Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Paratuberculosis (Johne’s disease) is caused by Mycobacterium avium subspecies paratuberculosis (MAP), a highly resistant, extremely fastidious, Gram-positive acid-fast bacilli. The disease is characterized by reduced productivity, loss in body weight, and can be experienced with (in cattle and buffaloes) or without (in goats and sheep) diarrhea. Animals acquire infection in the early stages (prenatal) through semen or in utero, with colostrum and milk, or from the environment after birth via the fecal–oral route.
- bioactive compounds
- Mycobacterium avium subspecies paratuberculosis (MAP)
1. Pathogenesis
Johne’s disease (JD) is characterized by persistent diarrhea and a mal absorption condition, which results in malnutrition and muscle atrophy (Figure 1). The fecal–oral pathway is the most common way for neonates and young animals to become infected. Milk feeding from an infected dam is another source of infection to neonates [1]. Calves up to the age of six months have a greater incidence of infection, but afterwards, the risk reduces [2]. According to animal research, M-cells and enterocytes both promote MAP adjunct to and transit through the gut mucosa upon consumption [3]. Tissue culture observations demonstrate that MAP influences the establishment of tight junctions in the intestinal mucosa, offering a mechanism for enhanced permeability [4]. Antigens 85 [5], 35 kDa [6], MAP oxidoreductase [7], MAP fibronectin-binding protein [8][9], and histone HupB [10] are all crucial in MAP epithelial cell adhesion and/or penetration, and host–pathogen interaction occurs consistently.
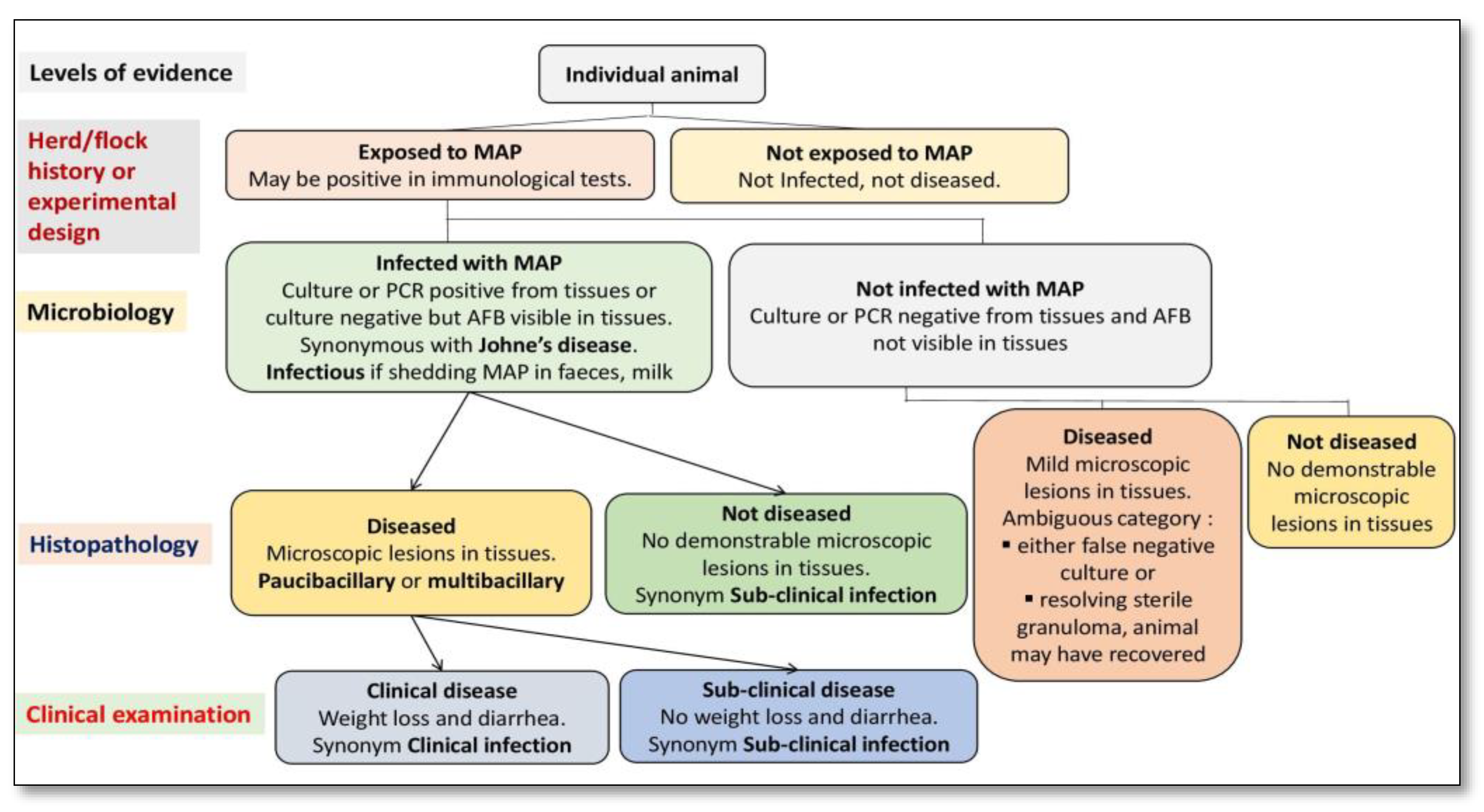
Figure 1. Primary classification of animals exposed to Mycobacterium avium subspecies paratuberculosis (MAP) using a systematic diagnostic approach.
The prior literature has shown that phagosome acidification stimulates interleukin (IL)-1 production, macrophage recruitment, and trans-epithelial migration in MAP-infected epithelial cells, utilizing the cow mammary epithelial cell line MAC-T [11] and bovine blood-monocyte-derived macrophages (BMDM) [12]. Bacilli (genus Bacillus) are subsequently phagocytosed in the sub- and intraepithelial spaces by these macrophages [13][14][15]. For pathogenesis, MAP’s capacity to persist and proliferate once inside phagocytic cells is fundamental [16][17]. Furthermore, researchers observed that the lipid content of MAP changes in macrophages that acquire a pro-inflammatory phenotype utilizing a culture passage model [18] (Figure 2).
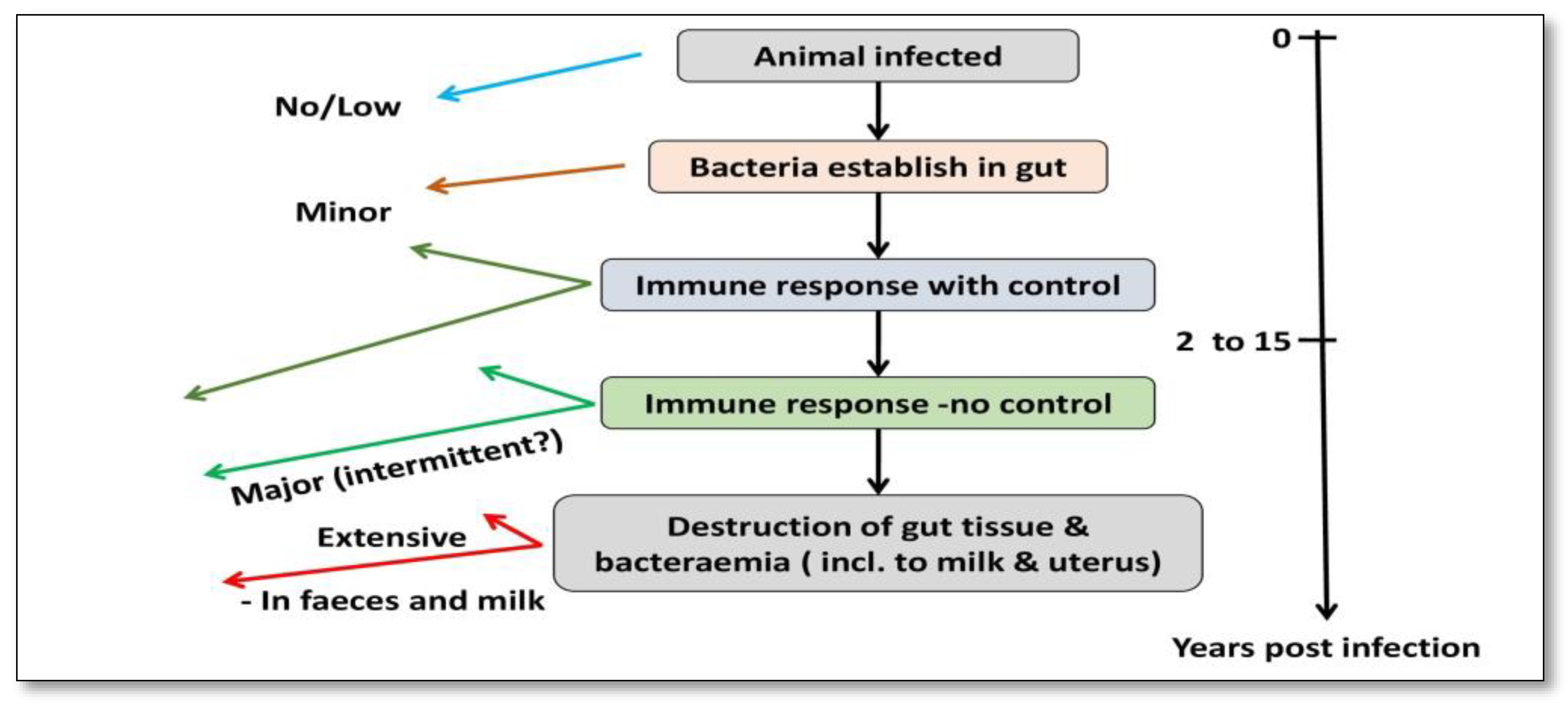
Figure 2. Pathogenesis of MAP infection in cattle.
The pathognomonic granulomatous enteritis of Johne’s illness [15], which is characterized by a wide and ridged intestinal wall as well as inflammatory lymph nodes, is the result of the ensuing host cellular immunological response. Toll-like receptors help tissue macrophages and dendritic cells recognize molecular patterns linked with pathogens in the innate phase, as well as the abstraction of cytokine-mediated cellular connections and antigen processing [19][20]. In the acquired immunity phase, Th1 T-helper cell responses and the concurrent stimulation of macrophages by interferon-gamma (INF) produced by Th1 T cells are used to reduce MAP infections. [21][22]. The inferential function of nitric oxide synthase has already been shown in cattle and is implicated in the killing process of these activated phagocytic cells [23].
In this condition, BMDM recovered from sub-clinically contaminated animals exhibits exceptionally high levels of nitric oxide generation [24] (Figure 3). MAP, on the other hand, affects the activity of bovine macrophages, as demonstrated by distinct profiles of mRNA expression [25], apoptosis suppression and antigen distribution [26], and diagnostic cytokine expression patterns [27]. In infected bovine T helper cells, MAP mostly generates a Th2 response, with an increase in production of IL-4, IL-5, IL-10, and tissues remodeling inhibitors [28][29]. This humoral response was confirmed in a newborn calf model [30]. In addition, in both ruminants and animals, regulatory T and Th17 cells have been involved in the immune pathogenesis of JD [26][31].
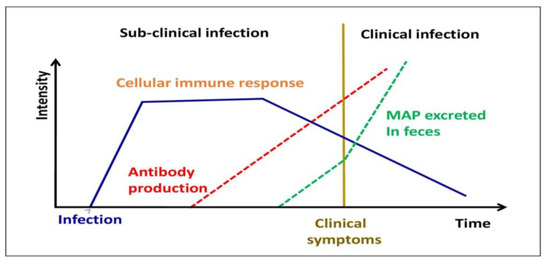
Figure 3. Different stages of MAP infection in domestic livestock.
MAP pathogenesis has been studied using a variety of models. MAP, on the other hand, produces immunological responses in ruminant hosts, which are not found in traditional in vitro models. MAP bacilli grow during 4–8 days in infected BMDM [21][32], although bacterial burdens are reduced over time after infection of the murine J774 macrophage cell line [21][32][33][34]. When researching, the interactions between MAP and phagocytic cells, it is preferable to use primary phagocytic cells. To follow the progression of MAP infection from the initial to final stages, Ileal loops have been employed to establish a prospective systems biology approach [35]. The host transcriptome profile following infection with M. avium subsp. avium and MAP were recently compared using this paradigm. Intestinal mucosal weakening, activation of a Th2 reaction, and phagocytosis suppression were all related to MAP transmission, which was not found with M. avium subsp. avium infection [36] (Figure 4).
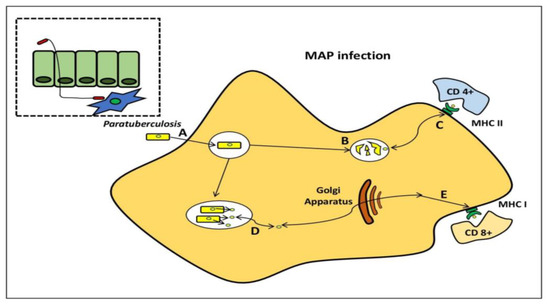
Figure 4. Mycobacterium avium subspecies paratuberculosis within the macrophage. MAP bacilli pass by the mucosal barrier, preferentially via M cells, and after which it is engulfed by sub-epithelial macrophage (A). MAP bacilli degrade within the phagosome (B) and also stimulateCD4+ immune responses via antigen presentation through the MHC class II pathway. (C) MAP evades destruction within phagosome through the inhibition of general killing mechanisms and proliferates. (D) MAP proteins are secreted from the phagosome to the cytosol and are subsequently available for presentation to (E) CD8+ immune responses via the MHC class I pathway.
2. Diagnosis and Control
Before any clinical indications, infected animals shed MAP in their feces, making them a prominent cause of infection for the herd’s other animals. To avoid the spread of JD, it is critical to diagnose the infection as soon as possible. Based on the detection of MAP both directly and indirectly, many diagnostic tests have been created [37]. The direct identification of MAP in clinical specimens can be achieved using (i) microscopy, (ii) culture-based MAP isolation, and (iii) PCR-based MAP DNA identification. Clinical samples have been analyzed using acid-fast or Ziehl–Neelsen staining. Acid-fast staining is the easiest, quickest, and most economical mode of diagnosis, but its accuracy and precision are inadequate since it is challenging to discern between MAP and some other acid-fast bacilli [38].
Although Ziehl–Neelsen staining can also be used to screen for MAP, it must be verified by additional procedures such as PCR and/or immunoassays. The “gold standard” for JD diagnosis is MAP isolation through culture. The fact that MAP requires mycobactin J to grow in a specific laboratory medium can be utilized to distinguish it from many other acid-fast bacteria. A novel growth media that increases MAP restoration and sensitivity by 1000-fold was recently divulged [39]. Due to the fact that MAP develops slowly (On solid medium, colony development takes 6–8 weeks.), culture-based diagnosis takes a long period. Consequently, a highly fast and precise PCR-based test was employed for MAP identification in environmental and clinical specimens [40][41][42]. IS900 is a 1.4 kb multi-copy insertion element that is sequence-specific to MAP. The primers used in this PCR are for IS900 [37][43]. Other mycobacteria with IS900-like insertion sequences, on the other hand, have been demonstrated to influence the specificity of this test, resulting in false-positive findings [41][44].
To prevent false-positive results, a multiplex PCR centered on the IS900, IS901, IS1245, and dnaJ genes was constructed, although the precision of this assay is restricted due to the reagent interference and primer-dimer generation [37][45]. Furthermore, PCR tests based on stool specimens hold only 70% sensitivity and 85% specificity [46]. There has been some advancement in identifying and utilizing more precise targets for PCR testing [47][48][49], and this comparative genomic technique has addressed an apprehension gap in MAP identification. Several of these objectives have made their way into commercial diagnostic tools. The immunological response of the host to infection is the basis for diagnostic MAP tests based on indirect detection. A Johnin pure protein derivative was used to produce the delayed-type hypersensitivity skin test [50].
However, because various environmental mycobacteria might sensitize the animal and provide false-positive findings, this test is not specific. As a result, delayed-type hypersensitivity skin tests cannot tell the difference between vaccinated animals and those that have been naturally affected. As previously established, MAP invasion triggers T helper cells, which secrete IFN-γ.The utilization of cultures with supernatants from day-old blood specimens which are treated with Johnin and co-stimulated with human IL-2and/or bovine IL-12 can also be used to diagnose JD using an enzyme-linked immune sorbent test (ELISA) [51]. Unfortunately, cross-reactivity issues arise because, in the INF-test, MAP pure proteins analogs are often used as antigens. A potential alternative MAP antigen for the research was L5P, a cell wall lipopeptide; however, the IFN-γ expression was reported to be weaker than that of Johnin [52].
Antibodies in milk and serum from diseased animals are detected using commercial ELISA kits such as (I)) ParaCheck (CSL/Biocor), (ii) HerdCheck M. paratuberculosis ELISA (IDEXX Laboratories, Inc., Westbrook, ME, USA), (iii)ID Screen®Paratuberculosis Indirect (ID Screen®Paratuberculosis Indirect (ID Screen (IDvet Genetics)and (iv) SERELISA ParaTB (Synbiotic Corp., Kansas City, MO, USA).In comparison to PCR testing, an ELISA seems to have a lower sensitivity of 50% but a far higher specificity of 99.8% [53][54]. To establish better sensitive immune-based tests for JD diagnosis, additional research is needed to uncover specialized MAP antigens. Vaccination (the most economical), screening, and improved herd control are all alternatives for avoiding JD, depending on a producer’s finances, infrastructure, and operations [55]. However, while JD vaccines can diminish systemic disease and discharge, their effectiveness is minimal, and none of them provide fairly long immunity.
In the United States, for instance, Mycopar® (BoehringerIngelheimVetmedica, Inc., St Joseph, MO, USA) has been the exclusively licensed vaccination for JD in cattle. Unfortunately, since strain 18 of M. avium subsp. avium was used to make the vaccine [56], it lacks an ideal antigenic repertoire. In Australia, Silirum® (Zoetis Animal Health, Parsippany-Troy Hills, NJ, USA), a different bacterin, is being investigated, and it has been licensed for restricted usage in cattle. The MAP 316F strain has been heat-killed in this vaccination. This formulation may contain a broader spectrum of antigenic; however, utilizing bacteria that have been destroyed by heat may lower efficacy while improving safety. Both Neoparasec® (Rhone-Merieux, Athens, GA, USA) and Gudair® (Zoetis Animal Health) contain the live-attenuated MAP strain 316F and are authorized for usage in goats and sheep. Vaccines that are currently available, on the other hand, are unable to discriminate between vaccinated and infected animals, impairing JD diagnostic testing [57], and strain 316F was created in the 1920s using random depreciation processes (e.g., passages in ox bile) that are currently being examined [58]. Eventually, to successfully manage JD, an elevated vaccination is necessary [59].
The latest batch of human anti-tuberculosis vaccines appear to provide better protection than subunit vaccines, according to testing results [60]. Because JD is induced by a bacteria called Mycobacterium, potential subunit or bacterin-based vaccines are likely to face a similar situation. The JD Integrative Protocol-Animal and Plant Health Inspection Service’s endeavors to establish a consistent vaccination testing program were spurred by this. In a three-phase investigation, investigators from New Zealand and the U.S provided 22 masked live-attenuated immunization candidates to be evaluated in mouse, BMDM, and goat models. Despite the substantial development of animal screening procedures [61], the bulk of the suppressed transposon variants investigated were from the first generation and had the Tn5367 transposase, causing destabilization. Furthermore, unknowns, including the ideal immunization path and dose plan, could not be determined before the commencement of the experiment. Despite this, crucial information and chemicals were created [57]. The design of a subunit vaccine that can manage infections by inducing the appropriate humoral immunity [62], specifically against antigens produced by the pro-inflammatory phenotype is not yet conceivable [63].
However, in the absence of a vaccine, the control of MAP infection in the human population can be accomplished either via the surgical removal of infected intestines or by medicines [64] using anti-tuberculosis drugs, which have had limited success [65][66]. The prolonged use of anti-tuberculosis drugs has resulted in drug resistance to all of the existing anti-mycobacterial molecules. Because of the increase in cases of animal and human infections, the demand for natural products as an alternative therapy for this chronic incurable disease has increased. This has encouraged researchers to find out bio-active (marker) compounds from plants with pharmacological properties against symptoms exhibited by MAP-infected domestic livestock populations, e.g., chronic progressive inflammation, etc. Prior studies have suggested that plant extracts can feasibly decrease the induction of TNF-α that modulates TNF-α mediated inflammatory pathways, and this may have potential against diseases arising due to chronic inflammation caused by MAP infection (paratuberculosis or Johne’s disease in animals and Crohn’s disease in humans). Plant extracts play major roles as immuno-modulators and immuno-stimulators and can increase or decrease the level of various pro-inflammatory and inflammatory cytokines during chronic inflammation.
The pre-2nd century ‘Charaka Samhita’ book reported that Ayurveda (Indian traditional medicine) herbal medicinal plants have been used to cure tuberculosis and other various ailments. Decoctions, Infusions, Tinctures, and macerations of herbal medicinal plant parts such as fruits and flowers, stem bark, roots, stems, and leaves have been used as part of traditional treatments for many centuries by native people worldwide. Even though ethnopharmacological and ethnobotanical studies have considered the wide use of herbal medicinal plants in the treatment of TB, most of them were established still to be therapeutic and safe doses. Most research studies have failed to give scientific proof to therapeutic practices and traditional beliefs. Consequently, this work aims to archive the traditional medicinal plants used to control TB and contrast the traditional therapeutic systems that have been used to cure TB, from the poorly documented oral Indian medicines to the well-documented Indian Ayurveda and so on.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28083490
References
- Streeter, R.N.; Hoffsis, G.F.; Bech-Nielsen, S.; Shulaw, W.P.; Rings, D.M. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am. J. Vet. Res. 1995, 56, 1322–1324.
- Windsor, P.A.; Whittington, R.J. Evidence for age susceptibility of cattle to Johne’s disease. Vet. J. 2010, 184, 37–44.
- Bermudez, L.E.; Petrofsky, M.; Sommer, S.; Barletta, R.G. Peyer‘s patch-deficient mice demonstrate that Mycobacterium avium subsp. paratuberculosis translocates across the mucosal barrier via both M cells and enterocytes but has inefficient dissemination. Infect. Immun. 2010, 78, 3570–3577.
- Bannantine, J.P.; Bermudez, L.E. No holes barred: Invasion of the intestinal mucosa by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 2013, 81, 3960–3965.
- Kuo, C.J.; Bell, H.; Hsieh, C.L.; Ptak, C.P.; Chang, Y.F. Novel mycobacteria antigen 85 complex binding motif on fibronectin. J. Biol. Chem. 2012, 287, 1892–1902.
- Bannantine, J.P.; Huntley, J.F.; Miltner, E.; Stabel, J.R.; Bermudez, L.E. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 2003, 149, 2061–2069.
- Alonso-Hearn, M.; Patel, D.; Danelishvili, L.; Meunier-Goddik, L.; Bermudez, L.E. The Mycobacterium avium subsp. paratuberculosis MAP3464 gene encodes an oxidoreductase involved in invasion of bovine epithelial cells through the activation of host cell Cdc42. Infect. Immun. 2008, 76, 170–178.
- Secott, T.E.; Lin, T.L.; Wu, C.C. Fibronectin attachment protein homologue mediates fibronectin binding by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 2001, 69, 2075–2082.
- Secott, T.E.; Lin, T.L.; Wu, C.C. Fibronectin attachment protein is necessary for efficient attachment and invasion of epithelial cells by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 2002, 70, 2670–2675.
- Katsube, T.; Matsumoto, S.; Takatsuka, M.; Okuyama, M.; Ozeki, Y.; Naito, M.; Nishiuchi, Y.; Fujiwara, N.; Yoshimura, M.; Tsuboi, T.; et al. Control of cell wall assembly by a histone-like protein in mycobacteria. J. Bacteriology 2007, 189, 8241–8249.
- Patel, D.; Danelishvili, L.; Yamazaki, Y.; Alonso, M.; Paustian, M.L.; Bannantine, J.P.; Meunier-Goddik, L.; Bermudez, L.E. The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect. Immun. 2006, 74, 2849–2855.
- Koets, A.P.; Eda, S.; Sreevatsan, S. The within host dynamics of Mycobacterium avium ssp. paratuberculosis infection in cattle: Where time and place matter. Vet. Res. 2015, 46, 1–7.
- Momotani, E.; Whipple, D.L.; Thiermann, A.B.; Cheville, N.F. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer‘s patches in calves. Vet. Pathol. 1988, 25, 131–137.
- Fujimura, Y.; Owen, R.L. M cells as portals of infection: Clinical and pathophysiological aspects. Infect. Agents Dis. 1996, 5, 144–156.
- Lugton, I.W. Mucosa-associated lymphoid tissues as sites for uptake, carriage and excretion of tubercle bacilli and other pathogenic mycobacteria. Immunol. Cell Biol. 1999, 77, 364–372.
- Kaufmann, S.H. Immunity to intracellular bacteria. Annu. Rev. Immunol. 1993, 11, 129–163.
- Zhao, B.E.; Collins, M.T.; Czuprynski, C.J. Effects of gamma interferon and nitric oxide on the interaction of Mycobacterium avium subsp. paratuberculosis with bovine monocytes. Infect. Immun. 1997, 65, 1761–1766.
- Everman, J.L.; Eckstein, T.M.; Roussey, J.; Coussens, P.; Bannantine, J.P.; Bermudez, L.E. Characterization of the inflammatory phenotype of Mycobacterium avium subspecies paratuberculosis using a novel cell culture passage model. Microbiology 2015, 161, 1420–1434.
- Chamy, E.L.L.; Leclerc, V.; Caldelari, I.; Reichhart, J.M. Sensing of “Danger Signals” and Pathogen Associated Molecular Patterns Defines Binary Signaling Pathways “Upstream” of Toll. Nat. Immunol. 2008, 9, 1165–1170.
- Coussens, P.M.; Verman, N.; Coussens, M.A.; Elftman, M.D.; McNulty, A.M. Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis: Evidence for an inherent proinflammatory gene expression pattern. Infect. Immun. 2004, 72, 1409–1422.
- Zhao, B.; Czuprynski, C.J.; Collins, M.T. Intracellular fate of Mycobacterium avium subspecies paratuberculosis in monocytes from normal and infected, interferon-responsive cows as determined by a radiometric method. Can. J. Vet. Res. 1999, 63, 56.
- Stabel, J.R. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 2000, 77, 465–473.
- Li, R.W.; Li, C.; Gasbarre, L.C. The vitamin D receptor and inducible nitric oxide synthase associated pathways in acquired resistance to Cooperia oncophora infection in cattle. Vet. Res. 2011, 42, 48.
- Khalifeh, M.S.; Al-Majali, A.M.; Stabel, J.R. Role of nitric oxide production in dairy cows naturally infected with Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunopathol. 2009, 131, 97–104.
- Tooker, B.C.; Burton, J.L.; Coussens, P.M. Survival tactics of M. paratuberculosis in bovine macrophage cells. Vet. Immunol. Immunopathol. 2002, 87, 429–437.
- Coussens, P.M.; Sipkovsky, S.; Murphy, B.; Roussey, J.; Colvin, C.J. Regulatory T cells in cattle and their potential role in bovine paratuberculosis. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 233–239.
- Weiss, D.J.; Evanson, O.A.; McClenahan, D.J.; Abrahamsen, M.S.; Walcheck, B.K. Regulation of expression of major histocompatibility antigens by bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium avium subsp. avium. Infect. Immun. 2001, 69, 1002–1008.
- Coussens, P.M.; Pudrith, C.B.; Skovgaard, K.; Ren, X.; Suchyta, S.P.; Stabel, J.R.; Heegaard, P.M. Johne‘s disease in cattle is associated with enhanced expression of genes encoding IL-5, GATA-3, tissue inhibitors of matrix metalloproteinases 1 and 2, and factors promoting apoptosis in peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 2005, 105, 221–234.
- Weiss, D.J.; Evanson, O.A.; Souza, C.D. Expression of interleukin-10 and suppressor of cytokine signaling-3 associated with susceptibility of cattle to infection with Mycobacterium avium subsp paratuberculosis. Am. J. Vet. Res. 2005, 66, 1114–1120.
- Diaz, F.E.; Guerra-Maupome, M.; McDonald, P.O.; Rivera-Pérez, D.; Kalergis, A.M.; McGill, J.L. A recombinant BCG vaccine is safe and immunogenic in neonatal calves and reduces the clinical disease caused by the respiratory syncytial virus. Front. Immunol. 2021, 12, 664212.
- Robinson, M.W.; O‘brien, R.; Mackintosh, C.G.; Clark, R.G.; Griffin, J.F. Immunoregulatory cytokines are associated with protection from immunopathology following Mycobacterium avium subspecies paratuberculosis infection in red deer. Infect. Immun. 2011, 79, 2089–2097.
- Woo, S.R.; Heintz, J.A.; Albrecht, R.; Barletta, R.G.; Czuprynski, C.J. Life and death in bovine monocytes: The fate of Mycobacterium avium subsp. paratuberculosis. Microb. Pathog. 2007, 43, 106–113.
- Kuehnel, M.P.; Goethe, R.; Habermann, A.; Mueller, E.; Rohde, M.; Griffiths, G.; Valentin-Weigand, P. Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: Phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cell. Microbiol. 2001, 3, 551–566.
- Bannantine, J.P.; Stabel, J.R. Killing of Mycobacterium avium subspecies paratuberculosis within macrophages. BMC Microbiol. 2002, 2, 1–7.
- Khare, S.; Lawhon, S.D.; Drake, K.L.; Nunes, J.E.; Figueiredo, J.F.; Rossetti, C.A.; Gull, T.; Everts, R.E.; Lewin, H.A.; Galindo, C.L.; et al. Systems biology analysis of gene expression during in vivo Mycobacterium avium paratuberculosis enteric colonization reveals role for immune tolerance. PLoS ONE 2012, 7, e42127.
- Khare, S.; Drake, K.L.; Lawhon, S.D.; Nunes, J.E.; Figueiredo, J.F.; Rossetti, C.A.; Gull, T.; Everts, R.E.; Lewin, H.A.; Adams, L.G. Systems analysis of early host gene expression provides clues for transient Mycobacterium avium ssp avium vs. persistent Mycobacterium avium ssp paratuberculosis intestinal infections. PLoS ONE 2016, 11, e0161946.
- Chaubey, K.K.; Gupta, R.D.; Gupta, S.; Singh, S.V.; Bhatia, A.K.; Jayaraman, S.; Kumar, N.; Goel, A.; Rathore, A.S.; Sahzad Sohal, J.S. Trends and advances in the diagnosis and control of paratuberculosis in domestic livestock. Vet. Q. 2016, 36, 203–227.
- Manning, E.J.; Collins, M.T. Mycobacterium avium subsp. paratuberculosis: Pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. 2001, 20, 133–150.
- Bull, T.J.; Munshi, T.; Mikkelsen, H.; Hartmann, S.B.; Sørensen, M.R.; Garcia, J.S.; Lopez-Perez, P.M.; Hofmann, S.; Hilpert, K.; Jungersen, G. Improved culture medium (TiKa) for Mycobacterium avium subspecies paratuberculosis (MAP) matches qPCR sensitivity and reveals significant proportions of non-viable MAP in lymphoid tissue of vaccinated MAP challenged animals. Front. Microbiol. 2017, 7, 2112.
- Vary, P.H.; Andersen, P.R.; Green, E.; Hermon-Taylor, J.; McFadden, J.J. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne‘s disease. J. Clin. Microbiol. 1990, 28, 933–937.
- Cousins, D.V.; Whittington, R.; Marsh, I.; Masters, A.; Evans, R.J.; Kluver, P. Mycobacteria distinct from Mycobacterium avium subsp. paratuberculosis isolated from the faeces of ruminants possess IS 900-like sequences detectable by IS 900 polymerase chain reaction: Implications for diagnosis. Mol. Cell. Probes 1999, 13, 431–442.
- Ellingson, J.L.; Stabel, J.R.; Bishai, W.R.; Frothingham, R.; Miller, J.M. Evaluation of the accuracy and reproducibility of a practical PCR panel assay for rapid detection and differentiation ofMycobacterium avium subspecies. Mol. Cell. Probes 2000, 14, 153–161.
- Harris, N.B.; Barletta, R.G. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 2001, 14, 489–512.
- Englund, S.; Bölske, G.; Johansson, K.E. An IS 900-like sequence found in a Mycobacterium sp. other than Mycobacterium avium subsp. paratuberculosis. FEMS Microbiol. Lett. 2002, 209, 267–271.
- Rachlin, J.; Ding, C.; Cantor, C.; Kasif, S. Computational tradeoffs in multiplex PCR assay design for SNP genotyping. BMC Genom. 2005, 6, 102.
- Clark Jr, D.L.; Koziczkowski, J.J.; Radcliff, R.P.; Carlson, R.A.; Ellingson, J.L. Detection of Mycobacterium avium subspecies paratuberculosis: Comparing fecal culture versus serum enzyme-linked immunosorbent assay and direct fecal polymerase chain reaction. J. Dairy Sci. 2008, 91, 2620–2627.
- Bannantine, J.P.; Baechler, E.; Zhang, Q.; Li, L.; Kapur, V. Genome scale comparison of Mycobacterium avium subsp. paratuberculosis with Mycobacterium avium subsp. avium reveals potential diagnostic sequences. J. Clin. Microbiol. 2002, 40, 1303–1310.
- Rajeev, S.; Zhang, Y.; Sreevatsan, S.; Motiwala, A.S.; Byrum, B. Evaluation of multiple genomic targets for identification and confirmation of Mycobacterium avium subsp. paratuberculosis isolates using real-time PCR. Vet. Microbiol. 2005, 105, 215–221.
- Stabel, J.R.; Bannantine, J.P. Development of a nested PCR method targeting a unique multicopy element, ISMap 02, for detection of Mycobacterium avium subsp. paratuberculosis in fecal samples. J. Clin. Microbiol. 2005, 43, 4744–4750.
- Maroudam, V.; Mohana Subramanian, B.; Praveen Kumar, P.; Dhinakar Raj, G. Paratuberculosis: Diagnostic methods and their constraints. J. Veterinar. Sci. Technol. 2015, 6, 1000259.
- Jungersen, G.; Mikkelsen, H.; Grell, S.N. Use of the johnin PPD interferon-gamma assay in control of bovine paratuberculosis. Vet. Immunol. Immunopathol. 2012, 48, 48–54.
- Holbert, S.; Branger, M.; Souriau, A.; Lamoureux, B.; Ganneau, C.; Richard, G.; Cochard, T.; Tholoniat, C.; Bay, S.; Winter, N.; et al. Interferon gamma response to Mycobacterium avium subsp. paratuberculosis specific lipopentapeptide antigen L5P in cattle. Res. Vet. Sci. 2015, 102, 118–121.
- Collins, M.T.; Wells, S.J.; Petrini, K.R.; Collins, J.E.; Schultz, R.D.; Whitlock, R.H. Evaluation of five antibody detection tests for diagnosis of bovine paratuberculosis. Clin. Vaccine Immunol. 2005, 12, 685–692.
- Collins, M.T. Diagnosis of paratuberculosis. Vet. Clin. North Am. Food Anim. Pract. 1996, 12, 357–371.
- Carter, M.A. Prevalence and prevention of paratuberculosis in North America. Jpn. J. Vet. Res. 2012, 60, S9–S18.
- Bastida, F.; Juste, R.A. Paratuberculosis control: A review with a focus on vaccination. J. Immune Based Ther. Vaccines 2011, 9, 8.
- Hines, M.E.; Turnquist, S.E.; Ilha, M.R.; Rajeev, S.; Jones, A.L.; Whittington, L.; Bannantine, J.P.; Barletta, R.G.; Gröhn, Y.T.; Katani, R.; et al. Evaluation of novel oral vaccine candidates and validation of a caprine model of Johne‘s disease. Front. Cell. Infect. Microbiol. 2014, 4, 26.
- Bull, T.J.; Schock, A.; Sharp, J.M.; Greene, M.; McKendrick, I.J.; Sales, J.; Linedale, R.; Stevenson, K. Genomic variations associated with attenuation in Mycobacterium avium subsp. paratuberculosisvaccine strains. BMC Microbiol. 2013, 13, 11.
- Lu, Z.; Schukken, Y.H.; Smith, R.L.; Gröhn, Y.T. Using vaccination to prevent the invasion of Mycobacterium avium subsp. paratuberculosis in dairy herds: A stochastic simulation study. Prev. Vet. Med. 2013, 110, 335–345.
- Franco, A.R.; Peri, F. Developing new anti-tuberculosis vaccines: Focus on adjuvants. Cells 2021, 10, 78.
- Hines, I.I.M.E.; Stiver, S.; Giri, D.; Whittington, L.; Watson, C.; Johnson, J.; Musgrove, J.; Pence, M.; Hurley, D.; Baldwin, C.; et al. Efficacy of spheroplastic and cell-wall competent vaccines for Mycobacterium avium subsp. paratuberculosis in experimentally-challenged baby goats. Vet. Microbiol. 2007, 120, 261–283.
- Achkar, J.M.; Casadevall, A. Antibody-mediated immunity against tuberculosis: Implications for vaccine development. Cell Host Microbe 2013, 13, 250–262.
- Everman, J.L.; Bermudez, L.E. Antibodies against invasive phenotype-specific antigens increase Mycobacterium avium subspecies paratuberculosis translocation across a polarized epithelial cell model and enhance killing by bovine macrophages. Front. Cell. Infect. Microbiol. 2015, 5, 58.
- Borody, T.J.; Leis, S.; Warren, E.F.; Surace, R. Treatment of severe Crohn‘s disease using antimycobacterial triple therapy—Approaching a cure? Dig. Liver Dis. 2002, 34, 29–38.
- Marcus, G.F.; Davis, E. Still searching for principles: A response to Goodman et al. (2015). Psychol. Sci. 2015, 26, 542–544.
- Singh, J.S.; Koushal, S.; Kumar, A.; Vimal, S.R.; Gupta, V.K. Book review: Microbial inoculants in sustainable agricultural productivity-Vol. II: Functional application. Front. Microbiol. 2016, 7, 2105.
This entry is offline, you can click here to edit this entry!
