Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Gynecological malignancies currently affect about 3.5 million women all over the world. Imaging of uterine, cervical, vaginal, ovarian, and vulvar cancer still presents several unmet needs when using conventional modalities such as ultrasound, computed tomography (CT), magnetic resonance, and standard positron emission tomography (PET)/CT.
- PET/CT
- long axial field of view
- total body
- gynecological
1. Assessment of Disease Extent and Quantitative Global Disease Assessment
After the clinical (patient’s history, physical examination, and lab tests) and histological diagnosis of cancer, defining the extent of the disease is a primary concern in oncology. Different imaging approaches should be considered and selected for specific issues.
Generally, gynecologists perform pelvic transvaginal US as a first-level examination in combination with pelvic palpation. For uterine cancer, pelvic MRI, which provides excellent soft tissue contrast, can be considered the gold standard for defining the local extension of the tumor, which is essential for surgical planning [1]. Despite the high accuracy of US, MRI is frequently used for adnexal mass evaluation with the aim of better defining the risk of malignancy and the anatomical relationship with pelvic organs. However, as far as LNs assessment is concerned, MRI has low sensitivity (about 50%) [2]. This issue is relevant since the presence of LNs metastases could drastically change the treatment approach between upfront surgery or chemo-radiation in cervical cancer or can require abdominal vs. minimally invasive surgery for endometrial and ovarian cancer patients, in which lymphadenectomy moves from staging to a cytoreductive step.
MRI is often followed by a CE-CT scan in cases of high-grade uterine carcinoma, in cervical carcinoma with revised 2018 FIGO [3] stages IB1–IB3, in vaginal and ovarian cancer when there is a clinical indication, and in vulvar cancer for T2 or larger primary tumor or when metastases are suspected. Guidelines require at least the abdomen and pelvis to be examined through CE-CT, while an X-ray is considered enough for thorax examination, unless differently indicated by the clinician and/or if distant metastases are suspected. In these cases, CE-CT is often supported by [18F]FDG PET/CT, which may be considered an alternative to CE-CT in particular clinical situations (essentially when CE-CT is contraindicated) and when metastatic disease is suspected.
In light of the great imaging potential that LAFOV PET/CT offers, regional assessment of cancer has limited value in the overall diagnosis and management of such serious and systemic human illnesses [4]. Indeed, simultaneous imaging of larger portions of the body, between 106 and 194 cm, opens the possibility of global disease assessment. In this regard, LAFOV PET/CT imaging is likely to be superior to all other conventional modalities, which individually do not reflect a measurement of overall disease activity. LAFOV scanners also allow dynamic imaging, providing simultaneous time-activity curves for organs or lesions not contained within the conventional PET/CT FOV. Entire body kinetics can be reconstructed and analyzed to create parametric images that may offer complementary information to typical Standardized Uptake Value (SUV)-scaled images [5]. Furthermore, LAFOV imaging has enhanced the potential role of global disease quantification and may be the right modality to enable quantification of a patient’s global disease burden [4]. Such data will represent an accurate simultaneous calculation of regional activity at all disease sites, a reduction in underestimation of regional and global values related to respiratory and cardiac motions, and the interaction between various biological systems. Finally, global disease quantification may be used to test and validate the efficacy of novel (pharmaceutical) interventions [4].
LAFOV scanners, thanks to their improved spatial resolution and sensitivity and their ability to offer dynamic imaging, may in the future become an alternative to surgical staging, providing detailed information regarding all relevant organs and lesions simultaneously in an extended field of view [6].
2. Differential Diagnosis between Physiological, Inflammatory, Benign, and Malignant Findings
In PET/CT studies with [18F]FDG, this radiolabeled glucose analogue accumulates in all metabolically active cells, both benign (e.g., the highly glucose-avid brain cells) and malignant (i.e., dividing cancer cells) in nature. Inflammatory and other physiologic biological processes also require glucose consumption, which can be detected by [18F]FDG PET/CT.
Many normal metabolic and structural changes occur in the female reproductive tract in pre- and post-menopausal women throughout their life span and periodically during the menstrual cycle. [18F]FDG PET/CT can detect these physiologic variations, especially in the uterus and ovaries. Such patterns, which are well described in the article by Dejanovic et al. [7], may be responsible for possible false-positive findings; therefore, being aware of them may avoid misinterpretation of PET images. Some examples include functional (on a hormonal basis) ovarian [18F]FDG uptake during the late follicular and early luteal phases of the menstrual cycle and functional uptake in the endometrial cavity during the ovulatory and menstrual phases [8]. Another possible false-positive finding is the accumulation of urine activity in the ureters, mimicking lymph node metastases. Moreover, physiological bowel or bladder activity can mask peritoneal lesions, causing false negative results [7]. In addition to physiologic uptake, many other processes may demonstrate increased glucose metabolism. For example, uterine leiomyomas, which are the most common benign uterine neoplasms, show highly variable FDG uptake and can be difficult to differentiate from uterine carcinomas or sarcomas. Inflammatory and infectious processes too, or other benign conditions such as endometriotic implants and ureteral diverticula, may be detected as areas of increased [18F]FDG uptake and be misdiagnosed as tumors. Several other pitfalls have been described [9]. However, the most common interpretation issue in this setting is probably represented by the differentiation between malignant and benign LNs. The use of delayed and dynamic (thus also parametric) imaging allowed by LAFOV PET/CT scanners may help the nuclear medicine physician avoid misdiagnosing. Indeed, based on experience gained by several research groups, it has become clear that FDG uptake in tumor lesions increases over time and reaches a plateau at 4 to 5 h, and several types of tumors have an uptake peak significantly later than the conventional 1-h uptake time used on SAFOV scanners [4][10][11]. At these delayed time points, there is a significant physiologic clearance of the radiotracer from organs and tissues such as the liver, kidneys, bladder, and blood, leading to an increase in tumor-to-background ratios. The improved sensitivity of LAFOV systems allows for delayed imaging with images of diagnostic quality and relatively low background noise, which may improve lesion detection and enable fundamental biologic insights, as described by Pantel et al. [12]. By testing the uptake curve of certain tissues and sites in “dual phase” acquisition protocols, a major impact could be observed in the optimal management of oncological patients, especially when the exact location and extent of the tumor are fundamental for surgical interventions or radiation therapy planning [13]. Some attempts at dual-time imaging have been made with conventional scanners, but with various limitations and few applications [14][15]. It could also be hypothesized that, in some cases, a delayed imaging timepoint will show previously unseen metastatic sites with different kinetics, suggesting biologic heterogeneity of the tumor, as previously described in other tumor entities like prostate cancer [12][16]. LAFOV PET/CT imaging may provide a full overview of tumor biological heterogeneity, guiding the performance of targeted biopsies or allowing for a reduction in their number, which are frequently and invasively performed in gynecological cancers. In this regard, LAFOV scanners have also shown potential for imaging more than one molecular target at the same time with dual-tracer examination protocols [17]. This kind of examination is still an unmet need in the evaluation and introduction of new radiopharmaceuticals in clinical practice. These novel radiopharmaceuticals, which explore specific biological characteristics of tumors, are currently under evaluation in gynecological cancers with the potentiality of identifying new prognostic factors and/or new personalized therapies. Among these, fibroblast activation protein inhibitors (FAPI) PET/CT [18] and fluoro-estradiol (FES) PET/CT [2][19] have shown preliminary promising results in gynecological cancer.
LAFOV technology has also opened new perspectives in dynamic imaging [5][20], providing simultaneous time-activity curves for organs and lesions that would not be included altogether in the FOV of conventional scanners [5], avoiding the need for simultaneous blood sampling (Figure 1).
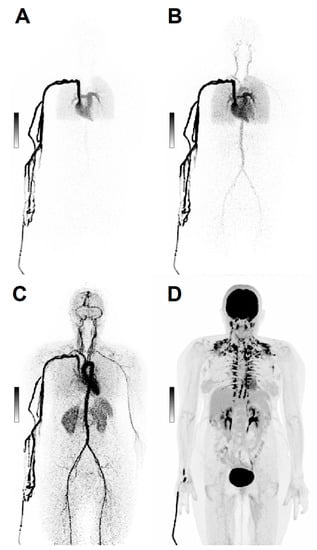
Figure 1. Dynamic Imaging. Maximum intensity projections (MIP) of a 69 y/o patient with ovarian cancer, scanned dynamically with 168 MBq [18F]FDG. After injection, the first 2 min of acquisition show the early biodistribution of the radiopharmaceutical (A–C). Image (D) shows [18F]FDG distribution at 60 min post-injection.
On a simple level, the dynamic acquisition of PET images makes it possible to clearly identify the [18F]FDG elimination path and thus distinguish with greater certainty the urine uptake in the bladder and ureters from neighboring sites of non-physiological uptake [21]. In the future, dynamic imaging may even become a complementary solution to conventional imaging protocols offered by LAFOV scanners. Indeed, the dynamic acquisition kinetic information may prove valuable for precision medicine. Entire body kinetics datasets can be reconstructed and analyzed to create parametric images that may offer complementary information to typical SUV-scaled images [5] and improve quantification [22]. Utilization rate (Ki) images of [18F]FDG can be provided to visualize glucose utilization in the target lesion, not confounded by contributions from [18F]FDG in plasma [22]. This kinetic information can be applied to identify small regions of tumoral infiltration, as such lesions may lack adequate contrast for detection in SUV images but sufficiently alter the kinetics within a voxel that they can be appreciated in parametric images [23]. Such a paradigm allows for the functional characterization of low-grade disease (cancers, inflammation/infection) that has so far represented a pitfall in [18F]FDG imaging [24]. Abbreviations of LAFOV scan protocols might allow overcoming the challenge of current long scan duration in dynamic imaging and might possibly lead to the implementation of dynamic imaging models into the clinical routine [5][25].
3. Detection of Peritoneal Carcinomatosis and Sub-Centimetric Metastases
Early detection of peritoneal carcinomatosis (i.e., seeding of metastatic implants in the peritoneal cavity) is an important step in staging and restaging gynecological malignancies, mainly ovarian cancer, as it is essential for complete cytoreductive surgery and prognostic information [26]. As already mentioned, the presence of peritoneal carcinomatosis in gynecological cancers is often assessed through surgical inspection. Anatomic imaging with CE-CT and MRI (also using DW sequences) plays an important role in non-invasively assessing peritoneal metastases. However, detection of peritoneal carcinomatosis is highly influenced by lesion size and site and the presence of ascites [26]. According to a recent meta-analysis, MRI and standard [18F]FDG PET/CT show similar diagnostic performance for the detection of peritoneal metastases in patients with ovarian cancer, with a pooled sensitivity and specificity of 92% and 85%, respectively, for DW-MRI and 80% and 90%, respectively, for PET/CT, with sensitivity values exceeding those of CT (68% sensitivity and 88% specificity) [27]. At PET/CT, patterns of visualization of peritoneal carcinomatosis include a focal pattern, with single or multiple areas of hypermetabolic activity that may become confluent, such as those involving paracolic gutters, small bowel mesentery, the pouch of Douglas, or the abdominal cavity among bowel loops; or a diffuse pattern with diffuse thickening and intense uptake, usually seen along the liver capsule or in the greater omentum (omental cake) [28]. These and other sites of metastases may go undetected on conventional PET/CT scanners if they are smaller than 1 cm in size, which is a major flaw associated with SAFOV [18F]FDG imaging.
The ultra-high sensitivity and the possibility to perform delayed imaging of LAFOV scanners have demonstrated higher diagnostic accuracy outputs compared to conventional PET imaging [4][5][6][12][29], allowing for the detection of small tumor deposits. Higher count statistics resulting in an improvement of signal-to-noise and tumor-to-background ratios simplify the detection of obscure small lesions [30][31] (Figure 2).
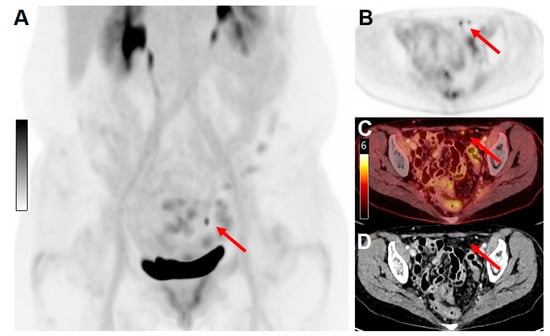
Figure 2. Example of the high sensitivity and spatial resolution of a long-axial field-of-view scanner. A 64 y/o female patient with cervical cancer was treated with surgery (hysterectomy and pelvic lymphadenectomy), followed by adjuvant radiation therapy, and systemic treatment with cisplatin. For response-to-treatment assessment, the patient underwent a PET/CT examination on the Biograph Vision Quadra scanner. (A) Maximum intensity projection (MIP) of images acquired 60 min after injection of 130 MBq [18F]FDG. (B) PET only, (C) fused PET/CT, and contrast-enhanced CT (D) images revealed the presence of two peritoneal metastatic lesions (red arrows, maximum diameter 4 mm) with clearly distinguishable [18F]FDG uptake (SUVmax 4.6).
If a downside may be pointed out, normal structures could also appear in a redefined way on LAFOV PET/CT images [5][24]. Hopefully, the implementation of LAFOV scanners in clinical settings will increase the nuclear medicine physicians’ experience in reading such high-definition images and avoid the risk of an increased number of false-positive results.
4. Effective Assessment of Post-Therapy Changes
Imaging techniques such as CT and MRI (even when integrated with DWI) cannot reliably distinguish residual disease from post-therapy changes (e.g., post-surgery and post-radiation therapy). A major flaw associated with this limitation is the generation of false-positive cases. In this setting, [18F]PET/CT has an excellent negative predictive value, while the number of false-positive results is still not optimal. In general, a 3-month interval is recommended for a reliable post-therapy functional assessment, as at this time radio-induced inflammation is thought to be resolved. Minimal residual disease and post-therapeutic local changes may have very different kinetic parameters that could be potentially detected by using parametric maps created by LAFOV PET datasets. This approach may in the future help to differentiate inflammation from tumor [24].
5. Motion Artifacts
Imaging on SAFOV PET/CT scanners requires between 15 and 20 min for a whole-body acquisition (skull-base to mid-thighs). During this time, the patient’s movements can lead to motion artifacts and disalignment between the PET and the CT images, with a consequent increased risk of misreadings. Other involuntary movements that may cause artifacts and interfere with image reading are represented by respiratory motion and heartbeat, bowel motion, and bladder filling with radioactive urine. Such issues are not to be underestimated in patients with a gynecological malignancy, as the pelvis represents a complex anatomical district with sites of physiological metabolic activity that may overlap with tumor lesions and vice versa, leading to false-positive or false negative results. LAFOV PET/CT scanners allow the implementation of fast and even ultra-fast acquisition protocols; the Biograph Vision Quadra system provided diagnostic-quality images, comparable to those of a 16-min scan on a SAFOV PET/CT, in 2 min [32]. For European Association of Nuclear Medicine Research Ltd. (EARL) standard compliant acquisition and reconstruction protocols, scan durations on the Biograph Vision Quadra could even be reduced to 1 min [31]. The uEXPLORER scanner provides high-quality images in an acquisition time of even 0.5 min, as found in the results of the NCT04110743 clinical trial (that has low-dose PET/CT scans, TB perfusion, and early biodistribution understanding in healthy volunteers among its aims) and further stated in an expert consensus on [18F]FDG PET/CT TB imaging [33]. Moreover, decreased acquisition time leads to a reduction in the need for sedation in debilitated patients, claustrophobic patients, and patients with difficulty laying still due to neurological, respiratory, or pain reasons, which would otherwise most commonly represent the cases in which motion artifacts interfere with correct imaging [6].
6. Radiation Exposure
One of the clear advantages of LAFOV PET/CT imaging and one of its most appreciated features is the already mentioned possibility to reduce the administered radiopharmaceutical activity down to a tenth of what is needed on conventional scanners and still obtain high-quality diagnostic images. The follow-up of oncological patients requires the repetition of the same examinations performed at baseline, for the correct interpretation of anatomical (and functional) changes, response to therapies, or evaluation of disease relapse. With LAFOV PET/CT imaging, patients will be able to undergo multiple scans for disease re-assessment with a substantial decrease in administered activity when compared to using conventional PET/CT systems [6][34]. An example can be found in the natural history of cervical cancer, which, in approximately one-third of patients, recurs within the first 2 years after therapy. The sites of recurrence are the vaginal vault (better distinguishable through dynamic acquisition), parametrial and pelvic walls, para-aortic and supraclavicular lymph nodes, and distant metastases, including peritoneal disease. The current survival rate is low, and cure efficacy is minimal in these patients [35]. Low-dose and more frequent follow-up PET/CT exams on LAFOV scanners could increase the early detection of recurrence and improve patients’ survival.
Ultra-low-dose protocols are already in use [33], and it can be expected that many applications will follow. In some cases, even their use for screening purposes has been hypothesized [36][37]. Subjects with a genetic-familial high-risk for ovarian cancer may be among the possible recipients of such an application, as the appropriateness of imaging modalities and scheduling in these patients is still under study [38].
Another of the most appealing current areas of interest for ultra-low-dose PET/CT exams is pregnancy. Cancer is diagnosed in approximately one in every 1000 pregnancies annually, with melanoma, breast cancer, cervical cancer, lymphomas, and leukemia being the most commonly associated malignancies [39]. LAFOV PET/CT could allow these patients to have a TB functional oncological assessment—and the best possible disease management—at unprecedented low-risk exposure to radiation.
Moreover, it is now being hypothesized that diagnostic PET images alone can be obtained with no or very low-dose CT (currently needed for attenuation correction) through deep learning and new reconstruction protocols [36][37][40][41][42].
7. Pain
One of the issues most associated with cancer is pain. Gynecological oncological patients can experience it too, especially in cases of bone metastases. The incidence of bone metastases in endometrial cancer is reported to be 6 to 15% [43][44], approximately 1.2% in ovarian cancer [45], and 1.1% in cervical neoplasms [46]. Improvements in surgery, radiation, and the development of novel chemotherapeutic agents have led to prolonged survival and an increase in the prevalence of bone metastases. Pain may prevent patients from undergoing important examinations like PET/CT. In these cases, even if the assessment leads to palliative treatments, it is still of utmost importance for physicians to put their effort into offering patients a solution for their dignity and quality of life. LAFOV PET/CT can represent a valuable solution because it can significantly shorten the acquisition time while maintaining good detectability of lesions and diagnostic image quality [47].
8. Detection of Cancer-Associated Vascular Complications
Oncological patients experience cancer-associated vascular complications, with a high incidence reported for both deep vein thrombosis and pulmonary embolism, up to a seven-fold increased risk compared to the non-oncological population [48]. Gynecological oncology patients are also affected and can experience recurrence of venous thromboembolism and/or pulmonary embolism despite treatment with edoxaban or a vitamin K antagonist, with no significant difference in outcomes among the different tumor types [49]. Cancer-associated vascular complications may result in an interruption of treatments or premature death. As conventional PET/CT fails to image the lower extremities, common sites of venous thrombosis often go unassessed, and clots go undetected. A delayed imaging of the total body may detect increased [18F]FDG uptake in blood clots as blood pool activity decreases in the venous system. Thus, delayed TB PET/CT imaging could act as a means of detection and may have a huge impact on the management of cancer patients and their outcome by identifying the clots earlier and with greater sensitivity [4][24].
9. Bone Metabolism and Osteoporosis Assessment
Women with gynecological cancer have an increased risk of cancer treatment-induced bone loss, which impacts their quality of life and overall survival [50]. Unfortunately, current imaging techniques have several limitations in the reliable assessment of bone metabolism and fail to detect a decreased bone mineral density at early time points. The currently most adopted modality is dual-energy x-ray absorptiometry (DXA), which, however, implies overestimating the results. Sodium fluoride (NaF) PET/CT is a promising imaging tool to fill this need [4], and its association with the already thoroughly discussed TB imaging advantages could represent the source of major benefits for patients.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15092407
References
- NCCN Guidelines. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 31 January 2023).
- Paredes, P.; Paño, B.; Díaz, B.; Vidal-Sicart, S. Endometrial Cancer. In Nuclear Medicine Manual on Gynaecological Cancers and Other Female Malignancies, 1st ed.; Collarino, A., Vidal-Sicart, S., Olmos, R.A.V., Eds.; Springer: Cham, Switzerland, 2022; Volume 1, pp. 71–88.
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO Staging for Carcinoma of the Cervix Uteri. Int. J. Gynecol. Obstet. 2019, 145, 129–135.
- Alavi, A.; Saboury, B.; Nardo, L.; Zhang, V.; Wang, M.; Li, H.; Raynor, W.Y.; Werner, T.J.; Høilund-Carlsen, P.F.; Revheim, M.-E. Potential and Most Relevant Applications of Total Body PET/CT Imaging. Clin. Nucl. Med. 2022, 47, 43–55.
- Nardo, L.; Pantel, A.R. Oncologic Applications of Long Axial Field-of-View PET/Computed Tomography. PET Clin. 2021, 16, 65–73.
- van Sluis, J.; Borra, R.; Tsoumpas, C.; van Snick, J.H.; Roya, M.; ten Hove, D.; Brouwers, A.H.; Lammertsma, A.A.; Noordzij, W.; Dierckx, R.A.J.O.; et al. Extending the Clinical Capabilities of Short- and Long-Lived Positron-Emitting Radionuclides through High Sensitivity PET/CT. Cancer Imaging 2022, 22, 69.
- Dejanovic, D.; Hansen, N.L.; Loft, A. PET/CT Variants and Pitfalls in Gynecological Cancers. Semin. Nucl. Med. 2021, 51, 593–610.
- De Gaetano, A.M.; Calcagni, M.L.; Rufini, V.; Valentini, A.L.; Gui, B.; Giordano, A.; Bonomo, L. Imaging of Gynecologic Malignancies with FDG PET–CT: Case Examples, Physiolocic Activity, and Pitfalls. Abdom. Imaging 2009, 34, 696–711.
- Hernandez Pampaloni, M.; Facchetti, L.; Nardo, L. Pitfalls in FDG PET Imaging in Gynecological Malignancies. Q. J. Nucl. Med. Mol. Imaging 2016, 60, 124–138.
- Basu, S.; Kung, J.; Houseni, M.; Zhuang, H.; Tidmarsh, G.F.; Alavi, A. Temporal Profile of Fluorodeoxyglucose Uptake in Malignant Lesions and Normal Organs over Extended Time Periods in Patients with Lung Carcinoma: Implications for Its Utilization in Assessing Malignant Lesions. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 9–19.
- Zhuang, H.; Pourdehnad, M.; Lambright, E.S.; Yamamoto, A.J.; Lanuti, M.; Li, P.; Mozley, P.D.; Rossman, M.D.; Albelda, S.M.; Alavi, A. Dual Time Point 18F-FDG PET Imaging for Differentiating Malignant from Inflammatory Processes. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2001, 42, 1412–1417.
- Pantel, A.R.; Viswanath, V.; Daube-Witherspoon, M.E.; Dubroff, J.G.; Muehllehner, G.; Parma, M.J.; Pryma, D.A.; Schubert, E.K.; Mankoff, D.A.; Karp, J.S. PennPET Explorer: Human Imaging on a Whole-Body Imager. J. Nucl. Med. 2020, 61, 144–151.
- Rao, Y.J.; Grigsby, P.W. The Role of PET Imaging in Gynecologic Radiation Oncology. PET Clin. 2018, 13, 225–237.
- Schillaci, O. Use of Dual-Point Fluorodeoxyglucose Imaging to Enhance Sensitivity and Specificity. Semin. Nucl. Med. 2012, 42, 267–280.
- Cheng, G.; Torigian, D.A.; Zhuang, H.; Alavi, A. When Should We Recommend Use of Dual Time-Point and Delayed Time-Point Imaging Techniques in FDG PET? Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 779–787.
- Alberts, I.; Prenosil, G.; Mingels, C.; Bohn, K.P.; Viscione, M.; Sari, H.; Rominger, A.; Afshar-Oromieh, A. Feasibility of Late Acquisition Ga-PSMA-11 PET/CT Using a Long Axial Field-of-View PET/CT Scanner for the Diagnosis of Recurrent Prostate Cancer-First Clinical Experiences. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4456–4462.
- Alberts, I.; Schepers, R.; Zeimpekis, K.; Sari, H.; Rominger, A.; Afshar-Oromieh, A. Combined Ga-PSMA-11 and Low-Dose 2-FDG PET/CT Using a Long-Axial Field of View Scanner for Patients Referred for -PSMA-Radioligand Therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 951–956.
- Dendl, K.; Koerber, S.A.; Tamburini, K.; Mori, Y.; Cardinale, J.; Haberkorn, U.; Giesel, F.L. Advancement and Future Perspective of FAPI PET/CT In Gynecological Malignancies. Semin. Nucl. Med. 2022, 52, 628–634.
- Tsujikawa, T.; Makino, A.; Mori, T.; Tsuyoshi, H.; Kiyono, Y.; Yoshida, Y.; Okazawa, H. PET Imaging of Estrogen Receptors for Gynecological Tumors. Clin. Nucl. Med. 2022, 47, e481–e488.
- Sari, H.; Mingels, C.; Alberts, I.; Hu, J.; Buesser, D.; Shah, V.; Schepers, R.; Caluori, P.; Panin, V.; Conti, M.; et al. First Results on Kinetic Modelling and Parametric Imaging of Dynamic 18F-FDG Datasets from a Long Axial FOV PET Scanner in Oncological Patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1997–2009.
- Narayanan, P.; Sahdev, A. The Role of 18 F-FDG PET CT in Common Gynaecological Malignancies. Br. J. Radiol. 2017, 90, 20170283.
- Wang, G.; Nardo, L.; Parikh, M.; Abdelhafez, Y.G.; Li, E.; Spencer, B.A.; Qi, J.; Jones, T.; Cherry, S.R.; Badawi, R.D. Total-Body PET Multiparametric Imaging of Cancer Using a Voxelwise Strategy of Compartmental Modeling. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1274–1281.
- Saboury, B.; Morris, M.A.; Farhadi, F.; Nikpanah, M.; Werner, T.J.; Jones, E.C.; Alavi, A. Reinventing Molecular Imaging with Total-Body PET, Part I: Technical Revolution in Evolution. PET Clin. 2020, 15, 427–438.
- Katal, S.; Eibschutz, L.S.; Saboury, B.; Gholamrezanezhad, A.; Alavi, A. Advantages and Applications of Total-Body PET Scanning. Diagnostics 2022, 12, 426.
- Viswanath, V.; Sari, H.; Pantel, A.R.; Conti, M.; Daube-Witherspoon, M.E.; Mingels, C.; Alberts, I.; Eriksson, L.; Shi, K.; Rominger, A.; et al. Abbreviated Scan Protocols to Capture 18F-FDG Kinetics for Long Axial FOV PET Scanners. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3215–3225.
- Campos, N.M.F.; Almeida, V.; Curvo Semedo, L. Peritoneal Disease: Key Imaging Findings That Help in the Differential Diagnosis. Br. J. Radiol. 2022, 95, 20210346.
- van’t Sant, I.; Engbersen, M.P.; Bhairosing, P.A.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; van Driel, W.J.; Aalbers, A.G.J.; Kok, N.F.M.; Lahaye, M.J. Diagnostic Performance of Imaging for the Detection of Peritoneal Metastases: A Meta-Analysis. Eur. Radiol. 2020, 30, 3101–3112.
- De Gaetano, A.M.; Calcagni, M.L.; Rufini, V.; Valenza, V.; Giordano, A.; Bonomo, L. Imaging of Peritoneal Carcinomatosis with FDG PET-CT: Diagnostic Patterns, Case Examples and Pitfalls. Abdom. Imaging 2009, 34, 391–402.
- Nardo, L.; Abdelhafez, Y.G.; Spencer, B.A.; Badawi, R.D. Clinical Implementation of Total-Body PET/CT at University of California, Davis. PET Clin. 2021, 16, 1–7.
- Prenosil, G.A.; Sari, H.; Fürstner, M.; Afshar-Oromieh, A.; Shi, K.; Rominger, A.; Hentschel, M. Performance Characteristics of the Biograph Vision Quadra PET/CT System with a Long Axial Field of View Using the NEMA NU 2-2018 Standard. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 476–484.
- van Sluis, J.; van Snick, J.H.; Brouwers, A.H.; Noordzij, W.; Dierckx, R.A.J.O.; Borra, R.J.H.; Slart, R.H.J.A.; Lammertsma, A.A.; Glaudemans, A.W.J.M.; Boellaard, R.; et al. EARL Compliance and Imaging Optimisation on the Biograph Vision Quadra PET/CT Using Phantom and Clinical Data. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4652–4660.
- Alberts, I.; Hünermund, J.-N.; Prenosil, G.; Mingels, C.; Bohn, K.P.; Viscione, M.; Sari, H.; Vollnberg, B.; Shi, K.; Afshar-Oromieh, A.; et al. Clinical Performance of Long Axial Field of View PET/CT: A Head-to-Head Intra-Individual Comparison of the Biograph Vision Quadra with the Biograph Vision PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2395–2404.
- Yu, H.; Gu, Y.; Fan, W.; Gao, Y.; Wang, M.; Zhu, X.; Wu, Z.; Liu, J.; Li, B.; Wu, H.; et al. Expert Consensus on Oncological FDG Total-Body PET/CT Imaging (Version 1). Eur. Radiol. 2022, 33, 615–626.
- Sachpekidis, C.; Pan, L.; Kopp-Schneider, A.; Weru, V.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Application of the Long Axial Field-of-View PET/CT with Low-Dose FDG in Melanoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 1158–1167.
- Feudo, V.; Collarino, A.; Arciuolo, D.; Lorusso, M.; Ferrandina, G.; Rufini, V. Cervical Cancer. In Nuclear Medicine Manual on Gynaecological Cancers and Other Female Malignancies, 1st ed.; Collarino, A., Vidal-Sicart, S., Olmos, R.A.V., Eds.; Springer: Cham, Switzerland, 2022; Volume 1, pp. 53–70.
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12.
- Slart, R.H.J.A.; Tsoumpas, C.; Glaudemans, A.W.J.M.; Noordzij, W.; Willemsen, A.T.M.; Borra, R.J.H.; Dierckx, R.A.J.O.; Lammertsma, A.A. Long Axial Field of View PET Scanners: A Road Map to Implementation and New Possibilities. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4236–4245.
- Lowry, K.P.; Lee, J.M.; Kong, C.Y.; McMahon, P.M.; Gilmore, M.E.; Cott Chubiz, J.E.; Pisano, E.D.; Gatsonis, C.; Ryan, P.D.; Ozanne, E.M.; et al. Annual Screening Strategies in BRCA1 and BRCA2 Gene Mutation Carriers: A Comparative Effectiveness Analysis. Cancer 2012, 118, 2021–2030.
- Hepner, A.; Negrini, D.; Hase, E.A.; Exman, P.; Testa, L.; Trinconi, A.F.; Filassi, J.R.; Francisco, R.P.V.; Zugaib, M.; O’Connor, T.L.; et al. Cancer During Pregnancy: The Oncologist Overview. World J. Oncol. 2019, 10, 28–34.
- Hu, Y.; Zheng, Z.; Yu, H.; Wang, J.; Yang, X.; Shi, H. Ultra-Low-Dose CT Reconstructed with the Artificial Intelligence Iterative Reconstruction Algorithm (AIIR) in 18F-FDG Total-Body PET/CT Examination: A Preliminary Study. EJNMMI Phys. 2023, 10, 1.
- Sari, H.; Teimoorisichani, M.; Mingels, C.; Alberts, I.; Panin, V.; Bharkhada, D.; Xue, S.; Prenosil, G.; Shi, K.; Conti, M.; et al. Quantitative Evaluation of a Deep Learning-Based Framework to Generate Whole-Body Attenuation Maps Using LSO Background Radiation in Long Axial FOV PET Scanners. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4490–4502.
- Guo, R.; Xue, S.; Hu, J.; Sari, H.; Mingels, C.; Zeimpekis, K.; Prenosil, G.; Wang, Y.; Zhang, Y.; Viscione, M.; et al. Using Domain Knowledge for Robust and Generalizable Deep Learning-Based CT-Free PET Attenuation and Scatter Correction. Nat. Commun. 2022, 13, 5882.
- Abdul-Karim, F.W.; Kida, M.; Wentz, W.B.; Carter, J.R.; Sorensen, K.; Macfee, M.; Zika, J.; Makley, J.T. Bone Metastasis from Gynecologic Carcinomas: A Clinicopathologic Study. Gynecol. Oncol. 1990, 39, 108–114.
- Rose, P.G.; Steven Piver, M.; Tsukada, Y.; Lau, T. Patterns of Metastasis in Uterine Sarcoma. An Autopsy Study. Cancer 1989, 63, 935–938.
- Cormio, G.; Rossi, C.; Cazzolla, A.; Resta, L.; Loverro, G.; Greco, P.; Selvaggi, L. Distant Metastases in Ovarian Carcinoma. Int. J. Gynecol. Cancer 2003, 13, 125–129.
- Thanapprapasr, D.; Nartthanarung, A.; Likittanasombut, P.; Na Ayudhya, N.I.; Charakorn, C.; Udomsubpayakul, U.; Subhadarbandhu, T.; Wilailak, S. Bone Metastasis in Cervical Cancer Patients Over a 10-Year Period. Int. J. Gynecol. Cancer 2010, 20, 373–378.
- Zhang, Y.-Q.; Hu, P.-C.; Wu, R.-Z.; Gu, Y.-S.; Chen, S.-G.; Yu, H.-J.; Wang, X.-Q.; Song, J.; Shi, H.-C. The Image Quality, Lesion Detectability, and Acquisition Time of 18F-FDG Total-Body PET/CT in Oncological Patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2507–2515.
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of Cancer-Associated Venous Thrombosis. Blood 2013, 122, 1712–1723.
- Odajima, S.; Seki, T.; Kato, S.; Tomita, K.; Shoburu, Y.; Suzuki, E.; Takenaka, M.; Saito, M.; Takano, H.; Yamada, K.; et al. Efficacy of Edoxaban for the Treatment of Gynecological Cancer-Associated Venous Thromboembolism: Analysis of Japanese Real-World Data. J. Gynecol. Oncol. 2022, 33, e62.
- O’Gorman, C.A.; Minnock, S.; Mulhall, J.; Gleeson, N. Attention to Bone Health in Follow-up of Gynaecological Cancers in Tertiary Care. Womens Health 2022, 18, 17455065211070747.
This entry is offline, you can click here to edit this entry!
