Vulvovaginal candidosis (VVC) is a frequently occurring infection of the lower female genital tract, mostly affecting immuno-competent women at childbearing age. Candida albicans is the most prevalent pathogenic yeast—apart from other non-albicans species—related to this fungal infection. Different virulence factors of C. albicans have been identified, which increase the risk of developing VVC. To initiate treatment and positively influence the disease course, fast and reliable diagnosis is crucial. In this narrative review, we cover the existing state of understanding of the epidemiology, pathogenesis and diagnosis of VVC. However, treatment recommendations should follow current guidelines.
- Candida albicans
- diagnosis
- epidemiology
- fungal infection
- review
- vulvovaginal candidosis
1. Introduction
Vulvovaginal candidosis (VVC) is a disease that affects women of all ethnicities and social classes[1][2]. The exact epidemiology of this disease is based on unreliable data, although based on what is currently known, 70–75% of women experience VVC at least once in their lifetime[2][3]. Numerous risk factors for VVC have been described. However, the pathological mechanism underlying the progression from colonization to infection is not fully understood[2], partly because most cases do not present a specific trigger[4][5]. The estrogenized vagina of asymptomatic women is often colonized with Candida species, although colonization does not necessarily lead to an infection[6]. The predominant species in 90–95% of cases is Candida albicans, followed by non-albicans species, such as C. glabrata, C. tropicalis, C. krusei and C. parapsilosis[7]. If VVC is caused by non-albicans species, it mostly manifests as a mild infection[4].
VVC causes distress in many affected women, and it is the reason for many consultations in gynecological offices worldwide.
2. Pathogenesis
Recognition of the importance of the innate response in driving inflammatory responses associated with VVC has led to many recent insights regarding the pathogenesis of VVC. It is known that cells of the innate host express receptors that recognize pathogen-associated molecular patterns (PAMPs)[8]. The major classes of receptors that recognize Candida-associated molecular patterns are those of the Toll-like receptors and lectin-like receptors families[9]. A mannose-binding lectin (MBL) recognizes and binds to Candida surface mannan, increases complement activation and inhibits Candida growth[10][11] Similarly, macrophages, dendritic cells and epithelial cells play an important role and exert a protective function. Macrophages and dendritic cells have specific surface receptors that recognize MBL and promote opsonization of MBL-bound microorganisms[12].
Adaptive immunity—including fungus-specific defense mechanisms—is developed directly or indirectly through cell-mediated immunity (T-cells)[13]. Decreased T-cell-mediated immunity is associated with increased susceptibility to VVC in women who are immunocompromised (e.g., due to HIV infection, previous organ transplantation, glucocorticoid therapy or antineoplastic chemotherapy)[14][15][16]. B-cells and immunoglobulin-secreting plasma cells migrate into the vaginal epithelium[17], protecting against pathogenic microorganisms. The exact mechanism by which antibodies protect against Candida is unknown [18][19]. Immunoglobulin A (IgA) and IgG are the predominant immunoglobulin classes found in vaginal secretions[20].
During the pathogenic process, Candida undergoes reversible yeast-to-hyphae transition, which causes changes in the type of surface carbohydrates, affecting the adhesion and invasion of vaginal epithelial cells (Figure 1). Candida either infects vaginal epithelial cells directly through the invasion of hyphae or indirectly through the contact of PAMPs with pattern recognition receptors (PRRs). In response to this, contact inflammatory immune mediators—chemokines, cytokines, antimicrobial peptides or damage-associated molecular patterns—are secreted, subsequently recruiting innate immune cells, such as macrophages, dendritic cells and neutrophils. These cells also recognize PAMPs through PRRs on their surfaces, bind to the pathogen, and stimulate its removal by phagocytosis.
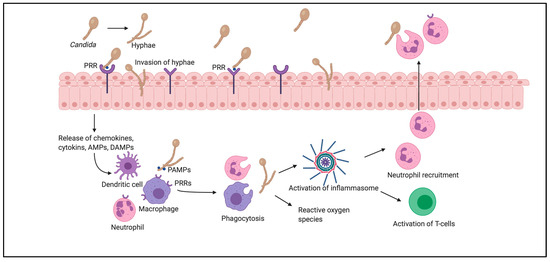
Figure 1. Pathogenesis of vulvovaginal candidosis.
This mechanism involves the production of reactive oxygen species, which further regulate all stages of inflammation. The phagocytized pathogenic components activate inflammasomes, which induce the release of proinflammatory cytokines, and subsequently promote T-cell activation and neutrophil recruitment. Neutrophils enter through the vaginal epithelium and promote phagocytosis of Candida on the epithelial surfaces of the vagina[21][22][23].
For Candida colonization on the host surface, adhesion to vaginal epithelial cells is crucial and contributes to infection and persistence[24]. This process is promoted by cell-surface components (adhesins), which recognize host ligands, such as serum proteins, in the extracellular matrix of host tissues (e.g., laminin, fibronectin, collagen, vitronectin and entactin)[25]. A major group of adhesins from the agglutinin-like sequence (ALS) gene family is encoded in C. albicans; this group consists of 8 members (ALS 1–7, ALS 9)[26]. Cheng et al.[27] found that ALS 1–3 and ALS 9 occurred more frequently in women with VVC.
2.1. The Role of Enzymes
Candida spp. secrete several hydrolytic enzymes[28]. Among the most important enzymes are aspartyl proteinases (Saps), which facilitate adhesion[24][29]. In recent years, 10 Sap genes were identified in C. albicans [30], 3 in C. parapsilosis[31], and 4 in C. tropicalis[32][33]. Saps only have proteinase activity in acidic environments[34]; Sap 1–3 show a specific correlation with VVC and the occurrence of C. albicans[35][36][37].
Phospholipases similarly play an important role in pathogenicity by damaging host cell membranes and contributing to adhesion. Mohandas and Ballal[38] observed a greater number of phospholipase-producing strains from vaginal isolates in patients with candidosis than in patients without candidosis. Moreover, different phospholipase genes (PLA, PLB1–2, PLC1–3, and PLD1) were described in infected women[39].
2.2. From Colonization to Infection
Vaginal colonization with C. albicans is common, and women with colonization are often asymptomatic[40]. However, when colonization progresses to infection, women frequently report vaginal itching, burning, pain, and redness. Typical VVC symptoms are often accompanied by vaginal discharge, consisting of shed epithelium, immune cells, yeast and vaginal fluid[41]. Candida invasion requires a transition from the yeast to the hyphae form; however, the ability to produce hyphae varies among different species[24]. In vitro studies showed that C. albicans without hyphae formation has a lower rate of tissue invasion[42]. In addition, the toxin candidalysin contributes to this transition, as it has a cytotoxic effect on the host cells and promotes invasion, attracting leukocytes[22][43]. Multiple virulence and host-specific factors may play a role in the development from colonization to infection.
3. Virulence Factors
VVC episodes cannot be attributed to a specific trigger[4][5]. The individual infection susceptibility depends on intrinsic and extrinsic factors. Host-specific risk factors, such as local defense mechanisms, age and hormonal status, pregnancy, allergies, psychosocial stress, metabolic issues, immunosuppression and individual genetic susceptibility, are important[2][44][45][46][47][48]. Additionally, behavioral risk factors, such as the use of oral contraceptives, antibiotics, glucocorticoids, inhibitors of the sodium glucose co-transporter-2 (SGLT2), intrauterine devices (IUDs), spermicides and condoms, as well as sexual, hygienic and dressing habits, need to be addressed [1][2][49].
3.1. Immunologic Factors
Genetic factors contribute to the development of VVC or its relapse. Foxman et al.[50] reported that women of African ethnicity showed an increased risk for VVC. This might be the result of a reduced occurrence of lactobacilli that happens more frequently in women of African ethnicity[51]. Moreover, genetic polymorphisms in blood group antigens and MBL have been identified in cases of increased susceptibility to RVVC[52][53][54][55]. A loss of the last 9 amino-acids in the carbohydrate recognition domain of the Dectin-1 gene has been associated with the occurrence of RVVC[56]. This mutation leads to insufficient production of cytokines (IL-17, tumor necrosis factor and IL-6) when it comes in contact with Candida (78). Moreover, women with atopic diathesis and type I allergies experience VVC more frequently than healthy individuals[57]. The typical VVC symptoms, such as itching and redness, may equally be regarded as signs of an allergic phenomenon [2][58].
Apart from common immunologic factors, pregnancy increases the likelihood of experiencing VVC; its incidence increases from 9% to 54% between the first and the third trimesters of pregnancy [59][60][61]. This rise may be partly attributed to immunologic factors, but also to the increase in sex hormones[44][45]. The occurrence of VVC during pregnancy is generally not considered dangerous with regard to preterm birth[45]. Recurrent candidosis showed an association with preterm birth in a large retrospective trial [60]. However, preterm birth is a multifactorial event, and it is likely that chronic inflammation but not VVC itself contributes to this event[60]. Of important note, almost all infants born from mothers with VVC during pregnancy show “diaper rash” or oral thrush due to the vertical mother-to-infant transmission [62].
3.2. Hormonal Factors
Glycogen serves as a nutrient substrate for fungi in the vaginal epithelium. There is a relationship between the respective hormonal cycle phase and the occurrence of VVC influenced by estrogen. Therefore, most women experience VVC cyclically or during the luteal phase. When estrogen levels drop during menstruation, symptoms often disappear[63]. Women who are on oral contraceptives and postmenopausal women who receive hormone replacement therapy are more likely to develop VVC than others[64]. Several studies report a higher incidence of colonization with Candida and VVC in women who use oral contraceptive pills [3][14][65]. The intake of contraceptives increases the level of vaginal glycogen, providing a better condition for Candida growth [66]. Miller et al. [67] demonstrated that IUDs compromise the vaginal defense against infections. Donders et al. [68] reported that the risk of developing VVC increased during the first 5 years after placement of levonorgestrel intrauterine systems, with the risk particularly high during the first year of placement[68]. Progesterone, however, reduces the ability of C. albicans to develop hyphae forms.
3.3. Metabolic Factors
Women with diabetes mellitus have an increased risk of experiencing VVC. This risk is exacerbated when their serum glucose levels are not within the normal range. Hyperglycemia leads to increased fungal adhesion and growth, and a glycemic index of 10–11 mmol/L can impair the host’s defense mechanisms[69]. Similarly, C. albicans shows a high ability to bind to vaginal epithelial cells in in vitro studies[70][71]. The yeast exhibits a glucose-inducible surface protein that promotes its adhesion to vaginal epithelial cells[69], and an increase in this protein impairs the recognition of neutrophil phagocytes[72]. Therefore, the migration of neutrophils is reduced and their functions, including phagocytosis, adhesion, chemotaxis, and intracellular killing, are impaired, increasing the sensitivity to VVC[3][5][47][68].
From a clinical perspective, women with diabetes are often unresponsive to standard antifungal treatment[46][47]. Compared with non-diabetic women, diabetic women are frequently colonized with non-albicans species, such as C. glabrata[73][74][75], impacting their treatment concept[73]. In diabetic women with RVVC, discontinuation of the antidiabetic agent should be considered[22]. The undesirable side effect of hyperglycemia caused by glucocorticoids has a similar effect to that of non-drug-induced hyperglycemia[76] because the steroid hormones suppress the immune response, increasing the susceptibility to VVC[77]. In pregnant women with gestational diabetes, their diabetic state impairs metabolic control and leukocyte function[5] [78].
3.4. Lifestyle Factors
Various lifestyle factors can have a significant influence on the development of VVC. There is weak evidence for the impact of nutrition on Candida growth, although some studies have reported that the consumption of food rich in sugar and carbohydrates, as well as dairy products, can lead to increased fungal growth[78][79]. In contrast, others have reported that yogurt, oat bran and flaxseed might have positive effects in preventing fungal growth [7]. Despite their theoretical basis, these findings are not well substantiated.
Sexual behavior, especially oral sex, plays a role particularly for re-infections[81][82]. Reed et al. demonstrated that oral microorganisms could be transmitted from the oral cavity to the vagina[81][83][84]. Genital hygiene can also become a risk factor for VVC, when poor personal hygiene and a high frequency of sexual intercourse increase the likelihood for colonization[80], VVC[85] and RVVC[86][87]. Women should be advised to avoid excessive washing of the genital area and the use of potential irritants such as perfumed soaps, bubble baths, powders or vaginal sprays[88].
The use of tight clothing and synthetic underwear might also promote fungal growth due to the increased perineal moisture and temperature[49][85].
3.5. Other Exogenous Factors
Women who are colonized with Candida have a 33% higher risk of developing VVC after antibiotic treatment than non-colonized women[80][89][90][91]. However, the routine use of antimycotic treatment after antibiotic therapy should be avoided because it fosters drug-resistant fungi[92]. There are different theories about why VVC occurs more frequently after antibiotic treatment. One of the theories involves the reduction or eradication of vaginal and intestinal lactobacilli caused by antibiotics. As a result, affected patients lack protection from pathogenic microorganisms because lactobacilli have the ability to adhere to vaginal epithelial cells and inhibit pathogenic fungal growth[93][94].
Some lactobacilli have antagonistic effects on Candida[95][96]; their vaginal administration may lead to an adequate colonization and reduction in fungal load[22][97]. In vitro, lactobacilli have also shown direct fungicidal and immuno-stimulatory effects[98]. The interactions are manifold: lactobacilli can block the passage of pathogenic microbes from the gastrointestinal tract into the vagina, modulate the host’s immune response, influence epithelial defense and thus affect the expression of VVC-induced inflammatory genes. Probiotics take advantage of these effects[99].
This entry is adapted from the peer-reviewed paper 10.3390/jof6040267
References
- Barajas, J.F.; Wehrs, M.; To, M.; Cruickshanks, L.; Urban, R.; McKee, A.; Gladden, J.; Goh, E.-B.; Brown, M.E.; Pierotti, D.; et al. Isolation and Characterization of Bacterial Cellulase Producers for Biomass Deconstruction: A Microbiology Laboratory Course. J. Microbiol. Biol. Educ. 2019, 20.
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971.
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2015, 42, 905–927.
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347.
- Yano, J.; Sobel, J.D.; Nyirjesy, P.; Sobel, R.; Williams, V.L.; Yu, Q.; Noverr, M.C.; Fidel, P.L. Current patient perspectives of vulvovaginal candidiasis: Incidence, symptoms, management and post-treatment outcomes. BMC Women’s Health 2019, 19, 1–9.
- Nyman, G.S.A.; Tang, M.; Inerot, A.; Osmancevic, A.; Malmberg, P.; Hagvall, L. Contact allergy to beeswax and propolis among patients with cheilitis or facial dermatitis. Contact Dermat. 2019, 81, 110–116.
- Felix, T.C.; Röder, D.V.D.D.B.; Pedroso, R.D.S. Alternative and complementary therapies for vulvovaginal candidiasis. Folia Microbiol. 2019, 64, 133–141.
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145.
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A.R. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Genet. 2008, 6, 67–78.
- Ip, W.K.; Lau, Y.L. Role of Mannose-Binding Lectin in the Innate Defense against Candida albicans: Enhancement of Complement Activation, but Lack of Opsonic Function, in Phagocytosis by Human Dendritic Cells. J. Infect. Dis. 2004, 190, 632–640.
- Pellis, V.; de Seta, F.; Crovella, S.; Bossi, F.; Bulla, R.; Guaschino, S.; Radillo, O.; Garred, P.; Tedesco, F. Mannose binding lectin and C3 act as recognition molecules for infectious agents in the vagina. Clin. Exp. Immunol. 2005, 139, 120–126.
- Babovic-Vuksanovic, D.; Snow, K.; Ten, R.M. Mannose-binding lectin (MBL) deficiency. Variant alleles in a Midwestern population of the United States. Ann. Allergy Asthma Immunol. 1999, 82, 134–143.
- Wira, C.R.; Fahey, J.V.; Sentman, C.L.; Pioli, P.A.; Shen, L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol. Rev. 2005, 206, 306–335.
- Apalata, T.; Longo-Mbenza, B.; Sturm, A.; Carr, W.; Moodley, P. Factors associated with symptomatic vulvovaginal candidiasis: A study among women attending a primary healthcare clinic in Kwazulu-Natal, South Africa. Ann. Med. Health Sci. Res. 2014, 4, 410–416.
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73.
- Nwadioha, I. Risk factors for vaginal candidiasis among women attending primary health care centers of Jos, Nigeria. J. Clin. Med. Res. 2010, 2, 110–113.
- Pudney, J.; Quayle, A.J.; Anderson, D.J. Immunological Microenvironments in the Human Vagina and Cervix: Mediators of Cellular Immunity are Concentrated in the Cervical Transformation Zone1. Biol. Reprod. 2005, 73, 1253–1263.
- Moragues, M.D.; Omaetxebarria, M.J.; Elguezabal, N.; Sevilla, M.J.; Conti, S.; Polonelli, L.; Pontón, J. A Monoclonal Antibody Directed against a Candida albicans Cell Wall Mannoprotein Exerts Three Anti-C. albicans Activities. Infect. Immun. 2003, 71, 5273–5279.
- Russell, M.W.; Mestecky, J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002, 4, 667–677.
- De Carvalho, R.J.V.; Cunha, C.M.; Silva, D.A.D.O.; Sopelete, M.C.; Urzedo, J.E.; Moreira, T.A.; Moraes, P.D.S.A.; Taketomi, E.A. IgA, IgE and IgG subclasses to Candida albicans in serum and vaginal fluid from patients with vulvovaginal candidiasis. Revista Associação Médica Brasileira 2004, 49, 434–438.
- Lewis, R.E.; Viale, P.; Kontoyiannis, D.P. The potential impact of antifungal drug resistance mechanisms on the host immune response to Candida. Virulence 2012, 3, 368–376.
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27.
- Kalia, N.; Singh, J.; Kaur, M. Immunopathology of Recurrent Vulvovaginal Infections: New Aspects and Research Directions. Front. Immunol. 2019, 10.
- Silva, S.C.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305.
- Verstrepen, K.J.; Klis, F.M. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 2006, 60, 5–15.
- Hoyer, L.L.; Green, C.B.; Oh, S.-H.; Zhao, X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family—A sticky pursuit. Med. Mycol. 2008, 46, 1–15.
- Cheng, G.; Wozniak, K.L.; Wallig, M.A.; Fidel, P.L.; Trupin, S.R.; Hoyer, L.L. Comparison between Candida albicans Agglutinin-Like Sequence Gene Expression Patterns in Human Clinical Specimens and Models of Vaginal Candidiasis. Infect. Immun. 2005, 73, 1656–1663.
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377.
- Pichová, I.; Pavlíčková, L.; Dostál, J.; Dolejší, E.; Hrušková-Heidingsfeldová, O.; Weber, J.; Ruml, T.; Souček, M. Secreted aspartic proteases of Candida albicans, Candida tropicalis, Candida parapsilosis and Candida lusitaniae. J. Biol. Inorg. Chem. 2001, 268, 2669–2677.
- Odds, F.C. Secreted proteinases and Candida albicans virulence. Microbiology 2008, 154, 3245–3246.
- Merkerová, M.D.; Dostã¡l, J.; Hradilek, M.; Pichovã¡, I.; Hrušková-Heidingsfeldová, O. Cloning and characterization of Sapp2p, the second aspartic proteinase isoenzyme from Candida parapsilosis. FEMS Yeast Res. 2006, 6, 1018–1026.
- Togni, G.; Sanglard, D.; Falchetto, R.; Monod, M. Isolation and nucleotide sequence of the extracellular acid protease gene (ACP) from the yeast Candida tropicalis. FEBS Lett. 1991, 286, 181–185.
- Zaugg, C.; Zepelin, M.B.-V.; Reichard, U.; Sanglard, D.; Monod, M. Secreted Aspartic Proteinase Family of Candida tropicalis. Infect. Immun. 2001, 69, 405–412.
- Williams, D.W.; Kuriyama, T.; Silva, S.; Malic, S.; Lewis, M.A.O. Candida biofilms and oral candidosis: Treatment and prevention. Periodontology 2000 2010, 55, 250–265.
- Schaller, M.; Bein, M.; Korting, H.C.; Baur, S.; Hamm, G.; Monod, M.; Beinhauer, S.; Hube, B. The Secreted Aspartyl Proteinases Sap1 and Sap2 Cause Tissue Damage in an in vitro Model of Vaginal Candidiasis Based on Reconstituted Human Vaginal Epithelium. Infect. Immun. 2003, 71, 3227–3234.
- Naglik, J.R.; Rodgers, C.A.; Shirlaw, P.J.; Dobbie, J.L.; Fernandes-Naglik, L.L.; Greenspan, D.; Agabian, N.; Challacombe, S.J. Differential Expression of Candida albicans Secreted Aspartyl Proteinase and Phospholipase B Genes in Humans Correlates with Active Oral and Vaginal Infections. J. Infect. Dis. 2003, 188, 469–479.
- Lian, C.H.; da Liu, W. Differential expression of Candida albicans secreted aspartyl proteinase in human vulvovaginal candidiasis. Mycoses 2007, 50, 383–390.
- Mohandas, V.; Ballal, M. Distribution of Candida Species in different clinical samples and their virulence: Biofilm formation, proteinase and phospholipase production: A study on hospitalized patients in Southern India. J. Glob. Infect. Dis. 2011, 3, 4–8.
- Samaranayake, Y.H.; Dassanayake, R.S.; Cheung, B.P.K.; Jayatilake, J.A.M.S.; Yeung, K.W.S.; Yau, J.Y.Y.; Samaranayake, L.P. Differential phospholipase gene expression by Candida albicans in artificial media and cultured human oral epithelium. APMIS 2006, 114, 857–866.
- Achkar, J.M.; Fries, B.C. Candida Infections of the Genitourinary Tract. Clin. Microbiol. Rev. 2010, 23, 253–273.
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903.
- Jayatilake, J.A.M.S.; Samaranayake, Y.H.; Cheung, L.K.; Samaranayake, L.P. Quantitative evaluation of tissue invasion by wild type, hyphal and SAP mutants of Candida albicans, and non-albicans Candida species in reconstituted human oral epithelium. J. Oral Pathol. Med. 2006, 35, 484–491.
- Naglik, J.R.; Gaffen, S.L.; Hube, B. Candidalysin: Discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019, 52, 100–109.
- Sobel, J.D.; Faro, S.; Force, R.W.; Foxman, B.; Ledger, W.J.; Nyirjesy, P.R.; Reed, B.D.; Summers, P.R. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 1998, 178, 203–211.
- Cotch, M.F.; Hillier, S.L.; Gibbs, R.S.; Eschenbach, D.A. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Am. J. Obstet. Gynecol. 1998, 178, 374–380.
- Goswami, R.; Dadhwal, V.; Tejaswi, S.; Datta, K.; Paul, A.; Haricharan, R.; Banerjee, U.; Kochupillai, N. Species-specific Prevalence of Vaginal Candidiasis Among Patients with Diabetes Mellitus and its Relation to their Glycaemic Status. J. Infect. 2000, 41, 162–166.
- Bohannon, N.J.V. Treatment of Vulvovaginal Candidiasis in Patients with Diabetes. Diabetes Care 1998, 21, 451–456.
- Meyer, H.; Goettlicher, S.; Mendling, W. Stress as a cause of chronic recurrent vulvovaginal candidosis and the effectiveness of the conventional antimycotic therapy. Mycoses 2006, 49, 202–209.
- Patel, D.A.; Gillespie, B.; Sobel, J.D.; Leaman, D.; Nyirjesy, P.; Weitz, M.; Foxman, B. Risk factors for recurrent vulvovaginal candidiasis in women receiving maintenance antifungal therapy: Results of a prospective cohort study. Am. J. Obstet. Gynecol. 2004, 190, 644–653.
- Geiger, A.M.; Foxman, B. Risk Factors for Vulvovaginal Candidiasis. Epidemiology 1996, 7, 182–187.
- Antonio, M.A.D.; Hawes, S.E.; Hillier, S.L. The Identification of VaginalLactobacillusSpecies and the Demographic and Microbiologic Characteristics of Women Colonized by These Species. J. Infect. Dis. 1999, 180, 1950–1956.
- Chaim, W.; Foxman, B.; Sobel, J.D. Association of recurrent vaginal candidiasis and secretory ABO and Lewis phenotype. J. Infect. Dis. 1997, 176, 828–830.
- Hilton, E.; Chandrasekaran, V.; Rindos, P.; Isenberg, H.D. Association of Recurrent Candidal Vaginitis with Inheritance of Lewis Blood Group Antigens. J. Infect. Dis. 1995, 172, 1616–1619.
- Babula, O.; Lazdāne, G.; Kroica, J.; Linhares, I.M.; Ledger, W.J.; Witkin, S.S. Frequency of Interleukin-4 (IL-4) -589 Gene Polymorphism and Vaginal Concentrations of IL-4, Nitric Oxide, and Mannose-Binding Lectin in Women with Recurrent Vulvovaginal Candidiasis. Clin. Infect. Dis. 2005, 40, 1258–1262.
- Donders, G.G.; Babula, O.; Bellen, G.; Linhares, I.M.; Witkin, S.S. Mannose-binding lectin gene polymorphism and resistance to therapy in women with recurrent vulvovaginal candidiasis. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1225–1231.
- Mendling, W.; Friese, K.; Mylonas, I.; Weissenbacher, E.R.; Brasch, J.; Schaller, M.; Mayser, P.; Effendy, I.; Ginter-Hanselmayer, G.; Hof, H.; et al. Vulvovaginal Candidosis (excluding chronic mucocutaneous candidosis). Guideline of the German Society of Gynecology and Obstetrics (AWMF Registry No. 015/072, S2k Level, December 2013). Geburtshilfe Frauenheilkunde 2015, 75, 342–354.
- Neves, N.A.; Carvalho, L.P.; de Oliveira, M.A.M.; Giraldo, P.C.; Bacellar, O.; Cruz, A.A.; Carvalho, E.M. Association between atopy and recurrent vaginal candidiasis. Clin. Exp. Immunol. 2005, 142, 167–171.
- Witkin, S.S.; Giraldo, P.C.; Linhares, I.M. New insights into the immune pathogenesis of recurrent vulvovaginal candidiasis. Ital. J. Gynaecol. Obstet. 2000, 12, 114–118.
- Morton, R.S.; Rashid, S. Candidal Vaginitis: Natural History, Predisposing Factors and Prevention. Proc. R. Soc. Med. 1977, 70, 3–6.
- Farr, A.; Kiss, H.; Holzer, I.; Husslein, P.; Hagmann, M.; Petricevic, L. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet. Gynecol. Scand. 2015, 94, 989–996.
- Chew, S.Y.; Than, L.T.L. Vulvovaginal candidosis: Contemporary challenges and the future of prophylactic and therapeutic approaches. Mycoses 2016, 59, 262–273.
- Blaschke-Hellmessen, R. Epidemiological studies of the occurrence of yeasts in children and their mothers. Mykosen 1968, 11, 611–616.
- Dennerstein, G.J.; Ellis, D.H. Oestrogen, glycogen and vaginal candidiasis. Aust. N. Z. J. Obstet. Gynaecol. 2001, 41, 326–328.
- Fischer, G.; Bradford, J. Vulvovaginal Candidiasis in Postmenopausal Women. J. Low. Genit. Tract Dis. 2011, 15, 263–267.
- Çetin, M.; Ocak, S.; Güngören, A.; Hakverdi, A.U. Distribution of Candida species in women with vulvovaginal symptoms and their association with different ages and contraceptive methods. Scand. J. Infect. Dis. 2007, 39, 584–588.
- Reed, B.D. Risk Factors for Candida Vulvovaginitis. Obstet. Gynecol. Surv. 1992, 47, 551–560.
- Miller, L.; Patton, D.L.; Meier, A.; Thwin, S.S.; Hooton, T.M.; Eschenbach, D.A. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet. Gynecol. 2000, 96, 431–439.
- Donders, G.G.G.; Bellen, G.; Ruban, K.; van Bulck, B. Short- and long-term influence of the levonorgestrel-releasing intrauterine system (Mirena(R)) on vaginal microbiota and Candida. J. Med. Microbiol. 2018, 67, 308–313.
- Hostetter, M.K. Handicaps to host defense. Effects of hyperglycemia on C3 and Candida albicans. Diabetes 1990, 39, 271–275.
- Kalo, A.; Segal, E. Interaction of Candida albicans with genital mucosa: Effect of sex hormones on adherence of yeasts in vitro. Can. J. Microbiol. 1988, 34, 224–228.
- Segal, E.; Soroka, A.; Schechter, A. Correlative relationship between adherence of Candida albicans to human vaginal epithelial cellsin vitroand candidal vaginitis. Med. Mycol. 1984, 22, 191–200.
- Gilmore, B.J.; Retsinas, E.M.; Lorenz, J.S.; Hostetter, M.K. An iC3b Receptor on Candida albicans: Structure, Function, and Correlates for Pathogenicity. J. Infect. Dis. 1988, 157, 38–46.
- Goswami, D.; Goswami, R.; Banerjee, U.; Dadhwal, V.; Miglani, S.; Lattif, A.A.; Kochupillai, N. Pattern of Candida species isolated from patients with diabetes mellitus and vulvovaginal candidiasis and their response to single dose oral fluconazole therapy. J. Infect. 2006, 52, 111–117.
- Ray, D.; Goswami, R.; Banerjee, U.; Dadhwal, V.; Goswami, D.; Mandal, P.; Sreenivas, V.; Kochupillai, N. Prevalence of Candida glabrata and its Response to Boric Acid Vaginal Suppositories in Comparison with Oral Fluconazole in Patients with Diabetes and Vulvovaginal Candidiasis. Diabetes Care 2007, 30, 312–317.
- Peer, A.K.; Hoosen, A.A.; Seedat, M.A.; Ende, J.V.D.; Omar, M.A. Vaginal yeast infections in diabetic women. S. Afr. Med. J. 1993, 83, 727–729.
- Ferris, H.A.; Kahn, C.R. New mechanisms of glucocorticoid-induced insulin resistance: Make no bones about it. J. Clin. Investig. 2012, 122, 3854–3857.
- Barnes, P.J. Glucocorticosteroids: Current and future directions. Br. J. Pharmacol. 2011, 163, 29–43.
- Donders, G.G.G.; Prenen, H.; Verbeke, G.; Reybrouck, R. Impaired tolerance for glucose in women with recurrent vaginal candidiasis. Am. J. Obstet. Gynecol. 2002, 187, 989–993.
- Cruickshank, R. Acquired Immunity: Bacterial Infections. Mod. Trends Immunol. 1963, 1, 107–129.
- Eckert, L.O. Vulvovaginal candidiasis: Clinical manifestations, risk factors, management algorithm. Obstet. Gynecol. 1998, 92, 757–765.
- Rylander, E.; Berglund, A.L.; Krassny, C.; Petrini, B. Vulvovaginal candida in a young sexually active population: Prevalence and association with oro-genital sex and frequent pain at intercourse. Sex. Transm. Infect. 2004, 80, 54–57.
- Reed, B.D.; Zazove, P.; Pierson, C.L.; Gorenflo, D.W.; Horrocks, J. Candida Transmission and Sexual Behaviors as Risks for a Repeat Episode of Candida Vulvovaginitis. J. Women’s Health 2003, 12, 979–989.
- Reed, B.D.; Gorenflo, D.W.; Gillespie, B.W.; Pierson, C.L.; Zazove, P. Sexual Behaviors and Other Risk Factors for Candida vulvovaginitis. J. Women’s Health Gender-Based Med. 2000, 9, 645–655.
- Bradshaw, C.S.; Morton, A.N.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Fairley, C.K. Higher-Risk Behavioral Practices Associated with Bacterial Vaginosis Compared with Vaginal Candidiasis. Obstet. Gynecol. 2005, 106, 105–114.
- Foxman, B. The epidemiology of vulvovaginal candidiasis: Risk factors. Am. J. Public Health 1990, 80, 329–331.
- Spinillo, A.; Pizzoli, G.; Colonna, L.; Nicola, S.; de Seta, F.; Guaschino, S. Epidemiologic characteristics of women with idiopathic recurrent vulvovaginal candidiasis. Obstet. Gynecol. 1993, 81, 721–727.
- Spinillo, A.; Capuzzo, E.; Nicola, S.; Baltaro, F.; Ferrari, A.; Monaco, A. The impact of oral contraception on vulvovaginal candidiasis. Contraception 1995, 51, 293–297.
- Watson, C.J.; Calabretto, H. Comprehensive review of conventional and non-conventional methods of management of recurrent vulvovaginal candidiasis. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 262–272.
- Pirotta, M.V.; Gunn, J.M.; Chondros, P. “Not thrush again!” Women’s experience of post-antibiotic vulvovaginitis. Med. J. Aust. 2003, 179, 43–46.
- Pirotta, M.V.; Garland, S.M. Genital Candida Species Detected in Samples from Women in Melbourne, Australia, before and after Treatment with Antibiotics. J. Clin. Microbiol. 2006, 44, 3213–3217.
- Xu, J.; Schwartz, K.; Bartoces, M.; Monsur, J.; Severson, R.K.; Sobel, J.D. Effect of antibiotics on vulvovaginal candidiasis: A MetroNet study. J. Am. Board. Fam. Med. 2008, 21, 261–268.
- Shukla, A.; Sobel, J.D. Vulvovaginitis Caused by Candida Species Following Antibiotic Exposure. Curr. Infect. Dis. Rep. 2019, 21, 44.
- Santos, C.M.A.; Pires, M.C.V.; Leão, T.L.; Hernández, Z.P.; Rodriguez, M.L.; Martins, A.K.S.; Miranda, L.S.; Martins, F.S.; Nicoli, J.R. Selection of Lactobacillus strains as potential probiotics for vaginitis treatment. Microbiology 2016, 162, 1195–1207.
- Swidsinski, A.; Guschin, A.; Tang, Q.; Dörffel, Y.; Verstraelen, H.; Tertychnyy, A.; Khayrullina, G.; Luo, X.; Sobel, J.D.; Jiang, X. Vulvovaginal candidiasis: Histologic lesions are primarily polymicrobial and invasive and do not contain biofilms. Am. J. Obstet. Gynecol. 2019, 220, 91.
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Veyver, I.V.D.; Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466.
- Hummelen, R.; Macklaim, J.M.; Bisanz, J.E.; Hammond, J.-A.; McMillan, A.; Vongsa, R.; Koenig, D.; Gloor, G.B.; Reid, G. Vaginal Microbiome and Epithelial Gene Array in Post-Menopausal Women with Moderate to Severe Dryness. PLoS ONE 2011, 6, e26602.
- Yano, J.; Peters, B.M.; Noverr, M.C.; Fidel, P.L. Novel Mechanism behind the Immunopathogenesis of Vulvovaginal Candidiasis: “Neutrophil Anergy”. Infect. Immun. 2017, 86.
- Mailänder-Sánchez, D.; Wagener, J.; Schaller, M. Potential role of probiotic bacteria in the treatment and prevention of localised candidosis. Mycoses 2011, 55, 17–26.
- Kosgey, J.C.; Jia, L.; Fang, Y.; Yang, J.; Gao, L.; Wang, J.; Nyamao, R.; Cheteu, M.; Tong, D.; Wekesa, V.; et al. Probiotics as antifungal agents: Experimental confirmation and future prospects. J. Microbiol. Methods 2019, 162, 28–37.
