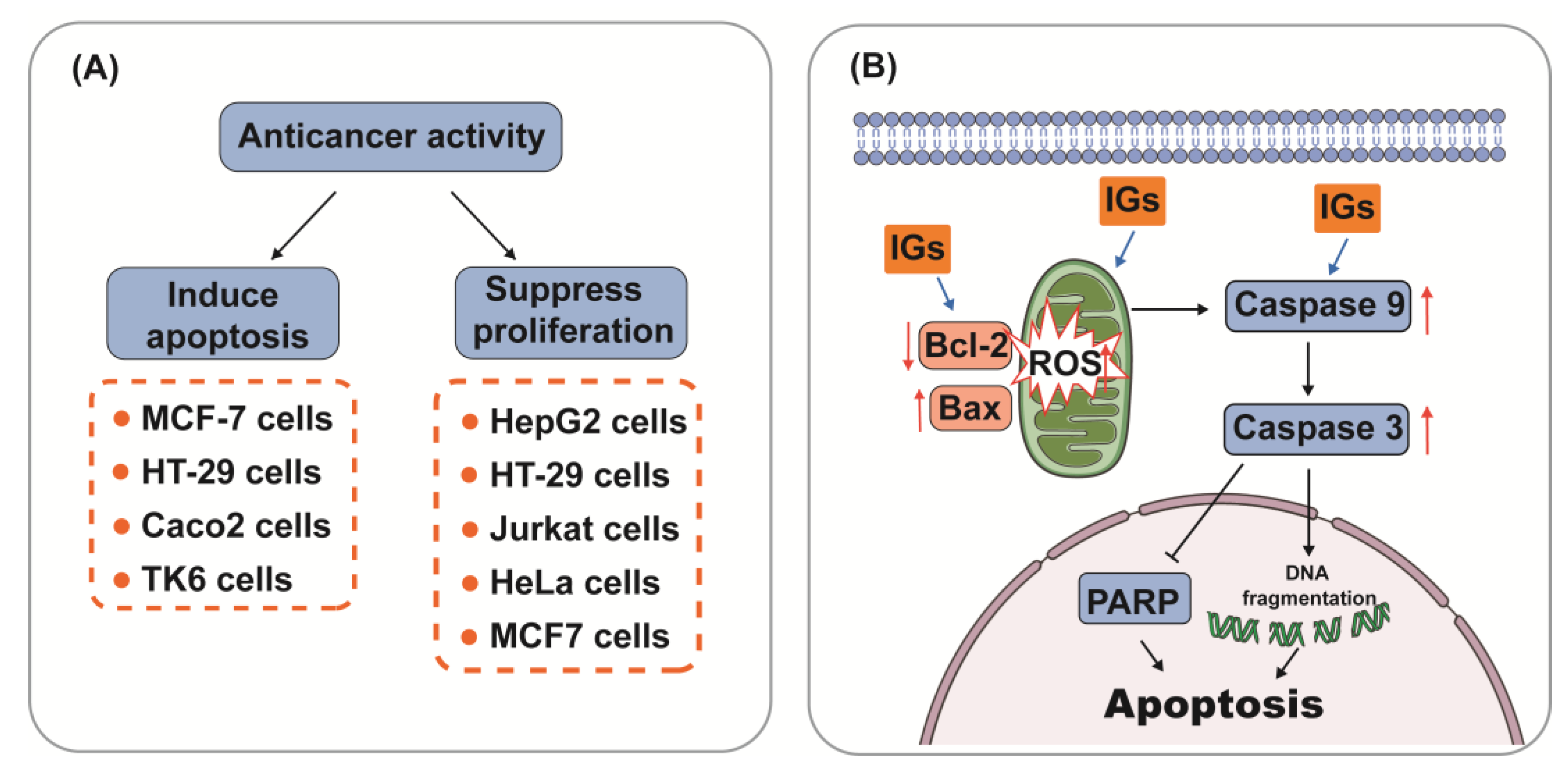
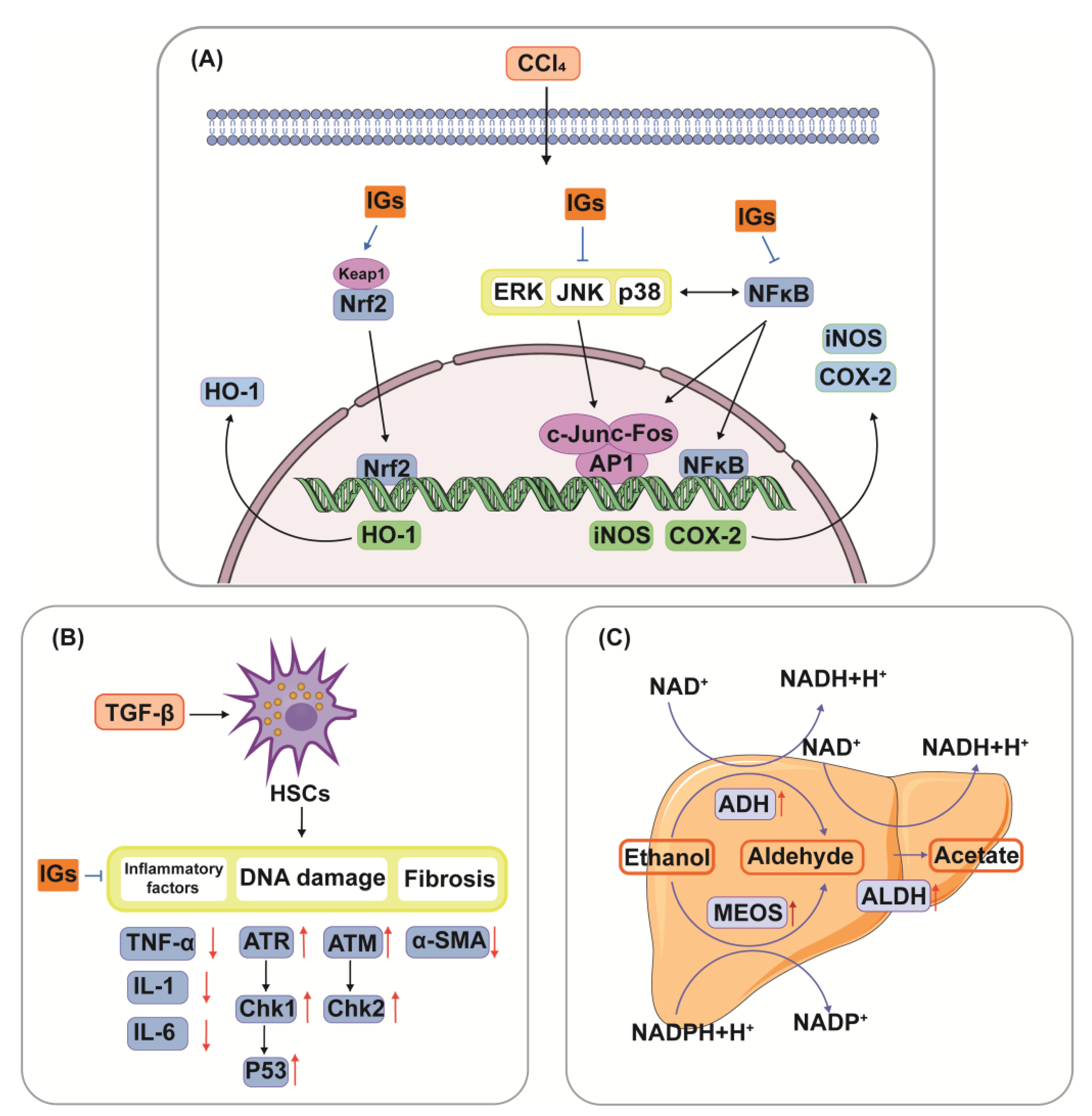
5.4. Hepatoprotective Ability
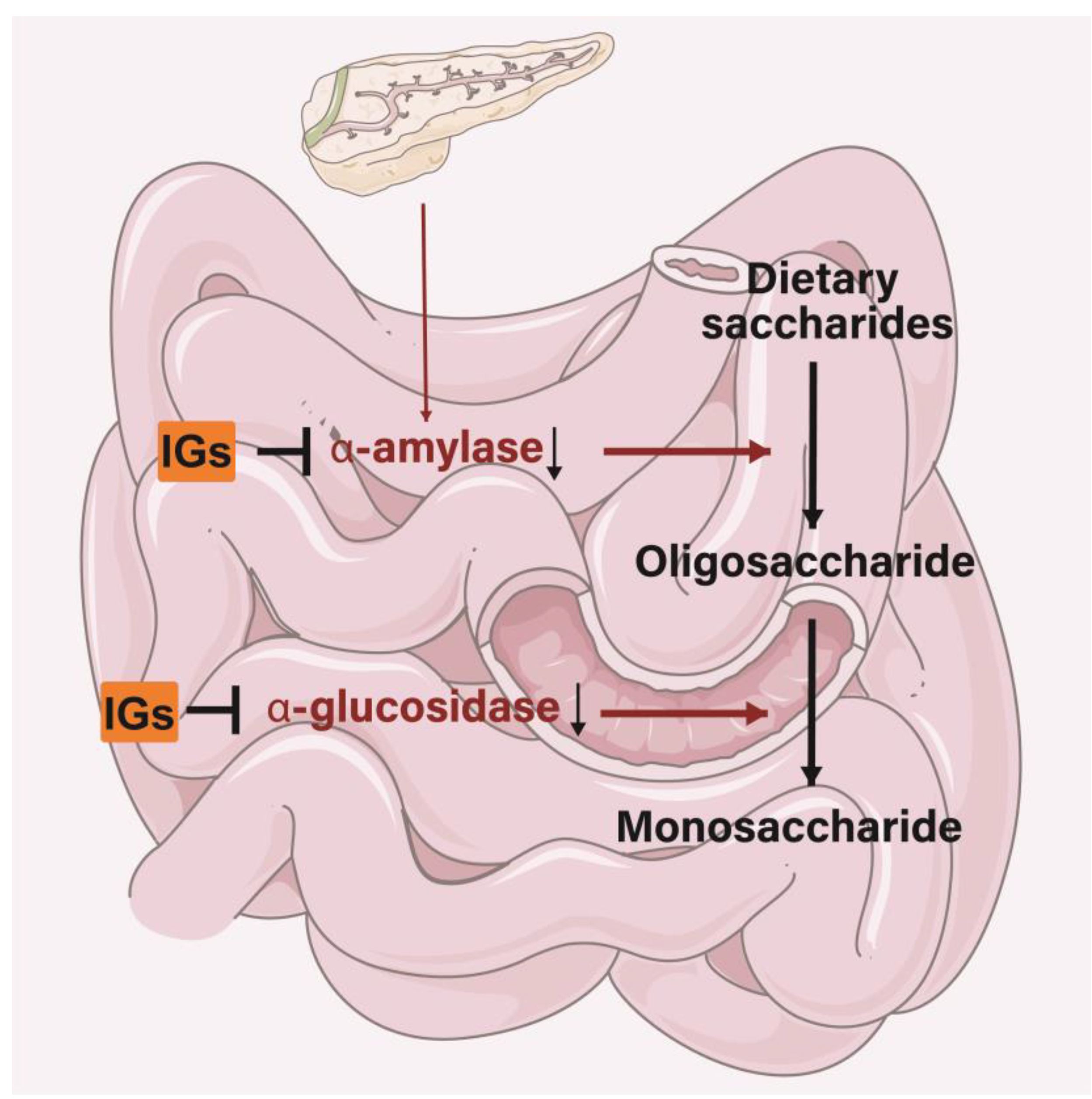
5.5. Antidiabetic Activity
5.6. Anti-Obesity Activity
5.7. Antithrombotic Activity
5.8. Toxic Effects
6. Bioaccessibility of IGs
This entry is adapted from the peer-reviewed paper 10.3390/nu15081947
References
- Monjotin, N.; Amiot, M.; Fleurentin, J.; Morel, J.; Raynal, S. Clinical Evidence of the Benefits of Phytonutrients in Human Healthcare. Nutrients 2022, 14, 1712.
- Valente, I.; Cabrita, A.; Malushi, N.; Oliveira, H.; Papa, L.; Rodrigues, J.; Fonseca, A.; Maia, M. Unravelling the phytonutrients and antioxidant properties of European Vicia faba L. seeds. Food Res. Int. 2019, 116, 888–896.
- Saraei, R.; Marofi, F.; Naimi, A.; Talebi, M.; Ghaebi, M.; Javan, N.; Salimi, O.; Hassanzadeh, A. Leukemia therapy by flavonoids: Future and involved mechanisms. J. Cell. Physiol. 2018, 234, 8203–8220.
- Roche, A.; Ross, E.; Walsh, N.; O’Donnell, K.; Williams, A.; Klapp, M.; Fullard, N.; Edelstein, S. Representative literature on the phytonutrients category: Phenolic acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096.
- Zhou, D.; Bai, Z.S.; Guo, T.T.; Li, J.Y.; Li, Y.W.; Hou, Y.; Chen, G.; Li, N. Dietary flavonoids and human top-ranked diseases: The perspective of in vivo bioactivity and bioavailability. Trends Food Sci. Technol. 2022, 120, 374–386.
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34.
- Tao, H.; Li, L.; He, Y.; Zhang, X.; Zhao, Y.; Wang, Q.; Hong, G. Flavonoids in vegetables: Improvement of dietary flavonoids by metabolic engineering to promote health. Crit. Rev. Food Sci. Nutr. 2022.
- Marilena, A.R.; César, R.-R.; Janet, G.-U.; Eduardo, C.C.E.; Sergio, S.-S. Bioaccessibility, Intestinal Permeability and Plasma Stability of Isorhamnetin Glycosides from Opuntia ficus-indica (L.). Int. J. Mol. Sci. 2017, 18, 1816.
- Wang, L.; Fan, X.; Jian, Y.; Dong, M.; Yang, Q.; Meng, D.; Fu, Y. A sensitive and selective multiple reaction monitoring mass spectrometry method for simultaneous quantification of flavonol glycoside, terpene lactones, and biflavonoids in Ginkgo biloba leaves. J. Pharm. Biomed. Anal. 2019, 170, 335–340.
- Ma, X.; Laaksonen, O.; Zheng, J.; Yang, W.; Trépanier, M.; Kallio, H.; Yang, B. Flavonol glycosides in berries of two major subspecies of sea buckthorn (Hippophaë rhamnoides L.) and influence of growth sites. Food Chem. 2016, 200, 189–198.
- Hyun, S.; Jung, Y.; Chung, H.; Jung, H.; Choi, J. Isorhamnetin glycosides with free radical and ONOO-scavenging activities from the stamens of Nelumbo nucifera. Arch. Pharmacal. Res. 2006, 29, 287–292.
- Abdel Motaal, A.; Salem, H.; Almaghaslah, D.; Alsayari, A.; Bin Muhsinah, A.; Alfaifi, M.; Elbehairi, S.; Shati, A.; El-Askary, H. Flavonol Glycosides: In Vitro Inhibition of DPPIV, Aldose Reductase and Combating Oxidative Stress are Potential Mechanisms for Mediating the Antidiabetic Activity of Cleome droserifolia. Molecules 2020, 25, 5864.
- Cho, J.; Song, N.; Nam, T.; Shrestha, S.; Park, H.; Lyu, H.; Kim, D.; Lee, G.; Woo, Y.; Jeong, T.; et al. Flavonoids from the grains of C1/R-S transgenic rice, the transgenic Oryza sativa spp. japonica, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 10354–10359.
- Ku, S.K.; Han, M.S.; Bae, J.S. Down-regulation of endothelial protein C receptor shedding by persicarin and isorhamnetin-3-O-galactoside. Thromb. Res. 2013, 132, e58–e63.
- Antunes-Ricardo, M.; Hernández-Reyes, A.; Uscanga-Palomeque, A.C.; Rodríguez-Padilla, C.; Martínez-Torres, A.C.; Gutiérrez-Uribe, J.A. Isorhamnetin glycoside isolated from Opuntia ficus-indica (L.) MilI induces apoptosis in human colon cancer cells through mitochondrial damage. Chem. Biol. Interact. 2019, 310, 108734.
- Kim, D.W.; Cho, H.I.; Kim, K.M.; Kim, S.J.; Choi, J.S.; Kim, Y.S.; Lee, S.M. Isorhamnetin-3-O-galactoside Protects against CCl4-Induced Hepatic Injury in Mice. Biomol. Ther. 2012, 20, 406–412.
- Hussain, H.; Green, I.; Abbas, G.; Adekenov, S.; Hussain, W.; Ali, I. Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: Patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 689–702.
- Barba, F.J.; Garcia, C.; Fessard, A.; Munekata, P.E.S.; Lorenzo, J.M.; Aboudia, A.; Ouadia, A.; Remize, F. Opuntia Ficus Indica Edible Parts: A Food and Nutritional Security Perspective. Food Rev. Int. 2022, 38, 930–952.
- Wang, K.; Xu, Z.; Liao, X. Bioactive compounds, health benefits and functional food products of sea buckthorn: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6761–6782.
- Ciesarova, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolkova, B.; Koplik, R.; Belajova, E.; Kukurova, K.; Dasko, L.; Panovska, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170.
- Yang, M.C.; Choi, S.Z.; Lee, S.O.; Chung, A.K.; Nam, J.H.; Lee, K.H.; Lee, K.R. Flavonoid constituents and their antioxidant activity of Laportea bulbifera Weddell. Saengyak Hakhoechi 2003, 34, 18–24.
- Jia, Z.; Zhu, G.; Wang, J. Flavonoid constituents of the seeds of Nitraria tangutorum Bolor. Lanzhou Daxue Xuebao Ziran Kexueban 1991, 27, 102.
- Park, Y.-K.; Lee, C.Y. Identification of Isorhamnetin 4’-Glucoside in Onions. J. Agric. Food Chem. 1996, 44, 34.
- Fattorusso, E.; Iorizzi, M.; Lanzotti, V.; Taglialatela-Scafati, O. Chemical composition of shallot (Allium ascalonicum Hort.). J. Agric. Food Chem. 2002, 50, 5686–5690.
- Kassem, M.E.S.; Afifi, M.S.; Marzouk, M.M.; Mostafa, M.A. Two new flavonol glycosides and biological activities of Diplotaxis harra (Forssk.) Boiss. Nat. Prod. Res. 2013, 27, 2272–2280.
- Aquino, R.; Behar, I.; D’Agostino, M.; De Simone, F.; Schettino, O.; Pizza, C. Phytochemical investigation on Mercurialis annua. Biochem. Syst. Ecol. 1987, 15, 667.
- Bechlem, H.; Mencherini, T.; Bouheroum, M.; Benayache, S.; Cotugno, R.; Braca, A.; De Tommasi, N. New Constituents from Gymnocarpos decander. Planta Med. 2017, 83, 1200–1206.
- Batyuk, V.S.; Vasil’chenko, E.A.; Kovaleva, S.N. Flavonoids of Solidago virgaurea L. and S. canadensis L. and their pharmacological properties. Rastit. Resur. 1988, 24, 92.
- Litvinenko, V.I.; Bubenchikova, V.N. Phytochemical study of Centaurea cyanus. Chem. Nat. Compd. 1988, 792, 672–674.
- Oksuz, S.; Putun, E. Flavonoids of Centaurea kotschyi var. kotschyi. Doga: Kim. Ser. 1987, 11, 66–71.
- Singh, K.N.; Pandey, V.B. Isorhamnetin 7-glucoside from Cnicus wallichi. Phytochemistry 1986, 25, 2683.
- Butayarov, A.V.; Batirov, E.K.; Tadzhibaev, M.M.; Melibaev, S.; Malikov, V.M. Flavonoids from aerial parts of Russowia sogdiana. Chem. Nat. Compd. 1993, 29, 807–808.
- Abdala, L.R. Flavonoids of the aerial parts from Tagetes lucida (Asteraceae). Biochem. Syst. Ecol. 1999, 27, 753–754.
- He, A.; Wang, M. Flavonoids from stringy stonecrop (Sedum sarmentosum). Zhongcaoyao 1997, 28, 517–522.
- Grace, M.H.; Mohamed, T.K.; Khattab, A.M. Flavonoids of Carduncellus eriocephalus. Egypt. J. Pharm. Sci. 1999, 39, 409–416.
- Zhang, Z.-x.; Zhang, J.; Luo, J.-q.; Zhang, T.-h. Chemical constituents of the seeds of Atriplex centralasiatica. Shenyang Yaoke Daxue Xuebao 2008, 25, 708–710.
- Awaad, A.S.; Grace, M.H. Flavonoids and pharmacological activity of Vernonia galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert. Egypt. J. Pharm. Sci. 2001, 40, 117–128.
- Krzeminski, K.; Krzeminska, K. Flavonoid heterosides in the herb of Raphanus raphanistrum L. Herba Pol. 1977, 23, 291.
- Zhang, S.; Shi, J.; Sun, Z.; Hu, C. Studies on chemical constituents from Caragana intermedia. Zhongyaocai 2006, 29, 19–21.
- Paskhov, D.; Marichkova, L. Flavonoids of Astragalus centralpinus and their effect on the smooth muscle of the gastrointestinal tract. Probl. Farm. 1983, 11, 36–42.
- Christensen, L.P.; Kaack, K.; Frette, X.C. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2008, 227, 293–305.
- Vidal-Ollivier, E.; Elias, R.; Faure, F.; Babadjamian, A.; Crespin, F.; Balansard, G.; Boudon, G. Flavonol glycosides from Calendula officinalis flowers. Planta Med. 1989, 55, 73.
- Merfort, I.; Wendisch, D. Flavonoid glucuronides from the flowers of Arnica montana. Planta Med. 1988, 54, 247.
- Kim, S.Y.; Park, J.Y.; Park, P.S.; Bang, S.H.; Lee, K.M.; Lee, Y.R.; Jang, Y.H.; Kim, M.J.; Chun, W.; Heo, M.Y.; et al. Flavonoid glycosides as acetylcholinesterase inhibitors from the whole plants of Persicaria thunbergii. Nat. Prod. Sci. 2014, 20, 191–195.
- Mezache, N.; Derbre, S.; Akkal, S.; Laouer, H.; Seraphin, D.; Richomme, P. Fast counter current chromatography of n-butanolic fraction from Senecio giganteus (Asteraceae). Nat. Prod. Commun. 2009, 4, 1357–1362.
- Granica, S.; Czerwinska, M.E.; Zyzynska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188.
- Cheng, W.; Sui, C.; Yuan, J.; Zhang, H. Flavonoids of argun groundsel (Senecio argunensis). Zhongcaoyao 1999, 30, 727–729.
- Zaki, A.A.; Xu, X.; Wang, Y.; Shie, P.-H.; Qiu, L. A new anti-inflammatory flavonoid glycoside from Tetraena aegyptia. Nat. Prod. Res. 2021, 35, 1985–1990.
- Cheng, J.; Wu, J.; Azi, g.; Li, R.; Lin, G. Chemical constituents of Aertaihuanqi (Astragalus altaicus). Zhongcaoyao 1994, 25, 563.
- Saleh, N.A.M.; Mansour, R.M.A.; Markham, K.R. An acylated isorhamnetin glycoside from Aerva javanica. Phytochemistry 1990, 29, 1344.
- Jia, S.; Liu, Y.; Ma, C.; Yang, S.; Zhou, H.; Zhao, D.; Liu, D.; Li, S. Flavonoid constituents of the pollen of Typha angustfolia L. (Puhuang). Yaoxue Xuebao 1986, 21, 441.
- Olszewska, M.A.; Kwapisz, A. Metabolite profiling and antioxidant activity of Prunus padus L. flowers and leaves. Nat. Prod. Res. 2011, 25, 1115–1131.
- Wang, S.; Shi, P.; Qu, L.; Ruan, J.; Yang, S.; Yu, H.; Zhang, Y.; Wang, T. Bioactive constituents obtained from the seeds of Lepidium apetalum willd. Molecules 2017, 22, 540.
- Hoerhammer, L.; Wagner, H.; Kraemer, H.; Farkas, L. Isorhamnetin glycosides. II. Isolation and composition of new glycosides from Brassica napus and Sinapis arvensis. Chem. Ber. 1967, 100, 2301.
- Halim, A.F.; Saad, H.-E.A.; Hashish, N.E. Flavonol glycosides from Nitraria retusa. Phytochemistry 1995, 40, 349.
- Tang, Y.; Lou, F.; Wang, J.; Li, Y.; Zhuang, S. Coumaroyl flavonol glycosides from the leaves of Ginkgo biloba. Phytochemistry 2001, 58, 1251–1256.
- Wang, D.-M.; Pu, W.-J.; Wang, Y.-H.; Zhang, Y.-J.; Wang, S.-S. A new isorhamnetin glycoside and other phenolic compounds from Callianthemum taipaicum. Molecules 2012, 17, 4595–4603.
- Schoensiegel, I.; Egger, K. Flavonol glycosides in the petals of Narcissus pseudonarcissus. Z. Naturforsch. B 1969, 24, 1215.
- Zhang, Z.-l.; Cai, M.-t.; Zuo, Y.-m.; Wang, Y.-y. Studies on chemical constituents in fruits of Trillium tschonoskii Maxim. Shizhen Guoyi Guoyao 2014, 25, 541–543.
- Yoshitama, K.; Shida, Y.; Oyamada, T.; Takasaki, N.; Yahara, S. "Studies of the flavonoids of the genus Trillium". 3. Flavonol glycosides in the leaves of Trillium apetalon Makino and T. kamtschaticum Pallas. J. Plant Res. 1997, 110, 443–448.
- Rodriguez, E.; Shen, M.C.; Mabry, T.J.; Dominguez, X.A. Isorhamnetin 3-0-glucoside 7-0-arabinoside from Eschscholzia mexicana. Phytochemistry 1973, 12, 2069.
- Li, H.; Kim, U.H.; Yoon, J.H.; Ji, H.S.; Park, H.M.; Park, H.Y.; Jeong, T.S. Suppression of Hyperglycemia and Hepatic Steatosis by Black-Soybean-Leaf Extract via Enhanced Adiponectin-Receptor Signaling and AMPK Activation. J. Agric. Food Chem. 2019, 67, 90–101.
- Kijima, H.; Ide, T.; Otsuka, H.; Takeda, Y. Alangiflavoside, a new flavonol glycoside from the leaves of Alangium premnifolium. J. Nat. Prod. 1995, 58, 1753.
- Yasukawa, K.; Sekine, H.; Takido, M. Studies of the constituents of genus Lysimachia. Part 4. Two flavonol glycosides from Lysimachia fortunei. Phytochemistry 1989, 28, 2215.
- Liu, H.; Mou, Y.; Zhao, J.; Wang, J.; Zhou, L.; Wang, M.; Wang, D.; Han, J.; Yu, Z.; Yang, F. Flavonoids from Halostachys caspica and their antimicrobial and antioxidant activities. Molecules 2010, 15, 7933–7945.
- Kaouadji, M.; Doucoure, A.; Mariotte, A.M.; Chulia, A.J.; Thomasson, F. Flavonol triglycosides from Blackstonia perfoliata. Phytochemistry 1990, 29, 1283.
- El-Sayed, N.H.; Abu Dooh, A.M.; El-Khrisy, E.A.M.; Mabry, T.J. Flavonoids of Cassia italica. Phytochemistry 1992, 31, 2187.
- Trineeva, O.V.; Perova, I.B.; Slivkin, A.I.; Eller, K.I. Study the composition of flavonoids fruits of sea buckthorn. Sorbtsionnye Khromatogr. Protsessy 2017, 17, 87–93.
- Skalski, B.; Lis, B.; Pecio, L.; Kontek, B.; Olas, B.; Zuchowski, J.; Stochmal, A. Isorhamnetin and its new derivatives isolated from sea buckthorn berries prevent H(2)O(2)/Fe—Induced oxidative stress and changes in hemostasis. Food Chem. Toxicol. 2019, 125, 614–620.
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133.
- Choi, J.; Jung, M.; Park, H.; Chung, H.; Kang, S. Further isolation of peroxynitrite and 1,1-diphenyl-2-picrylhydrazyl radical scavenging isorhamnetin 7-O-glucoside from the leaves of Brassica juncea L. Arch. Pharmacal Res. 2002, 25, 625–627.
- Hawas, U.; Abou El-Kassem, L.; Shaher, F.; Al-Farawati, R. In vitro inhibition of Hepatitis C virus protease and antioxidant by flavonoid glycosides from the Saudi costal plant. Nat. Prod. Res. 2019, 33, 3364–3371.
- Chen, P.; Cao, Y.; Bao, B.; Zhang, L.; Ding, A. Antioxidant capacity of Typha angustifolia extracts and two active flavonoids. Pharm. Biol. 2017, 55, 1283–1288.
- Yang, C.; Yang, Y.; Aisa, H.A.; Xin, X.; Ma, H.; Yili, A.; Zhao, Y. Bioassay-guided isolation of antioxidants from Astragalus altaicus by combination of chromatographic techniques. J. Sep. Sci. 2012, 35, 977–983.
- Qi, J.; Gui, X.; Chen, G. Research on active component extraction of nitraria sibirica pall. fruit and their antioxidant activity. Mod. Chin. Med. 2013, 15, 827–831.
- Bouhlel, I.; Limem, I.; Skandrani, I.; Nefatti, A.; Ghedira, K.; Dijoux-Franca, M.; Leila, C. Assessment of isorhamnetin 3-O-neohesperidoside from Acacia salicina: Protective effects toward oxidation damage and genotoxicity induced by aflatoxin B1 and nifuroxazide. J. Appl. Toxicol. JAT 2010, 30, 551–558.
- Demirkiran, O.; Sabudak, T.; Ozturk, M.; Topcu, G. Antioxidant and tyrosinase inhibitory activities of flavonoids from Trifolium nigrescens Subsp. petrisavi. J. Agric. Food Chem. 2013, 61, 12598–12603.
- Hassan, R.; Tawfik, W.; Abou-Setta, L. The flavonoid constitunts of Leucaena leucocephala. Growing in Egypt, and their biological activity. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2014, 11, 67–72.
- Yuca, H.; Özbek, H.; Demirezer, L.; Kasil, H.G.; Güvenalp, Z. trans-Tiliroside: A potent α-glucosidase inhibitor from the leaves of Elaeagnus angustifolia L. Phytochemistry 2021, 188, 112795.
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.W.; Wang, H.Q.; Yu, J.Q.; Duan, J.A. Flowers of Astragalus membranaceus var. mongholicus as a Novel High Potential By-Product: Phytochemical Characterization and Antioxidant Activity. Molecules 2019, 24, 434.
- Yokozawa, T.; Kim, H.; Cho, E.; Choi, J.; Chung, H. Antioxidant effects of isorhamnetin 3,7-di-O-beta-d-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J. Agric. Food Chem. 2002, 50, 5490–5495.
- Boubaker, J.; Ben Sghaier, M.; Skandrani, I.; Ghedira, K.; Chekir-Ghedira, L. Isorhamnetin 3-O-robinobioside from Nitraria retusa leaves enhance antioxidant and antigenotoxic activity in human chronic myelogenous leukemia cell line K562. BMC Complement. Altern. Med. 2012, 12, 135.
- Abdallah, H.; Esmat, A. Antioxidant and anti-inflammatory activities of the major phenolics from Zygophyllum simplex L. J. Ethnopharmacol. 2017, 205, 51–56.
- Bouhlel, I.; Skandrani, I.; Nefatti, A.; Valenti, K.; Ghedira, K.; Mariotte, A.M.; Hininger-Favier, I.; Laporte, F.; Dijoux-Franca, M.G.; Chekir-Ghedira, L. Antigenotoxic and antioxidant activities of isorhamnetin 3-O neohesperidoside from Acacia salicina. Drug Chem. Toxicol. 2009, 32, 258–267.
- Yeskaliyeva, B.; Mesaik, M.; Abbaskhan, A.; Kulsoom, A.; Burasheva, G.; Abilov, Z.; Choudhary, M.; Atta-ur-Rahman. Bioactive flavonoids and saponins from Climacoptera obtusifolia. Phytochemistry 2006, 67, 2392–2397.
- Matias, A.; Nunes, S.; Poejo, J.; Mecha, E.; Serra, A.; Madeira, P.; Bronze, M.; Duarte, C. Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice. Food Funct. 2014, 5, 3269–3280.
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chem. 2017, 221, 997–1003.
- Andersson, U.; Yang, H.; Harris, H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases? Expert Opin. Ther. Targets 2018, 22, 263–277.
- Kim, T.; Ku, S.; Bae, J. Anti-inflammatory activities of isorhamnetin-3-O-galactoside against HMGB1-induced inflammatory responses in both HUVECs and CLP-induced septic mice. J. Cell. Biochem. 2013, 114, 336–345.
- Yong, H.; Koh, M.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905.
- Park, J.; Kim, S.; Lee, H.; Kim, S.; Kwon, Y.; Chun, W. Isorhamnetin-3-O-Glucuronide Suppresses JNK and p38 Activation and Increases Heme-Oxygenase-1 in Lipopolysaccharide-Challenged RAW264.7 Cells. Drug Dev. Res. 2016, 77, 143–151.
- Ahmed, A.F.; Wen, Z.-H.; Bakheit, A.H.; Basudan, O.A.; Ghabbour, H.A.; Al-Ahmari, A.; Feng, C.-W. A Major Diplotaxis harra-Derived Bioflavonoid Glycoside as a Protective Agent against Chemically Induced Neurotoxicity and Parkinson’s Models; In Silico Target Prediction; and Biphasic HPTLC-Based Quantification. Plants 2022, 11, 648.
- Fu, Y.; Jia, Y.; Sun, Y.; Liu, X.; Yi, J.; Cai, S. Dietary Flavonoids Alleviate Inflammation and Vascular Endothelial Barrier Dysfunction Induced by Advanced Glycation End Products In Vitro. Nutrients 2022, 14, 1026.
- Jin, H.; Ko, H.; Chowdhury, M.; Lee, D.; Woo, E. A new indole glycoside from the seeds of Raphanus sativus. Arch. Pharmacal Res. 2016, 39, 755–761.
- Osman, S.; El Kashak, W.; Wink, M.; El Raey, M. New Isorhamnetin Derivatives from Salsola imbricata Forssk. Leaves with Distinct Anti-inflammatory Activity. Pharmacogn. Mag. 2016, 12, S47–S51.
- Ameur, A.S.; Negab, I.; Zouzou, F.; Legseir, B. Anti-inflammatory and antispasmodic activities of isorhamnetin glycosides isolated from Opuntia ficus-indica (L.) mill. Flowers. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 432–437.
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.; Martínez-Vitela, C.; Serna-Saldívar, S. Topical anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica. BioMed Res. Int. 2015, 2015, 847320.
- Ren, Q.-C.; Li, X.-H.; Li, Q.-Y.; Yang, H.-L.; Wang, H.-L.; Zhang, H.; Zhao, L.; Jiang-Yong, S.-L.; Meng, X.-L.; Zhang, Y.; et al. Total flavonoids from sea buckthorn ameliorates lipopolysaccharide/cig arette smoke-induced airway inflammation. Phytother. Res. PTR 2019, 33, 2102–2117.
- Raffa, D.; Maggio, B.; Raimondi, M.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228.
- Mohammed, M.; El-Sharkawy, E.R.; Matloub, A.A. Cytotoxic flavonoids from Diplotaxis harra (Forssk.) Boiss. growing in Sinai. J. Med. Plant Res. 2013, 520, 5099–5103.
- Tofighi, Z.; Asgharian, P.; Goodarzi, S.; Hadjiakhoondi, A.; Ostad, S.N.; Yassa, N. Potent cytotoxic flavonoids from Iranian Secur. Securidaca. Med. Chem. Res. 2014, 23, 1718–1724.
- Tundis, R.; Loizzo, M.; Bonesi, M.; Menichini, F.; Statti, G.; Menichini, F. In vitro cytotoxic activity of Salsola oppositifolia Desf. (Amaranthaceae) in a panel of tumour cell lines. Z. Fur Naturforschung. C J. Biosci. 2008, 63, 347–354.
- Liu, Y.; Xiao, Z.Y.; Liu, P.; Huang, J.; Algradi, A.M.; Pan, J.; Guan, W.; Zhou, Y.Y.; Yang, B.Y.; Kuang, H.X. New flavonoids from the aerial part of Bupleurum chinense DC. Fitoterapia 2020, 147, 104739.
- Pfeffer, C.; Singh, A. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448.
- Yao, N.; Li, Y.; Lei, Y.; Hu, N.; Chen, W.; Yao, Z.; Yu, M.; Liu, J.; Ye, W.; Zhang, D. A piperazidine derivative of 23-hydroxy betulinic acid induces a mitochondria-derived ROS burst to trigger apoptotic cell death in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. CR 2016, 35, 192.
- Rademaker, G.; Boumahd, Y.; Peiffer, R.; Anania, S.; Wissocq, T.; Liégeois, M.; Luis, G.; Sounni, N.; Agirman, F.; Maloujahmoum, N.; et al. Myoferlin targeting triggers mitophagy and primes ferroptosis in pancreatic cancer cells. Redox Biol. 2022, 53, 102324.
- Mendes, S.; Sá, R.; Magalhães, M.; Marques, F.; Sousa, M.; Silva, E. The Role of ROS as a Double-Edged Sword in (In)Fertility: The Impact of Cancer Treatment. Cancers 2022, 14, 1585.
- Highton, A.; Schuster, I.; Degli-Esposti, M.; Altfeld, M. The role of natural killer cells in liver inflammation. Semin. Immunopathol. 2021, 43, 519–533.
- Lücke, J.; Sabihi, M.; Zhang, T.; Bauditz, L.; Shiri, A.; Giannou, A.; Huber, S. The good and the bad about separation anxiety: Roles of IL-22 and IL-22BP in liver pathologies. Semin. Immunopathol. 2021, 43, 591–607.
- Sayed, A.M.; Hassanein, E.; Hassan, S.; Hussein, O.E.; Mahmoud, A.M. Flavonoids-mediated SIRT1 signaling activation in hepatic disorders. Life Sci. 2020, 259, 118173.
- Igarashi, K.; Mikami, T.; Takahashi, Y.; Sato, H. Comparison of the preventive activity of isorhamnetin glycosides from atsumi-kabu (red turnip, Brassica, campestris L.) leaves on carbon tetrachloride-induced liver injury in mice. Biosci. Biotechnol. Biochem. 2008, 72, 856–860.
- Galati, E.; Mondello, M.; Lauriano, E.; Taviano, M.; Galluzzo, M.; Miceli, N. Opuntia ficus indica (L.) Mill. fruit juice protects liver from carbon tetrachloride-induced injury. Phytother. Res. PTR 2005, 19, 796–800.
- Maheshwari, D.; Yogendra Kumar, M.; Verma, S.; Singh, V.; Singh, S. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2422–2428.
- Kuang, H.; Tang, Z.; Wang, X.; Yang, B.; Wang, Z.; Wang, Q. Chemical constituents from Sambucus williamsii Hance fruits and hepatoprotective effects in mouse hepatocytes. Nat. Prod. Res. 2018, 32, 2008–2016.
- Ben Saad, A.; Dalel, B.; Rjeibi, I.; Smida, A.; Ncib, S.; Zouari, N.; Zourgui, L. Phytochemical, antioxidant and protective effect of cactus cladodes extract against lithium-induced liver injury in rats. Pharm. Biol. 2017, 55, 516–525.
- Rodríguez-Rodríguez, C.; Torres, N.; Gutiérrez-Uribe, J.; Noriega, L.; Torre-Villalvazo, I.; Leal-Díaz, A.; Antunes-Ricardo, M.; Márquez-Mota, C.; Ordaz, G.; Chavez-Santoscoy, R.; et al. The effect of isorhamnetin glycosides extracted from Opuntia ficus-indica in a mouse model of diet induced obesity. Food Funct. 2015, 6, 805–815.
- Guo, Z.; Cheng, J.; Zheng, L.; Xu, W.; Xie, Y. Mechanochemical-Assisted Extraction and Hepatoprotective Activity Research of Flavonoids from Sea Buckthorn (Hippophaë rhamnoides L.) Pomaces. Molecules 2021, 26, 7615.
- Zhang, G.; Liu, Y.; Liu, P. Active Components from Sea Buckthorn (Hippophae rhamnoides L.) Regulate Hepatic Stellate Cell Activation and Liver Fibrogenesis. J. Agric. Food Chem. 2018, 66, 12257–12264.
- Hur, J.M.; Park, S.H.; Choi, J.W.; Park, J.C. Effects of extract and isorhamnetin glycoside from Brassica juncea on hepatic alcohol-metabolizing enzyme system in rats. Nat. Prod. Sci. 2012, 18, 190–194.
- Ran, B.; Guo, C.; Li, W.; Li, W.; Wang, Q.; Qian, J.; Li, H. Sea buckthorn (Hippophae rhamnoides L.) fermentation liquid protects against alcoholic liver disease linked to regulation of liver metabolome and the abundance of gut microbiota. J. Sci. Food Agric. 2021, 101, 2846–2854.
- Tanveer, A.; Akram, K.; Farooq, U.; Hayat, Z.; Shafi, A. Management of diabetic complications through fruit flavonoids as a natural remedy. Crit. Rev. Food Sci. Nutr. 2017, 57, 1411–1422.
- Nan, X.; Jia, W.; Zhang, Y.; Wang, H.; Lin, Z.; Chen, S. An on-line detection system for screening small molecule inhibitors of α-Amylase and α-Glucosidase in Prunus mume. J. Chromatogr. A 2022, 1663, 462754.
- Tundis, R.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Inhibitory effects on the digestive enzyme alpha-amylase of three Salsola species (Chenopodiaceae) in vitro. Pharm. Die 2007, 62, 473–475.
- Pham, A.T.; Malterud, K.E.; Paulsen, B.S.; Diallo, D.; Wangensteen, H. alpha-Glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimp toxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014, 52, 1166–1169.
- Gómez-Maqueo, A.; García-Cayuela, T.; Fernández-López, R.; Welti-Chanes, J.; Cano, M. Inhibitory potential of prickly pears and their isolated bioactives against digestive enzymes linked to type 2 diabetes and inflammatory response. J. Sci. Food Agric. 2019, 99, 6380–6391.
- Siegień, J.; Buchholz, T.; Popowski, D.; Granica, S.; Osińska, E.; Melzig, M.; Czerwińska, M. Pancreatic lipase and α-amylase inhibitory activity of extracts from selected plant materials after gastrointestinal digestion in vitro. Food Chem. 2021, 355, 129414.
- Fujimura, Y.; Watanabe, M.; Morikawa-Ichinose, T.; Fujino, K.; Yamamoto, M.; Nishioka, S.; Inoue, C.; Ogawa, F.; Yonekura, M.; Nakasone, A.; et al. Metabolic Profiling for Evaluating the Dipeptidyl Peptidase-IV Inhibitory Potency of Diverse Green Tea Cultivars and Determining Bioactivity-Related Ingredients and Combinations. J. Agric. Food Chem. 2022, 70, 6455–6466.
- Kwon, E.; Lee, J.; Kim, Y.; Do, A.; Choi, J.; Cho, S.; Jung, U.; Lee, M.; Park, Y.; Choi, M. Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity. Nutrients 2017, 9, 569.
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.; Ferreres, F.; Moreno, D.; Nowicka, P. UPLC-PDA-Q/TOF-MS profiling of phenolic and carotenoid compounds and their influence on anticholinergic potential for AChE and BuChE inhibition and on-line antioxidant activity of selected Hippophaë rhamnoides L. cultivars. Food Chem. 2020, 309, 125766.
- Chen, Y.G.; Li, P.; Li, P.; Yan, R.; Zhang, X.Q.; Wang, Y.; Zhang, X.T.; Ye, W.C.; Zhang, Q.W. α-Glucosidase Inhibitory Effect and Simultaneous Quantification of Three Major Flavonoid Glycosides in Microctis folium. Molecules 2013, 18, 4221–4232.
- Yang, Z.; Wen, X.; Li, Y.; Matsuzaki, K.; Kitanaka, S. Inhibitory effects of the constituents of Hippophae rhamnoides on 3T3-L1 cell differentiation and nitric oxide production in RAW264.7 cells. Chem. Pharm. Bull. 2013, 61, 279–285.
- Kong, C.S.; Seo, Y. Antiadipogenic activity of isohamnetin 3-O-beta-d-glucopyranoside from Salicornia herbacea. Immunopharmacol. Immunotoxicol. 2012, 34, 907–911.
- Furie, B.; Furie, B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008, 359, 938–949.
- Furie, B.; Furie, B.C. In vivo thrombus formation. J. Thromb. Haemost. JTH 2007, 5 (Suppl. S1), 12–17.
- Mega, J.L.; Simon, T. Pharmacology of antithrombotic drugs: An assessment of oral antiplatelet and anticoagulant treatments. Lancet 2015, 386, 281–291.
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303.
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Che Jalil, N.A.; Wan Ghazali, W.S.; Mohamed, M. Bee bread attenuates the progression of atherosclerosis by activating Nrf2/Keap1 and modulating TNF-alpha/NF-kappabeta-associated mast cell migration and a mitochondrial-dependent apoptotic pathway in the obese rat model. Food Funct. 2022, 13, 8119–8130.
- Sobral, F.; Calhelha, R.C.; Barros, L.; Duenas, M.; Tomas, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I.C. Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal. Molecules 2017, 22, 248.
- Budzianowska, A.; Toton, E.; Romaniuk-Drapala, A.; Kikowska, M.; Budzianowski, J. Cytotoxic Effect of Phenylethanoid Glycosides Isolated from Plantago lanceolata L. Life 2023, 13, 556.
- Wu, Q.; Kroon, P.A.; Shao, H.; Needs, P.W.; Yang, X. Differential Effects of Quercetin and Two of Its Derivatives, Isorhamnetin and Isorhamnetin-3-glucuronide, in Inhibiting the Proliferation of Human Breast-Cancer MCF-7 Cells. J. Agric. Food Chem. 2018, 66, 7181–7189.
- Naksuriya, O.; Daowtak, K.; Tima, S.; Okonogi, S.; Mueller, M.; Toegel, S.; Khonkarn, R. Hydrolyzed Flavonoids from Cyrtosperma johnstonii with Superior Antioxidant, Antiproliferative, and Anti-Inflammatory Potential for Cancer Prevention. Molecules 2022, 27, 3226.
- Peanparkdee, M.; Borompichaichartkul, C.; Iwamoto, S. Bioaccessibility and antioxidant activity of phenolic acids, flavonoids, and anthocyanins of encapsulated Thai rice bran extracts during in vitro gastrointestinal digestion. Food Chem. 2021, 361, 130161.
- Mrudulakumari Vasudevan, U.; Lee, E.Y. Flavonoids, terpenoids, and polyketide antibiotics: Role of glycosylat ion and biocatalytic tactics in engineering glycosylation. Biotechnol. Adv. 2020, 41, 107550.
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S.
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Busselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934.
- Yang, B.; Liu, H.L.; Yang, J.L.; Gupta, V.K.; Jiang, Y.M. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124.
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Curto, R.L.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion—ScienceDirect. Food Chem. 2010, 122, 1083–1088.
- Gómez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants 2020, 9, 164.
- De Santiago, E.; Gill, C.I.; Carafa, I.; Tuohy, K.M.; De Peña, M.P.; Cid, C. Digestion and Colonic Fermentation of Raw and Cooked Opuntia ficus-indica Cladodes Impacts Bioaccessibility and Bioactivity. J. Agric. Food Chem. 2019, 67, 2490–2499.
- Kim, H.Y.; Lee, J.M.; Yokozawa, T.; Sakata, K.; Lee, S. Protective activity of flavonoid and flavonoid glycosides against gluc ose-mediated protein damage. Food Chem. 2010, 126, 892–895.
- Yu, L.; Chen, C.; Wang, L.-F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.-R. Neuroprotective effect of kaempferol glycosides against brain injury a nd neuroinflammation by inhibiting the activation of NF-κB and STAT3 i n transient focal stroke. PLoS ONE 2013, 8, e55839.
- Michael, H.N.; Salib, J.Y.; Eskander, E.F. Bioactivity of Diosmetin Glycosides Isolated from the Epicarp of Date Fruits, Phoenix dactylifera, on the Biochemical Profile of Alloxan Diabetic Male Rats. Phytother. Res. 2012, 27, 699–704.
- Makino, T.; Kanemaru, M.; Okuyama, S.; Shimizu, R.; Tanaka, H.; Mizukami, H. Anti-allergic effects of enzymatically modified isoquercitrin (α-oligo glucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-oligoglu cosyl rutin, and quercetin, when administered orally to mice. J. Nat. Med. 2013, 67, 881–886.
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better bi ological significance? Crit. Rev. Food Sci. Nutr. 2015, 57, 1874–1905.
- Cao, S.; Ni, B.; Feng, L.; Yin, X.; Dou, H.; Fu, J.; Lin, L.; Ni, J. Simultaneous Determination of Typhaneoside and Isorhamnetin-3-O-Neohesperidoside in Rats After Oral Administration of Pollen Typhae Extract by UPLC-MS/MS. J. Chromatogr. Sci. 2015, 53, 866–871.
- Zeng, H.; Xue, P.; Su, S.; Huang, X.; Shang, E.; Guo, J.; Qian, D.; Tang, Y.; Duan, J. Comparative Pharmacokinetics of three major bioactive components in rats after oral administration of Typhae Pollen-Trogopterus Feces drug pair before and after compatibility. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2016, 24, 2.
- Lehtonen, H.M.; Lehtinen, O.; Suomela, J.P.; Viitanen, M.; Kallio, H. Flavonol glycosides of sea buckthorn (Hippophae rhamnoides ssp. sinensis) and lingonberry (Vaccinium vitis-idaea) are bioavailable in humans and monoglucuronidated for excretion. J. Agric. Food Chem. 2010, 58, 620–627.
- Aziz, A.A.; Edwards, C.A.; Lean, M.E.; Crozier, A. Absorption and excretion of conjugated flavonols, including quercetin-4’-O-beta-glucoside and isorhamnetin-4’-O-beta-glucoside by human volunteers after the consumption of onions. Free Radic. Res. 1998, 29, 257–269.
- Boyle, S.P.; Dobson, V.L.; Duthie, S.J.; Kyle, J.A.; Collins, A.R. Absorption and DNA protective effects of flavonoid glycosides from an onion meal. Eur. J. Nutr. 2000, 39, 213–223.
- Baell, J.B.; Holloway, G.A. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53, 2719–2740.
- de Matos, A.M.; Blazquez-Sanchez, M.T.; Sousa, C.; Oliveira, M.C.; de Almeida, R.F.M.; Rauter, A.P. C-Glucosylation as a tool for the prevention of PAINS-induced membrane dipole potential alterations. Sci. Rep. 2021, 11, 4443.
