Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Loco-regional hyperthermia at 40–44 °C is a multifaceted therapeutic modality with the distinct triple advantage of being a potent radiosensitizer, a chemosensitizer and an immunomodulator. Risk difference estimates from pairwise meta-analysis have shown that the local tumour control could be improved by 22.3% (p < 0.001), 22.1% (p < 0.001) and 25.5% (p < 0.001) in recurrent breast cancers, locally advanced cervix cancer (LACC) and locally advanced head and neck cancers, respectively by adding hyperthermia to radiotherapy over radiotherapy alone.
- low-middle-income group countries
- cancer
- hyperthermia
1. Cancer Status in Low-Middle-Income Group Countries
According to the Global Cancer Observatory of the World Health Organization (WHO), the total cancer incidence estimated in 2020 was 19.3 M and is expected to rise to 24.1 M in 2030 [1]. In 2020, 11.4 M (59%) of these cases were reported in the low-middle-income countries (LMICs) where the cancer burden is projected to escalate to 14.6 M (+28.2%) and 17.9 M (+56.8%) by 2030 and 2040, respectively. Of the 11.4 M cancer cases in LMICs, presently, cancers of breast, cervix and head and neck regions combined constitutes around 3 M (26.2%) of cases. Furthermore, the cancers in these sites in LMICs constitute 61.1%, 88.1% and 71.8% of the global cancers, respectively (Table 1). In view of the advanced stages of their presentation, most of these cases are inoperable. Thus, radiotherapy (RT) and/or chemotherapy (CT) forms the mainstay of their treatment, resulting in a %mortality/incidence at 36%, 58.7% and 38.2% in cancers of the breast, cervix and head and neck, respectively in LMICs (Figure 1, Table 1). Certainly, there is a need to explore other cost-effective options to improve these treatment outcomes in LMICs [2].
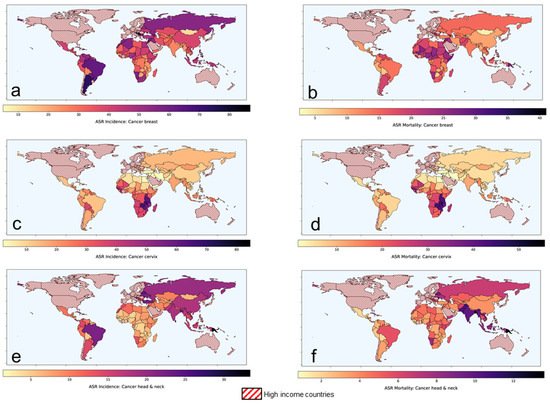
Figure 1. Age-standardized rates of (ASR) for incidence and mortality for (a,b) breast cancer (c,d) cervical cancer and (e,f) head and neck cancers, respectively in low-middle-income group countries. Based on data from Global Cancer Observatory [1].
Table 1. Estimated number of cancer cases and deaths as per the Global Cancer Observatory in ages (0–85+ years) pertaining to breast, cervix and head and neck region globally and in low-middle-income countries (LMICs) in 2020 [1]. Countries classified in various income groups based on the World Bank classification.
| Cancer Sites | Cancer Incidence | Cancer Mortality | % Mortality/Incidence in LMICs | ||||
|---|---|---|---|---|---|---|---|
| All Countries | LMICs Only | Proportion in LMICs (%) | All Countries | LMICs Only | Proportion in LMICs (%) | ||
| All sites | 19,292,789 | 11,441,886 | 59.3 | 9,958,133 | 7,063,070 | 70.9 | 61.7 |
| Breast | 2,261,419 | 1,381,539 | 61.1 | 684,996 | 497,496 | 72.6 | 36.0 |
| Cervix | 604,127 | 532,239 | 88.1 | 341,831 | 312,373 | 91.4 | 58.7 |
| Head and neck # | 1,518,133 | 1,090,262 | 71.8 | 510,771 | 416,206 | 81.5 | 38.2 |
# includes cancers of lip, oral cavity, nasopharynx, oropharynx, hypopharynx, larynx, salivary glands and thyroid; Data as on 12 September 2021 [1].
2. Hyperthermia as a “Potential Game-Changer”
Loco-regional hyperthermia (HT) or thermotherapy, at 40–44 °C, has been shown to be a potent radiosensitizer, a chemosensitizer and an immunomodulator with no significantly added side effects [3,4,5]. HT sensitizes the hypoxic tumour cells and inhibits the repair of RT- and/or CT-induced DNA damage. In addition, cells in radioresistant “S” phase are heat sensitive [3]. Furthermore, thermoradiobiologically, HT has been shown to impart high LET properties to low LET proton or photon beams [6]. The addition of HT to photons creates a radiobiological advantage in tumours akin to fast beam neutrons. The physiological vasodilation at temperatures of 39–45 °C allows rapid heat dissipation from normal tissues, thereby sparing the normal tissues from HT-induced morbidity. On the contrary, the chaotic and relatively rigid tumour vasculature results in heat retention leading to higher intratumoural temperatures. Consequently, the high LET attributes of HT with photon radiations are mostly limited to the confines of the heated tumour, while the normothermic normal tissues get irradiated with low LET photons. HT thereby augments photon therapy by conferring therapeutic advantages of high LET radiations to the tumours akin to neutrons, while the ‘heat-sink’ effect spares the normal tissues from thermal radiosensitization. Thus, photon thermoradiotherapy imparts radiobiological advantages selectively to tumours analogous to neutrons without exaggerating normal tissue morbidities.
Accordingly, HT could be an effective therapeutic modality in conjunction with RT and/or CT. Moderate HT, as defined by the Kadota Forum in 2008, is elevation of the tumour temperature between 39 °C and 45 °C [7]. The biological and physiological mechanisms involved in HT at 38–45 °C has been very aptly summarized by van Rhoon [8]. The thermodynamic changes are initiated at around 38 °C and results in a gradual increase in tumour blood flow and subsequent oxygenation while the thermoradiobiological mechanisms lead to direct cell kill, thermal sensitization and inhibition of DNA repair between 39 °C and 45 °C. Thus, at the usual clinically achievable temperature of 40–42 °C, HT could lead to appreciable radiosensitization, chemosensitization and immunomodulation along with RT ± CT.
Incorporating HT along with the standard therapeutic modalities, namely RT and/or CT, could thus be expected to augment therapeutic outcomes through the multifaceted actions of HT [3,4]. In LMICs, most patients present in relatively locally advanced stages, thereby limiting the role of primary surgical option. Thus, RT and/or CT forms the mainstay of management of these locally advanced tumours—namely of head and neck, cervix and breast. The treatment needs to be tolerable as patients usually have compromised nutritional status, especially in LMICs. In addition, due to limited health insurance coverage, most patients may have to bear the cost of their treatment through out-of-pocket resources. All these factors, enforces one to consider cost-effective strategies that are also tolerable with low acute and late morbidities. HT, a safe modality, with limited toxicities, and a known potentiator of RT and CT could thus be a possible therapeutic addendum in clinical settings in LMICs. The present report summarizes the available clinical evidence to justify the inclusion of HT in the management of these common cancers in LMICs along with RT ± CT. As evident, HT could indeed emerge as a potential game-changer by improving the therapeutic outcome in the common cancers prevalent in LMICs.
3. Locally Advanced Breast Cancers: Scope for Improvement with Hyperthermia
Locally advanced breast cancers (LABC) are a fairly common problem in LMICs. Most patients present in an advanced stage where primary surgical intervention is usually not feasible. Thus, patients are usually subjected to neoadjuvant chemotherapy (NACT) to enable tumour downstaging followed by mastectomy. Most CT drugs exhibit thermal synergism by (a) increasing the cellular uptake of drugs, (b) increased oxygen radical production, (c) increasing DNA damage, and (d) inhibiting chemotherapeutic-induced DNA damage [9,10,11]. HT inflicts oxidative damage and/or strand cross links, as well as single or double strand DNA breaks, along with CT agents, namely adriamycin, cyclophosphamide, 5-flurouracil and taxanes commonly used as NACT agents for LABC. Further, HT also interferes with the various DNA repair process involving excision repair, non-homologous end joining and/or homologous recombination [9,11].
Clinical Outcomes with Hyperthermia in Locally Advanced Breast Cancers
In a recently reported randomized clinical trial in stages IIB-IIIA breast cancers, patients treated with NACT (adriamycin, cyclophosphamide, 5-flurouracil) with loco-regional HT using 27.1 MHz, experienced a significant reduction in both primary tumour (+15.9%, p = 0.034) and axillary lymph nodes (+14.1%, p = 0.011) compared to those treated with NACT alone [12]. Further, a higher proportion of patients underwent breast conservative surgery (+13.6%) with NACT + HT following appreciable tumour regression. A significantly improved overall survival at 10-year was also evident in patients treated with NACT + HT (p = 0.009).
In a phase I/II study, Vujaskovic et al. [13], evaluated the safety and efficacy of a NACT with paclitaxel, liposomal doxorubicin and HT in LABC. A combined response rate of 72% was reported at the end of NACT with four of the 43 patients achieving a complete response (CR). A 4-year disease-free and overall survival rate of 63% and 75% were attained, respectively.
HT has been reported to increase the systolic blood flow in breast tumours by about 3.5 times compared to pre-HT blood flow [12].
Thus, NACT + HT could be a viable option for LABC and the consequence of its effects on the key outcomes need to be examined systematically in future studies. These should also incorporate a detailed histopathological evaluation to explore HT-induced immunomodulation.
4. Locally Advanced Head and Neck Cancers: Scope of Improvement with Hyperthermia
In 2020, 71.8% and 81.5% of all global incidence and mortalities in head and neck cancers were reported in the LMICs [1]. The %mortality/incidence in LMICs for these cancers are estimated at 38.2% (Figure 1, Table 1). As in the cervix, most patients present as locally advanced head and neck cancers (LAHNC), CTRT has been the mainstay of their treatment. CTRT has been shown to improve outcomes in successive reports of the Meta-analysis of Chemotherapy in Head and Neck (MACH-NC) collaborative group. In their latest update of 107 randomized trials with 19,085 patients published in 2021, a 6.5% absolute benefit at 5 years was demonstrated (hazard ratio: 0.83; 95% CI: 0.79–0.86) [25]. However, this benefit reduced with increasing patient age and poor performance status.
Clinical Outcomes with Hyperthermia in Locally Advanced Head and Neck Cancer
In LMICs, patients with LAHNC are often in poor performance status due to inadequate nutritional intake. This could have a bearing on the outcomes with CTRT. HT has been used with RT and outcomes compared with RT alone. In a meta-analysis of six clinical trials comprising 451 cases of LAHNC, HTRT improved the overall CR by 25.5% over RT alone (p < 0.0001) [19] (Figure 2). Acute and late morbidities appear similar.
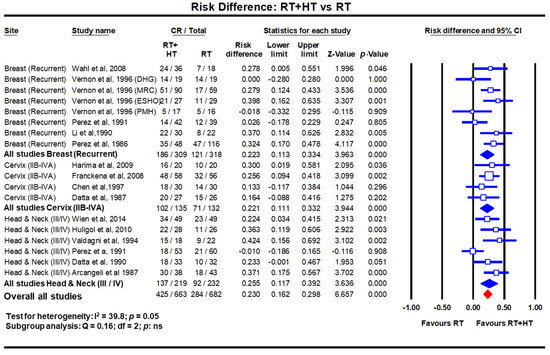
Figure 2. Forest plots depicting the risk difference for complete response with radiotherapy (RT) with hyperthermia (HT) versus RT alone in recurrent breast cancers, locally advanced cervical cancer (stages IIB-IVA) and locally advanced head and neck cancers (stages III/IV). Data extracted from Datta et al. [14,18,19] and replotted. Addition of hyperthermia to radiotherapy favours the outcome compared to radiotherapy alone in all sites with a risk difference of 23% (p < 0.001). (Q test: test for heterogeneity; df: degree of freedom and ns: not significant). For citations of the studies listed, please refer to [14,18,19].
The positive outcomes of HTCTRT in LACC, which also share a similar histology with LAHNC, should encourage patients to be recruited for phase III randomized trial with HTCTRT vs. CTRT alone. However, one of limitations could be lack of a proper HT unit for head and neck region that would allow adequate heating and monitoring of HT during individual treatment session. A dedicated HT delivery system working at 433 MHz–the HYPERCollar (Sensius, Rotterdam, The Netherlands) fills in the long-standing gap for a site-specific HT for LAHNC [26,27,28,29,30,31,32]. The system initially had 12 antennas, which was later upgraded to 20 antennas. Presently, an MR-compatible version of this applicator is being used within a 1.5 T MR system. This would allow online monitoring of the temperature using non-invasive thermometry with the proton resonance frequency shift method [33,34]. In addition, model-based and other new MR-thermometry temperature reconstruction methods are emerging which are quite promising [35,36,37]. The unit is currently being validated in clinics for HT delivery in head and neck regions [38].
Thus, LAHNC provides yet another common site in which HT, along with RT or CTRT, could be expected to improve therapeutic outcomes without any significant added toxicities. It is therefore highly desirable that HT should be evaluated systematically in LAHNC. As LMICs harbour more than two-thirds of global head and neck cancers, these patients need to be included in single/multicentric clinical trials for evaluating HTCTRT vs. CTRT alone.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14020315
This entry is offline, you can click here to edit this entry!
