Isorhamnetin glycosides (IGs) are a class of essential flavonoids derived from dietary and medicinal plants such as Opuntia ficus-indica, Hippophae rhamnoides, and Ginkgo biloba.
- isorhamnetin glycosides
- phytonutrients
- health-promoting effects
- sources
1. Introduction
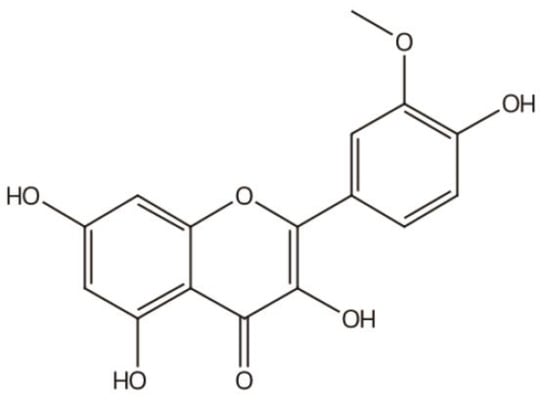
2. Structure of IGs

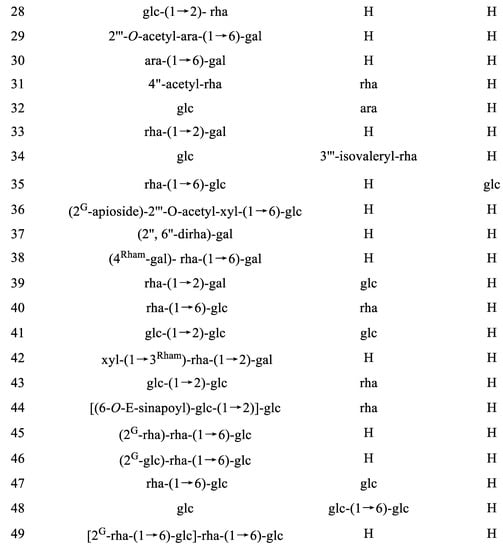
|
No. |
Name |
Trivial Name |
Source |
Ref. |
|---|---|---|---|---|
|
Monoglycosides |
||||
|
1 |
Isorhamnetin-7-O-β-d-glucoside |
Brassicin |
Centaurea cyanus Centaurea kotschyi var. kotschyi Cnicus wallichi Russowia Sogdiana Tagetes lucida (Asteraceae) Sedum sarmentosum Bunge Nitraria tangutorum Bolor |
[29] [30] [31] [32] [33] [34] [22] |
|
2 |
Isorhamnetin-7-O-α-l-rhamnoside |
Carduncellus eriocephalus Nitraria tangutorum Bolor Atriplex centralasiatica Laportea bulbifera Wedd. V. galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert Raphanus raphanistrum L. Caragana intermedia |
[35] [22] [36] [21] [37] [38] [39] |
|
|
3 |
Isorhamnetin-3-O-α-l-rhamnoside |
Laportea bulbifera Wedd. |
[21] |
|
|
4 |
Isorhamnentin-3-O-β-d-glucoside |
Astragalus centralpinus Solidago canadensis L. Hippophae rhamnoids Sambucus nigra L. Calendula officinalis |
[40] [28] [20] [41] [42] |
|
|
5 |
Isorhamnetin-3-O-β-d-glucuronide |
Arnica montana Persicaria thunbergii Senecio giganteus Polygonum aviculare L. Senecio argunensis Turcz. |
[43] [44] [45] [46] [47] |
|
|
6 |
Isorhamnetin-3-O-β-d-(2-acetyl-glucuronide) |
Polygonum aviculare L. |
[46] |
|
|
7 |
Isorhamnetin-3-O-β-d (6-acetyl-glucoside) |
Solidago canadensis L. |
[28] |
|
|
8 |
Isorhamnetin-3-O-β-d-galactoside |
Senecio argunensis Turcz. |
[47] |
|
|
9 |
Isorhamnetin-4′-O-β-d glucoside |
Allium cepa L. |
[23] |
|
|
Diglycosides |
||||
|
10 |
Isorhamnetin-3-O-[2‴-O-acetyl−β-d-xyloside-(1→6)-β-d-glucoside] |
Gymnocarpos decander |
[27] |
|
|
11 |
Isorhamnetin-3-O-[2‴,3‴-O-isopropylidene-α-l-rhamnoside]—(1→6)-β-d-glucoside |
Tetraena aegyptia |
[48] |
|
|
12 |
Isorhamnetin-7-O-α-l-rhamnoside-(1→2)-β-d-glucoside |
Isorhamnetin-7-O-β-neohesperidoside |
Cleome droserifolia |
[12] |
|
13 |
Isorhamnetin-7-O-β-d-glucoside-(1→6)-β-d-glucoside |
Astragaloside or Isorhamnetin-7-O-gentiobioside |
Astragalus altaicus |
[49] |
|
14 |
Isorhamnetin-3-O-β-(4‴-p-coumaroyl-α-rhamnosy]—(1→6)-galactoside) |
Aerva javanica |
[50] |
|
|
15 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→2)-β-d-glucoside |
Isorhamnetin-3-O-β-neohesperidoside |
Hippophae rhamnoids Typha augustifolia L. Calendula officinalis |
[20] [51] [42] |
|
16 |
Isorhamnetin-3-O-β-d-xylosidel-(1→2)-β-d-galactoside |
Prunus padus L. |
[52] |
|
|
17 |
Isorhamnetin-3,4′-O-β-d-diglucoside |
Allium ascalonicum Lepidium apetalum willd |
[24] [53] |
|
|
18 |
Isorhamnetin-3,7-O-β-d-diglucoside |
Sedum sarmentosum Bunge Carduncellus eriocephalus |
[34] [35] |
|
|
19 |
Isorhamnetin-3,7-O-α-l-dirhamnoside |
Laportea bulbifera Wedd. |
[21] |
|
|
20 |
Isorhamnetin-3-O-β-d-glucoside-7-O-α-l-rhamnoside |
Brassidine |
Sinapis arvensis Atriplex centralasiatica Hippophae rhamnoids |
[54] [36] [20] |
|
21 |
Isorhamnetin-3-O-β-d-glucoside-4′-O-β-d-xyloside |
Diplotaxis harra (Forssk.) Boiss |
[26] |
|
|
22 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-galactoside |
Isorhamnetin-3-O-robinobioside |
Nitraria retusa |
[55] |
|
23 |
Isorhamnetin-3-O-α-rhamnoside-(1→2)-rhamnoside |
Laportea bulbifera Wedd. |
[21] |
|
|
24 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside |
Narcissin Isorhamnetin-3-O-rutinoside |
V. galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert opuntia ficus-indica Hippophae rhamnoids Ginkgo biloba Sambucus nigra L. Calendula officinalis |
[37] [18] [20] [41] [42] |
|
25 |
Isorhamnetin-3-O-β-d-apioide (1→2)-β-d-galactoside |
V. galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert |
[37] |
|
|
26 |
Isorhamnetin-3-O-α-l-arabinoside-7-O-β-d-glucoside |
Callianthemum taipaicum Narcissus pseudonarcissus |
[57] [58] |
|
|
27 |
Isorhamnetin-3-O-β-d- (6‴-p-coumaroyl-α-glucoside-(1→2)-rhamnoside) |
Ginkgo biloba |
[56] |
|
|
28 |
Isorhamnetin-3-O-β-d-glucoside-(1→2)-α-l-rhamnoside |
Ginkgo biloba |
[56] |
|
|
29 |
Isorhamnetin-3-O-[2‴-O-acetyl−α-l-arabinoside-(1→6)-β-d-galactoside] |
Trillium tschonoskii Maxim. Trillium apetalon Makino. and T. kamtschaticum Pallas. |
[59] [60] |
|
|
30 |
Isorhamnetin-3-O−α-l-arabinoside-(1→6)-β-d-galactoside |
Trillium apetalon Makino. and T. kamtschaticum Pallas. |
[60] |
|
|
31 |
Isorhamnetin-3-O-α-(4″-acetyl-rhamnoside)-7-O-α-rhamnoside |
Cleome droserifolia |
[12] |
|
|
32 |
Isorhamnetin-3-O-β-d-glucoside-7-O-α-l-arabinoside |
Eschscholtzia mexicana Greene |
[61] |
|
|
33 |
Isorhamnetin-3-O-α-l-rhamnoside(1→2)]-β-d-galactoside |
Glycine max (L.) Merr. |
[62] |
|
|
34 |
Isorhamnetin-3-O-β-glucoside-7-O-α-(3″′-isovaleryl)-rhamnoside |
Lepidium apetalum |
[53] |
|
|
Triglycosides |
||||
|
35 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-4′-O-β-d-glucoside |
Isorhamnetin-3-rutinoside-4′-glucoside |
Mercurialis annua |
[26] |
|
36 |
Isorhamnetin-3-O-(2G-β-d-apiofuranosyl) [2‴-O-acetyl−β-d-xyloside-(1→6)-β-d-glucoside] |
Gymnocarpos decander |
[27] |
|
|
37 |
Isorhamnetin-3-O-(2″,6″-O-α-l-dirhamnoside)-β-d-galactoside |
Alangium premnifolium Lysimachia fortunei |
[63] [64] |
|
|
38 |
Isorhamnetin-3-O-(4Rham-β-d-galactosyl)-α-l-rhamnoside-(1→6)-β-d-galactoside] |
Isorhamnetin-3-O-4Rham-galactosyl-robinobioside |
Nitraria retusa |
|
|
39 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→2)-β-d-galactoside-7-O-β-d-glucoside |
Blackstonia perfoliata |
[66] |
|
|
40 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-7-O-α-l-rhamnoside |
Isorhamnetin-3-rutinoside-7-rhamnoside |
Cassia italica Hippophae rhamnoides |
[67] [68] |
|
41 |
Isorhamnetin-3-O-β-glucoside-(1→2)-β-d-glucoside-7-β-d-glucoside |
Brassicoside or Isorhamnetin-3-O-sophoroside-7-O-β-d-glucoside |
Brassica napus |
[54] |
|
42 |
Isorhamnetin-3-O-β-d-xyloside-(1→3Rham)-α-l-rhamnoside-(1→6)-β-d-galactoside |
Isorhamnetin 3-xylosyl-robinobioside |
Nitraria retusa |
[55] |
|
43 |
Isorhamnetin-3-O-β-glucoside-(1→2)-β-d-glucoside-7-O-α-l-rhamnoside |
Isorhamnetin-3-O-sophoroside-7-O-rhamnoside |
Hippophae rhamnoids |
[20] |
|
44 |
Isorhamnetin-3-O-[(6-O-E-sinapoyl)-β-d-glucoside-(1 → 2)]-β-d-glucoside-7-O-α-l-rhamnoside |
Hippophae rhamnoids |
[20] |
|
|
45 |
Isorhamnetin-3-O-(2G-α-l-rhamnoside)-α-l-rhamnoside-(1→6)-β-d-glucoside |
Typhaneoside |
Typha augustifolia L. Calendula officinalis |
[51] [42] |
|
46 |
Isorhamnetin-3-O-(2G-β-d-glucoside)-α-l-rhamnoside-(1→6)-β-d-glucoside |
Boldo Folium |
[69] |
|
|
47 |
Isorhammetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-7-O-β-d-glucoside |
Isorhammetin-3-rutinoside-7-glucoside |
Hippophae rhamnoids Mercurialis annua |
[20] [26] |
|
48 |
Isorhamnetin-3-O-β-d-glucoside-7-O-β-d-glucoside-(1→6)-β-d-glucoside |
Isorhamnetin-3-O-glucoside-7-O-gentiobioside |
Lepidium apetalum willd |
[53] |
|
Tetraglycosides |
||||
|
49 |
Isorhamnetin-3-O-[2G-α-l-rhamnoside-(1→6)-β-d-glucoside]-α-l-rhamnoside-(1→6)-β-d-glucoside |
Boldo Folium |
[69] |
|
3. Sources of IGs

4. IG Identification and Quantification Methods
4.1. Spectral Techniques and Mass Spectrometry
4.2. Chromatographic Techniques
5. The Health-Promoting Effects of IGs
5.1. Antioxidant Activity
5.2. Anti-Inflammatory Activity
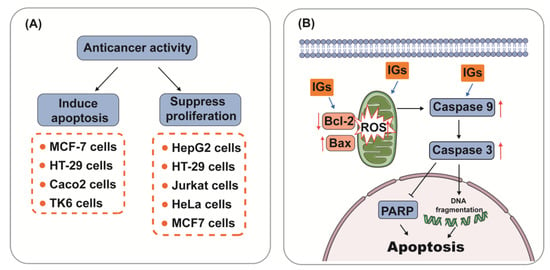
5.3. Anti-Cancer Activity
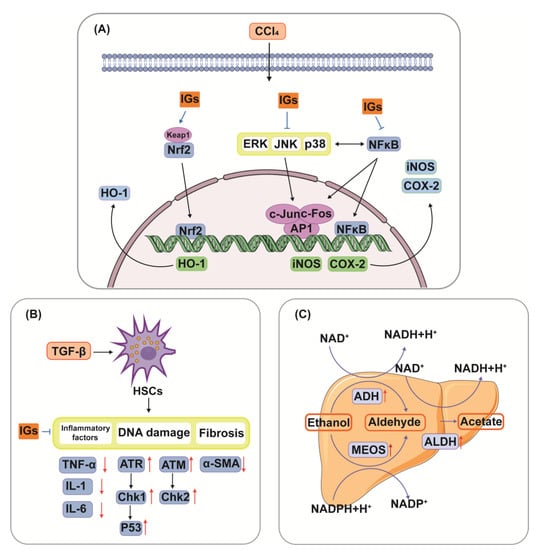
5.4. Hepatoprotective Ability
5.5. Antidiabetic Activity
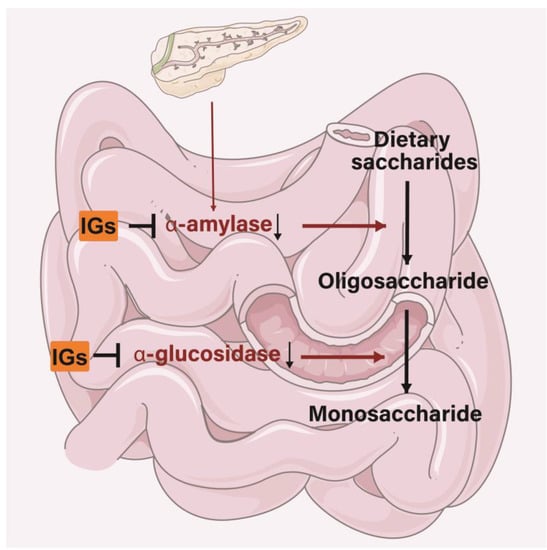
5.6. Anti-Obesity Activity
5.7. Antithrombotic Activity
5.8. Toxic Effects
Currently, there are no systematic toxicological studies on IGs, and further studies are needed. Bee bread (BB) is a fermented mixture of plant pollen, honey, and bee saliva, and is rich in flavonoid glycoside derivatives [204]. Filipa Sobral et al. collected a variety of BB samples, and the most abundant compounds in BB1 (>400 µg/mL) were isrohamnetin-O-hexosyl-O-rutinoside and isorhamnetin-O-pentosyl-hexoside. They found that the BB1 sample showed no toxicity to non-tumor porcine liver primary cells [205]. Isorhamnetin-3-rutinoside-4′ -glucoside (35), isolated from P. lanceolata inflorescences, showed signifificantlyless cytotoxicity towards the nontumorigenic cell line MCF-12A at a concentration of400 µM [206]. Isorhamnetin-3-O-β-D-galactopyranoside (8) and isorhamnetin-3-O-β-D-glucopyranoside (4) (100 µg/mL) isolated from Salsola imbricata Forssk. exhibited no cytotoxicity in RAW 264.7 macrophage cells [158]. Furthermore, it was demonstrated that the viability of PBMCs was slightly decreased after 48 h of incubation with isoretin-3-O-rutin(24) (0–180 µM) from Cyrtosperma johnstonii. However, the decrease in cell viability was no greater than 30% [207]. A brine shrimp toxicity assay of extracts and isolated compounds from Terminalia macroptera leaves showed that narcissin (24) was not toxic against brine shrimp larvae at the tested concentrations (200 µM) [182].
6. Bioaccessibility of IGs
The bioaccessibility of bioactive compounds refers to the maximum fraction of the compound released from the food matrix into the lumen of the gastrointestinal tract to be absorbed [208]. Most flavonoids exist in nature as glycosides, in which sugar residues modify the absorption mechanism and their ability to enter cells or interact with transporters and cellular lipoproteins [209,210]. Flavonoid glycosides exhibit better bioavailability both in vitro and in vivo, which is probably due to their higher aqueous solubility and stability during digestion [8]. At the same time, the gut microbiota plays an important role in improving the bioavailability and enhancing the absorption of flavonoids [211]. The deglycosylation of flavonoid glycosides by the gut microbiota enhances the bioavailability of flavonoids [212].
Compared with isorhamnetin aglycone, IGs have higher accessibility. Antunes Ricardo et al. found that glycosylation protected isorhamnetin from degradation during simulated digestion, and IGs were better retained in the circulatory system than aglycone [8]. Isorhamnetin-3-O-rutinoside (24) (93.2 ± 0.2%) and isorhamnetin 3-O-glucoside (4) (66.8 ± 1.7%) from almond skins showed higher bioaccessibility than isorhamnetin (25.1 ± 7.0%) after simulated digestion [213]. Isorhamnetin glucosyl-rhamnosylrhamnoside, isorhamnetin glucosyl-rhamnosyl-pentoside, isorhamnetin hexosyl-hexosyl-pentoside, and isorhamnetin glucosyl-pentoside showed high bioaccessibility in the peels of four prickly pear varieties during in vitro simulated gastrointestinal digestion [58]. Isorhamnetin glucosyl-rhamnosyl-rhamnoside and isorhamnetin glucosyl-pentoside in Opuntia fificus-indica cladodes showed bioaccessibility values of 58% and 38%[215].
It was also reported that the antidiabetic, anti-inflammatory, and antiallergic activities of flavonoid glycosides were similar or even higher than those of aglycones when provided orally [216,217,218,219]. The effect of flavonoid glycosides is benefificial, probably due to the fact that flavonoid glycosides maintain higher plasma concentrations and have a longer mean residence time in the blood than aglycones [220]. Typhaneoside (45) and isorhamnetin-3-O-neohesperidoside (15) were detected immediately after the oral administrations of pollen typhae extract in rats, indicating that they were rapidly absorbed after oral administration [86,221]. IGs in sea buckthorn berries were monoglucuronidated in humans and were readily bioavailable [222]. Following the ingestion of lightly fried onions, flavonols were absorbed into the plasma of humans as glycosides, with a higher accumulation of isorhamnetin-4′ -glucoside (9) in the plasma and urine than quercetin conjugates, which indicated that 9 may be preferentially absorbed [223]. Similarly, the results of a randomized crossover supplementation trial in female volunteers showed that 9 underwent significant elevation in the plasma after the ingestion of onion powder [224]. Antunes-Ricardo et al.reported that IGs found naturally in O. ficus-indica have a longer elimination half-life than isorhamnetin, suggesting that they can maintain constant plasma concentrations, and thus, prolong their biological effects [8].
Planar lipophilic polyphenols, such as curcumin, epigallocatechin gallate, quercetin, and genistein, are known as Pan-Assay Interference Compounds (PAINS) or Invalid Metabolic Panaceas (IMPS) because of their ability to interfere with membrane dipole potential [225]. Ana Marta de Matos et al. demonstrated that compounds produced via C-glycosylation are no longer able to alter the membrane dipole potential [226]. However, O-glycosylated compounds are easily hydrolyzed in the gut, so they are not suitable for this strategy. There are no more studies on the interference of isorhamnetin glycosides on membrane dipole potential, so further research in this field is warranted.
This entry is adapted from the peer-reviewed paper 10.3390/nu15081947
