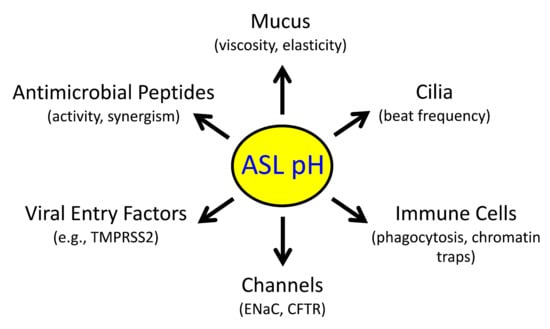
In the absence of rigorous in vivo assessments, the effects of inflammation on pH
ASL may be investigated in vitro by applying CF-relevant inflammatory stimuli to primary cultures of differentiated human airway epithelia. The cytokine interleukin-17 (IL-17) is an evolutionarily conserved molecule that drives neutrophilic airway inflammation [
80,
81]. In targeting the airway epithelium, IL-17 acts synergistically with other cytokines such as tumor necrosis factor-α (TNFα), IL-1β, etc. [
82]. These proinflammatory molecules are increased in established CF airway disease [
83,
84].
Inflammation may alter acid secretion into ASL. One study reported increased expression of ATP12A in CF airways with established disease [
88]. Exposure of airway epithelia to IL-4 or IL-13 also increased ATP12A expression, and thus H
+ secretion [
47,
86]. TNFα exposure had a similar effect, but IL-17 alone or combined IL-17/TNFα did not change H
+ secretion [
40]. Importantly, inhibiting ATP12A with apical ouabain decreases H
+ secretion, lowers ASL viscosity, and increases bacterial killing [
51,
55,
86]. Efforts to identify safer agents that reduce ATP12A activity are underway.
Loss of CFTR function also affects distal (small) airways. Small airway epithelia lack ATP12A, but instead use V-ATPase to secrete H
+. Effects of inflammation and infection on small airway H
+ secretion remain relatively unexplored.
P. aeruginosa infection may reduce V-ATPase-mediated H
+ secretion [
89,
90], or acidify ASL via apically expressed monocarboxylate transporter 2, a H
+-lactate cotransporter [
91]. Interestingly, Li et al. showed that pH
ASL regulates membrane localization of V-ATPase in porcine small airway epithelia [
41]. At neutral pH
ASL (7.4), the V-ATPase subunit ATP6V0D2 localizes in the apical membrane of small airway secretory cells.
2.5. HCO3− Secretion
2.5.1. CFTR-Mediated HCO3− Secretion
Several inflammatory cytokines have been shown to alkalinize pH
ASL. IL-17/TNFα, IL-4, IL-13, and IL-1β increase CFTR expression and activity, and increased CFTR-mediated anion secretion improves respiratory host defenses [
40,
47,
92,
93]. In CF epithelia, this component of host response is missing due to mutated, non-functional CFTR proteins. In contrast to above-mentioned cytokines, TGF-β reduces CFTR expression and transport activities in airway epithelia [
94,
95]. In vivo effects of inflammation are likely to be complex given that several cytokines elevated in CF airways target airway epithelium, alter HCO
3− transport, and thus modify pH
ASL.
2.5.2. Non-CFTR-Mediated HCO3− Secretion
An array of cytokines (IL-17/TNFα, IL-4, and IL-13) induce non-CFTR HCO
3− secretion across airway epithelia [
39,
47,
85,
92,
96]. This is achieved through pendrin, an apical Cl
−/HCO
3− exchanger, encoded by the gene
SLC26A4. Several aspects of this transport process are noteworthy. First, pendrin is minimally expressed in airway epithelia under basal conditions but is strongly upregulated by inflammatory cytokines. Second, pendrin is an electroneutral exchanger that does not mediate net anion secretion or absorption or change membrane potential. Third, in the absence of functional CFTR channels, pendrin alone can drive ASL alkalinization, though greater alkalinization is achieved with pendrin plus CFTR [
39,
40]. Fourth, some reports indicate structural or functional interactions between CFTR and pendrin, resulting in the increased activity of both [
85,
97].
2.5.3. Paracellular HCO3− Shunt
In addition to secretion by airway epithelial cells, HCO
3− can also move between the cells. Very few studies have explored the contribution of the paracellular pathway to pH
ASL, though it is often mentioned in the context of inflammation. A recent report showed that the paracellular pathway is as permeable to HCO
3− as it is to Cl
− [
98]. Under basal conditions, pH
ASL (6.6) is lower than the pH of the interstitial fluid (7.4) and the paracellular HCO
3− flux is towards the lumen; however, the paracellular HCO
3− flux decreases or even reverses as pH
ASL approaches or rises higher than the pH of the interstitial fluid. The paracellular pathway thus acts as a HCO
3− shunt that may oppose increased cellular HCO
3− secretion in inflamed airway epithelia [
99]. Whether paracellular HCO
3− permeability can be modulated to support ASL alkalinization is an interesting question and requires further investigation.
2.6. Other Regulatory Mechanisms
Inflammatory cytokines regulate diverse cellular mechanisms involved in HCO
3− secretion. In addition to changes in apical HCO
3− transporters, cytokines such as IL-17/TNFα, IL-13, or IL-4 also increase transcripts of several carbonic anhydrase and NBC isoforms [
39,
47]. Additional cytoplasmic mechanisms that regulate epithelial HCO
3− secretion also change in the presence of cytokines. The WNK (with-no-lysine [K]) kinases are master-regulators of pancreatic HCO
3− secretion [
100]. As reported recently, these kinases also control HCO
3− secretion across CF and non-CF airway epithelia [
101]. Secretory cells, key HCO
3− secreting cells in airway epithelia, express two WNK isoforms, WNK1 and WNK2. Reducing WNK kinase activity increases HCO
3− secretion, raises pH
ASL, and enhances CF epithelial host defenses. At a mechanistic level, WNK kinases regulate intracellular [Cl
−] through their control of the basolateral Na
+-K
+-2Cl
− cotransporter (NKCC1). Inhibiting WNK lowers intracellular [Cl
−], which in turn may act as a signaling ion to stimulate HCO
3− transport [
102,
103]. It is of note that combined IL-17/TNFα reduce WNK2 expression and raise pH
ASL and inhibiting residual WNK kinase activity further alkalinizes ASL.