Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Laccase belongs to the superfamily of multicopper oxidases and has been widely investigated in recent decades. Due to its mild and efficient oxidation of substrates, laccase has been successfully applied in organic catalytic synthesis, the degradation of harmful substances, and other green catalytic fields. Adding a mediator not only effectively improves the reaction efficiency of laccase but also expands the scope of the substrate.
- laccase
- laccase–mediator system
- green catalysis
1. Introduction
Laccase (EC 1.10.3.2) is a copper-containing polyphenol oxidase that belongs to the blue copper oxidase (MCO) family [1,2]. It was first discovered in the Japanese lacquer tree Rhus Vernicifera [3,4]. Subsequently, laccases were found in different plant species [5], microbes [6,7], and animals [8]. There have been more investigations on microbial laccases than on animal and plant laccases, for which there have been relatively few. Microbial laccases are divided into fungal laccases and bacterial laccases. Bacterial laccase mainly plays a role in melanin production, spore wall defense, morphological change, and copper ion detoxification [9,10]. Fungal laccase is mainly related to pigment generation, plant disease, and lignin degradation [11,12]. Plant laccase is closely related to lignin biosynthesis [13]. At the same time, the primary function of animal laccase protein is to control the ossification of the epidermis [14].
Laccase is a glycoprotein with a molecular mass ranging from 50 to 140 kDa. Their amino acid sequence can span from 220 to 800 amino acids and may contain three cupredoxin-like domains. These domains bind copper centers involved in intermolecular electron transfer reactions and constitute the catalytic core of laccases (Figure 1) [15]. The active copper center of laccase generally contains four copper ions: a type I copper ion (T1-Cu), a type II copper ion (T2-Cu), and two type III copper ions (T3-Cu). T1-Cu is a mononuclear center that can gain electrons from the substrate and then transfer them to the trinuclear cluster (TNC), and the oxidation of the substrate occurs there. T2-Cu is a single-electron acceptor, whereas T3-Cu forms coupled ion pairs and is a double-electron acceptor. T2-Cu and T3-Cu together form a trinuclear cluster (TNC). Oxygen accepts four electrons and four protons to form water, which joins the bulk solvent [16,17]. Laccases can perform the single-electron oxidation of the substrate without using hydrogen peroxide while reducing molecular oxygen to water; therefore, they have a surprisingly broad substrate spectrum and can oxidize simple diphenols, polyphenols, diamines, and aromatic amines. The optimum temperature and pH of laccases depend on the enzyme source and substrate properties; those ranges are, respectively, from 20 °C to 75 °C and 3 to 8 (Table 1).
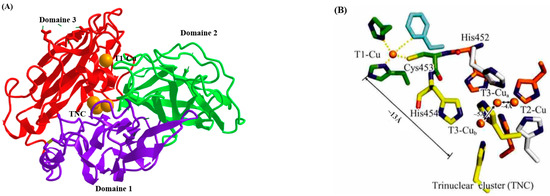
Figure 1. Crystal structure of the laccase (LccI) (PDB code: 1GYC) in Trametes versicolor. (A) The overall structure of LccI; (B) structure of the schematic representation of the four copper sites in LccI.
Table 1. Enzymatic properties of laccase from different sources.
As an oxidase, the ability of laccase to oxidize the substrate is directly related to its redox potential (E0) [33,34]. E0 is an important characteristic of the catalytic oxidation capacity of laccase, which is the energy required for laccase to capture an electron from a reducing substrate. The E0 is critical to the reactivity of laccase and the overall reaction characteristics. Laccase can directly oxidize substrates with low E0, whereas some mediators are needed to assist laccase in oxidizing substrates with high E0. Adding a mediator not only effectively improves the reaction efficiency of laccase but also expands the scope of the substrate. For instance, with the mediator’s help, laccase can oxidize nonphenolic structures with high E0 and is used in pulp bleaching [35]. Recently, laccase and the laccase–mediator system (LMS) have received extensive attention in green catalysis, such as synthesizing complex organic compounds, the selective modification of natural products, and the degradation of harmful substances [36,37,38,39]. For example, the C–N bond breakage of amines catalyzed by laccase is essential for synthesizing amino acids and nucleosides [40,41]. Existing research indicates that using Pleurotus ostreatus laccase and its natural mediator (syringaldehyde) to catalyze C–C bond breakage results in the removal of up to 100% and 85% of BPA at concentrations of 0.44 and 0.88 mmol/L in wastewater within 1 h [42].
2. The Effect of the Mediator System on Laccase Catalysis
Currently, some problems still need to be solved urgently to directly apply laccase to industrial production. For instance: (1) Numerous substrates cannot directly bind to laccase specifically. (2) With laccase, it is difficult to oxidize nonphenolic compounds with high E0 (E0 > 1.3 V) due to its low E0 (E0 < 0.8 V). Thus, the development of laccase in industries such as lignin degradation and bio-bleaching is limited [43,44]. In order to reduce the oxidation potential of substrates and improve the oxidation efficiency, some mediators can be used as an intermediate substrate for laccase to form new intermediate states to transport electrons [41,45]. These mediators are compounds with low molecular mass and low E0, such as 2,2′-Azino-bis-(3-ethylbenzothiazoline-sulphonate) (ABTS) and 2,2,6,6-Tetramethyl-1-piperidinylox (TEMPO), which can easily gain and lose electrons. They can form highly active and stable intermediates under the action of laccase and act on the substrate to is oxidized. In the LMS, laccase first oxidizes the mediator into a free radical. The oxidized mediator rapidly applies to compounds above the E0 of laccase and to those polymers that cannot directly access the laccase active center [46,47,48] (Figure 2).
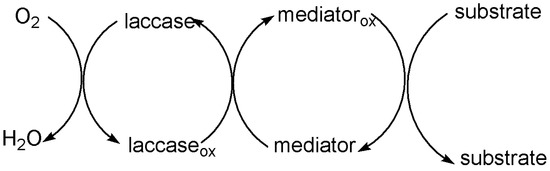
Figure 2. Oxidation of substrates by LMS.
Laccase mediators are usually divided into artificial and natural mediators (Figure 3) [49]. Due to their high efficiency and inexpensive availability, artificial mediators are widely used in lignin degradation, polycyclic aromatic hydrocarbon (PAH) oxidation, and dye decolorization. Common artificial mediators include ABTS, TEMPO, and 1-hydroxy-benzotriazole (HBT) (Figure 3B) [50,51,52]. Three mechanisms have been proposed for the function of mediators in the LMS: (1) hydrogen atom transfer (HAT), (2) electron transfer (ET), and (3) the ionic mechanism (IM). ABTS was the first mediator found to promote the laccase-catalyzed oxidation of nonphenolic lignin. The action mechanism of ABTS belongs to the electron transfer mechanism (ET), which undergoes two stages (Figure 4): forming an ABTS+· cationic radical and slowly oxidizing to ABTS2+. ABTS2+ with higher reduction potential (but not ABTS+·) performs a more critical function in the laccase–ABTS system, which mediates the oxidation of nonphenolic lignin substrates [53,54,55]. The HAT mechanism, which is generally the oxidation mechanism mediated by the N-OH type mediator, uses a form of nitryl (>N-O·) to perform oxidation, such as the HBT system. Meanwhile, the purpose of the ion mechanism (IM) is mainly to form an ammonium oxide ion (>N=O·) through the nitryl group (>N-O·) to carry out the oxidation, such as the TEMPO system [39,56].
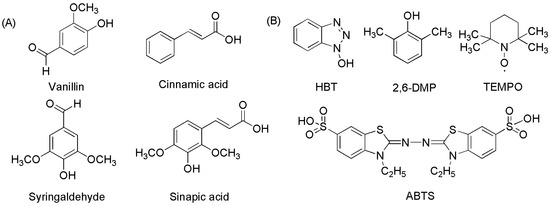
Figure 3. The chemical structures of several artificial and natural redox mediators in laccase-catalyzed oxidation reaction systems. (A) natural redox mediators; (B) artificial redox mediators.
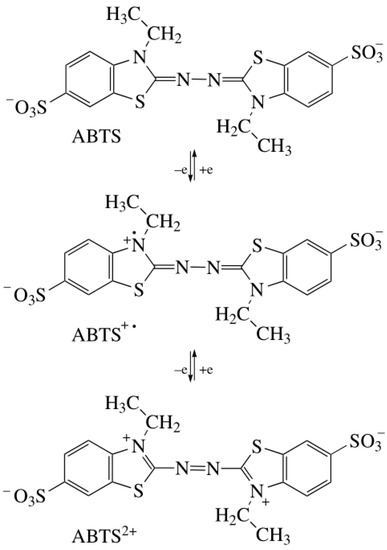
Figure 4. Oxidation of ABTS by Laccase.
Artificial mediators have potential applications in the areas of lignin degradation and polycyclic aromatic hydrocarbon (PAH) oxidation for dye decolorization, but some disadvantages limit their use. For instance: (1) poor stability, (2) potential toxicity [57], and (3) difficulty in regeneration when the molar ratio of mediators to substrates is as high as 40:1 [53,58]. Compared to artificial mediators, natural mediators have more economic value because they are readily obtained, environmentally friendly, and reproducible (Figure 3A) [35,53]. Some fungal metabolites and lignin derivatives could be used as natural mediators of laccase, including but not limited to vanillin, acetyl vanillin, acetosyringone, syringaldehyde, 2,4,6-trimethyl phenol, and p-coumaric acid [59]. Taking the laccase p-coumaric acid system as an example, it can remove 95% anthracene (80% with HBT) and benzoin anthracene within 24 h [57].
Besides indirectly assisting laccase-catalyzing substrates, mediators show synergism with each other, and the degradation efficiency increases with the increase in mediator concentration [60]. For example, the complex mediator system composed of laccase, ABTS, and HBT can oxidize phenanthrene with only one intermediary phase with a degradation rate that can be increased by 30–40% compared with a single-mediator system (such as the ABTS system or HBT system) [61]. Therefore, with intensive research on and development of the LMS, the biocatalytic substrates of laccase can be further expanded.
This entry is adapted from the peer-reviewed paper 10.3390/catal13040750
This entry is offline, you can click here to edit this entry!
