Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
Nanotechnology has the potential to revitalize both poorly performing marketed drugs and many of those pre-clinically promising candidates that were “beached” due to inadequate water solubility, in addition to novel therapeutic developments using components in the 1–100 nanometer range. As a result of developments in nanotechnology, researchers have been tackling this problem by formulating drugs with the aid of nanocarriers.
- solubility
- bioavailability
- prodrug
- oral drug delivery
1. Liposomes
Liposomes have been demonstrated to be one of the most promising drug delivery methods. The first liposomal (intravenous) formulation came out on the market in 1995 (Doxil®) and has been researched since then and integrated with various active molecules, including peptides and proteins [1]. Liposomes are created using amphipathic compounds, typically lipids; the synthesis process can be tweaked to control their size and form [2]. Liposomes are vesicles surrounded by phospholipid bilayers that can solubilize drugs that are insoluble in water in the lipid domain of the liposomal membrane [3]. The structural and compositional similarities of liposomes to biological membranes have encouraged their usage for the non-invasive oral delivery of weakly permeable drugs aided by their dissolving capability and biocompatibility [4][5]. Because liposomes may solubilize weakly water-soluble pharmaceuticals, safeguard the drug from GI tract degradation, and improve permeability across the epithelial cell membrane (boosting oral bioavailability), liposomal administration appears promising for the oral delivery of hydrophobic drugs [5]. For oral administration, liposomal membrane formulation can delay or regulate the drug’s release from the liposomes, resulting in a range of absorption rates. Bypassing the hepatic first-pass effect, liposomes can directly deliver the drug through the lymphatic route. By minimizing direct interaction with the intestinal environment by encapsulating into liposomes, drug-induced GI irritation may also be decreased [5][6]. They were first employed in the 1960s to examine biological membranes. Since then, their use has expanded to drug administration, cosmetic formulations, or the food industry, among other applications, as shown in Figure 1.
Figure 1. Mechanism of drug’s solubilization with the aid of liposomes.
Rao et al. has prepared a liposomal drug delivery system for the enhancement of the solubility and bioavailability of Efavirenz. Efavirenz, having poor aqueous solubility (0.0085 mg/mL) and high lipophilicity (log P: 5.4), belongs to BCS class II. It has been found in their studies that there is an improvement in the solubility of Efavirenz with increasing concentration of soya lecithin in the liposomal formulation (i.e., after the addition of 900 mg of soya lecithin) along with the drug and water; solubility went up to 27.82 ± 2.55 µg/mL. In addition, according to the in vivo pharmacokinetic study, it has been reported that the oral bioavailability of the liposomal formulation has increased 2-fold compared to the free drug [7]. To overcome the poor aqueous solubility of Apigenin, Telang et al. developed the phospholipid complex of apigenin (APLC) via the incubation of phospholipon 90H along with apigenin in a solution of 1,4-dioxane and methanol at 50 °C for 2 h and then redissolved in chloroform and methanol. This mixture was further precipitated in hexane followed by vacuum drying. It was found in their study that the newly formed complex showed an increase in solubility that may be because of the amorphous state of apigenin in the APLC complex. Namely, APLC showed a 37-fold solubility improvement in the water of apigenin (i.e., from 0.62 ± 0.88 µg/mL to 22.80 ± 1.40 µg/mL) [8].
2. Dendrimers
Dendrimers, a new class of polymers, possess excellent potential for drug solubility enhancement [9][10]. Dendrimers are made up of four domains: a central core, internal layers made up of repeating units that link to the vacant spaces, external surface groups, and core (generation, G) [11]. Dendrimers containing poly (amidoamine) (PAMAM) are the most extensively studied dendrimers as drug delivery systems. They consist of an ethylenediamine core and branched units made of methyl acrylate and ethylenediamine [12]. The capacity of these hyper-branched, mono-dispersed molecules to covalently bind drug molecules to their peripheral branches and encapsulate them within the dendritic structure is unique. Several published research studies have successfully employed dendrimers to increase the solubility of poorly soluble drugs. Using physical encapsulation or covalent conjugation, dendrimers may also increase the solubility of hydrophobic compounds [9][13]. As per literature, G0 PAMAM dendrimers can greatly improve the solubility of aceclofenac, a practically water insoluble anti-inflammatory drug [14]. According to the research by Patel et al., the solubility improvement was concentration-dependent and depended on the pH, concentration, temperature, and dendrimer generation. The solubility was improved in the following sequence via dendrimer synthesis at a constant pH: G3 > G2 > G1 > G0. The enhancement in aceclofenac solubility caused by the dendrimer pH may be the result of an electrostatic interaction between the NH2 groups of the dendrimer and the COOH group of the medication, and the temperature of the dendrimer solution was shown to have an inverse relationship with the solubility of aceclofenac [14].
Likewise, Gautam and Verma investigated the effect of a full-generation PAMAM (G4) dendrimer on the solubility of candesartan cilexetil (lipophilic calcium channel blocker agent). Purified water was used throughout the testing, which was conducted at room temperature, and the drug’s concentration was determined to be 2.63 g/mL. The maximum solubility of candesartan cilexetil increased approx. 373-fold at a 10 mg/mL PAMAM concentration, and it was shown that the enhancement in solubility relies on the concentration of the dendrimers [15]. Simvastatin was investigated with dendrimers by Kulhari et al. with the goal of assessing the effectiveness of three different G4 PAMAM dendrimers. It was found that PEGylated dendrimers had the highest solubilization (33-fold), followed by NH2- (23-overlap) as well as OH-ended (17.5-overlay) dendrimers. The solvency improved from 33.4 to 1093.25 mole/L with the introduction of 109.04 M (0.4%, w/v) PEGylated dendrimer complexes (i.e., 33-overlap) [16].
3. Nanosuspensions
Nanosuspensions are submicron colloidal dispersions of drug particles in an aqueous phase, colloidally stabilized with the aid of surfactants [17]. As an adjunct to lipidic systems, nanosuspensions are employed in the formulation of drugs that are insoluble in both water and organic solvents. Compounds with a high melting point, a high log p value, and high dosage strength are the best candidates to be formulated as nanosuspensions [18]. By delivering the nanosuspension orally or intravenously (IV), the rate of saturation of the active component increases and the optimal plasma level is more rapidly achieved. The size distribution of solid particles in nanosuspension ranges from 200 nm to 600 nm [19]. Aghrbi et al. developed cilostazol-incorporated nanosuspension in an attempt to enhance the in vitro solubility and dissolution rate via a wet milling method, and the results showed that at pH = 1.2, the particle size reduction significantly increased the maximum thermodynamic solubility of the drug (cilostazol) and had a two-fold improvement over unmilled and pure surfactant dispersion [20]. Albendazole exhibits a solubility of 4.1 mg/L at 25 °C in water and <5% bioavailability through the oral route. Thus, to improve the solubility of albendazole, Rao et al. formulated nanosuspension-encapsulated multiparticulates; they found in their studies that there was a 16-fold increase in aqueous solubility [21].
4. Micelles
The combination of a hydrophilic spherical shell composed of polar heads or a hydrophobic core composed of a polar tail produces an optimal environment for the solubilization of poorly water-soluble drugs [22][23]. Polymeric micelles, which are typically composed of amphiphilic block copolymers, have received a lot of interest in the last few decades in terms of delivering hydrophobic payloads. Polymeric micelles with central hydrophobic sections composed of hydrophobic moieties, such as poly (propylene oxide) (PPO), PCL, poly (ethylene imine) (PEI), PLA, poly (jasmine lactone) (PJL), and phosphatidylethanolamine (DSPE), and an outer hydrophilic shell (typically poly (ethylene oxide) (PEO) may self-assemble into micelles in an aqueous media with low critical micellar concentration (CMC) [24][25]. Due to the particular features of polymeric micelles, such as nanoscale size, distinctive structure, stability, and compatibility, they are suitable for many types of applications [26].
Bansal et al. has evaluated the solubilizing capability of poly-based (jasmine lactone) (PJL) polymeric micelles against Soluplus® and with poly (lactide) copolymer micelles. They found that after the introduction of -COOH groups to the polymeric chain of PJL, the aqueous solubility of clotrimazole was enhanced approx. 334-fold compared to Soluplus®. Hence, it was proposed that the solubilization capability of polymeric micelles can be enhanced drastically via the introduction of a free functional group on the polymer chain, which can interact with drugs electrostatically [27][28]. Zhou et al. synthesized griseofulvin-loaded core crosslinked micelles to increase solubility and stability. As early dissociation might be harmful to the micelles, linear dendritic polymers were crosslinked in this study to avoid both it and drug leakage. The results of the study suggest a 10-fold improvement in the solubilization and sustained-release behavior of griseofulvin in developed polymeric micelles [29].
5. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are two lipid-based nano-systems that have sparked a great deal of interest for a method of orally delivering hydrophobic drugs with low bioavailability [30]. These lipid nanocarriers offer several advantages including biocompatibility, simplicity of scale-up, enhanced lymphatic transport, and, hence, lower first-pass metabolism. SLNs are the 1st generation of lipidic nanoparticles, measuring around 50 to 1000 nm in diameter, and are composed of an aquatic lipid matrix dispersion (composed of approximately 0.1–30 (% w/w) solid fat dispersed in an aqueous phase) stabilized using surfactants, which is solid at both room and body temperatures [30]. Nevertheless, these systems have several disadvantages, which include inefficient drug loading and the potential for drug leakage [31]. NLCs, similar to SLNs, are innovative SLNs constructed out of solid lipids and liquid lipids. A larger payload, reduced drug leaching during storage, and improved performance in producing the final dosage forms, such as creams, tablets, capsules, and injectables, can be achieved. Moreover, suspensions of higher solid content (e.g., 30–50% solid) and the sustained release of medication are the advantages of NLCs. These liquid oils inside the solid–lipid matrix provide a matrix with a much lower lipid content, which allows for more cargo molecules to be accommodated. For the oral administration of hydrophobic drugs, several investigations have looked into SLN or NLC formulations [32]. Hu et al. developed solid lipid nanoparticles (SLNs) to improve the oral bioavailability of all-trans retinoic acid (ATRA), a poorly soluble drug used as the model drug, and the findings demonstrated that the absorption of ATRA is improved significantly by incorporating it into SLN formulations [33]. Khan et al. evaluated the potential of NLCs for the improvement of the solubility and bioavailability of tacrolimus (TL). He and his colleagues created a tacrolimus-loaded nanostructured lipid carrier for this purpose, and they discovered that it increased the relative bioavailability of TL-NLC by 7.2 times compared to TL suspension [34].
6. Supercritical Antisolvent (SAS)
SAS is far more effective than liquid solvent precipitation and may be utilized as a distinctive, environmentally friendly technique for producing nanomaterials. Supercritical CO2 has been widely used to create a wide range of materials, including polymers, biopolymers, superconductors, explosives, colorants, active pharmaceutical ingredients (APIs), and catalysts. If the processed compounds do not dissolve in the supercritical medium, an antisolvent is used to cause the controlled precipitation of solids to be dissolved in the conventional solvent. These chemicals dissolve in an organic liquid that is miscible with the supercritical antisolvent in the proper processing conditions. In order to produce extremely porous nanoparticles, SAS combines the advantages of the sol-gel method with the use of a supercritical CO2 antisolvent [35]. Conventional micronization techniques such as milling, grinding, and spray drying, which rely on mechanical and thermal stress to disaggregate the active compound, have the drawbacks of the overuse of solvent, the thermal and chemical degradation of pharmaceuticals, polymers, and biologically active proteins, a high concentration of residual solvent, and, most importantly, difficulty in controlling the particle size and distribution during processing. These disadvantages can be overcome via processes based on the use of supercritical fluids, the most common of which is supercritical carbon dioxide (scCO2). The SAS process is predicated on a few key prerequisites. Because scCO2 serves as an antisolvent in this technique, it must be completely miscible with the liquid solvent used. In the literature, the SAS process is most frequently used to create microparticles that increase APIs’ aqueous solubility. Rapid contact between the two media (i.e., antisolvent and polymer/drug solution) speeds up the process of nucleation and growth, resulting in the formation of very fine particles that gives SAS an advantage over all abovementioned conventional methods [36].
Traditional procedures can benefit from novel, more effective micronization techniques such as SCF-assisted particle formation, which can produce solvent-free products in comparatively normal circumstances. Supercritical antisolvent (SAS) methods have the capacity to improve the solubility and bioavailability of a number of medications that are poorly water soluble [36]. Although SCFs are incredibly dense and generally organic solvents are miscible with it, the essential mechanism of the SAS technique is dependent on critical parameters such as temperature, pressure, nature of the solvent, flow rate, and nozzle geometry [36][37]. A SAS experiment begins by pumping CO2 into the precipitator, which is then heated to the desired temperature. After the operating conditions have been stabilized, the pure solvent is delivered to the precipitator via a nozzle. The liquid solution containing the solute/solutes dissolved in the chosen solvent is then injected. The solute(s) precipitates on a filter as a result of supersaturation. The solvent/antisolvent mixture is recovered and separated downstream of the precipitator, where a vessel to collect the liquid solvent is located. After injecting the solution, the scCO2 continues to flow to eliminate the solvent residues. The precipitator is depressurized to atmospheric pressure at the end of this washing step, and the precipitated powder can be collected [38]. The critical conditions when SCFs are extremely dense and usually miscible with organic solvents determine the basic mechanism of the SAS process. Figure 2 displays a schematic of the SAS process.
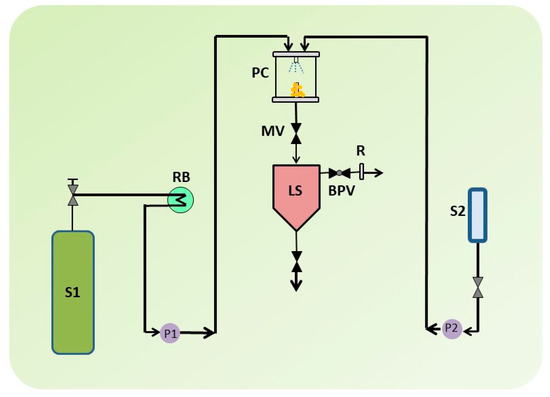
Figure 2. A schematic illustration of supercritical antisolvent (SAS) apparatus. S1: CO2 supply; S2: Liquid solution supply; P1 & P2; Pumps; RB: Refrigerating bath; LS: Liquid Separator; PC: Precipitation Chamber; MV: Micrometric Valve; BPV: Block-pressure Valve; R: Rotameter.
Telmisartan is a BCS class II drug with severely limited solubility in water, but it is easily soluble in strongly alkalized solutions. Telmisartan dissolves in only a few organic solvents. The most significant barrier to reaching the desired bioavailability is the issue of solubility. The SAS technique was utilized to micronize, amorphize, or solid disperse BCS class II drugs in several ways due to its unique properties. The SAS technique has been used for producing solid dispersions of hydroxy propyl methyl cellulose/polyvinylpyrrolidone (HPMC/PVP) at 1:0.5, 1:1, and 1:2 weight ratios of drug to polymer, while pure telmisartan had also been processed. In conformity with the researchers, this SAS technique might be a potential approach for accelerating the dissolving or improving the solubility of telmisartan after adjusting the solid dispersion formulation [39]. Likewise, in order to study fluconazole monohydrate using the SAS method, Park et al. varied the temperature (40, 60, and 80 °C), the pressure (8, 12, and 16 MPa), and the type of solvent (acetone, ethanol, and dichloromethane (DCM)). At high pressure, neither particle precipitation nor nucleation took place (16 MPa). Nevertheless, both were observed when the pressure was being reduced (12 MPa). This is caused by the increased solubility of fluconazole in SC-CO2 at high pressures. Additionally, the product yield increased gradually as the temperature was raised from 40 to 80 °C while maintaining the same pressure. At high temperatures, the organic solvent’s solubility in SC-CO2 increased, causing the solvent to be extracted more quickly, aiding in the drug’s precipitation [40].
7. Nanoemulsions
Nanoemulsions are heterogeneous, thermodynamically stable systems consisting of an oil phase and an aqueous phase with one dispersed in the other with the assistance of the surfactant, where the interfacial film formation of the surfactant provides colloidal stability to the system [41]. Having a droplet size lower than other that of colloidal systems (1–100 nm) confers a greater surface area (e.g., standard emulsions), which assists in improving solubility [42]. Reddy et al. has formulated nanoemulsions of febuxostat, a BCS class II drug used for solubility enhancement. The outcome of their in vitro dissolution study demonstrated that within 6 h, 42.37% of the drug was released from the formulated nanoemulsion; this indicated that the improved solubility of the drug is due to the developed formulation [43]. Ostwald ripening, a foremost destabilization process of nanoemulsions, is a process in which larger droplets grow at the smaller droplets’ expense. This process can be avoided or slowed down by using hydrophobic components in the oil phase followed by a decreased rate of coalescence. Wik et al. prepared a nanoemulsion having an oily phase of a renewable poly (δ-decalactone) (PDL) and Pluronic F-68 as a surfactant via the nanoprecipitation method for the evaluation of drug delivery potential using various hydrophobic drugs. They found that, compared to well-founded Pluronic micelles, the developed nanoemulsion (with droplet size < 200 nm) enhanced the aqueous solubility of the drugs by improving it from 3- to 10-fold [44][45].
8. Nanogels
Nanogels are three-dimensional hydrogel substances with a high capacity to hold water and are generated by crosslinked swellable polymer networks in the nanoscale size range without physically dispersing into the aqueous media [46]. Nanogels are created by physically or chemically crosslinking nanoscale-sized networks, which include networks made of neutral and cationic polymers such as poly (ethylene glycol) (PEG) and polyethylenimine (PEI) [47]. Particle sizes in nanogels range from 100 to 200 nm [48], and altering the solvent quality assists in maintaining the three-dimensional network of the nanogel [49]. They have drawn significant attention as versatile polymer-based nanodrug delivery systems, as they are capable of encapsulating both hydrophilic and hydrophobic molecules. Having large surface areas, good drug loading capacities, and effectiveness for the solubility augmentation of poorly soluble drugs makes nanogels a promising, effective, and safe nanotechnological approach for delivering drugs [50]. Yao et al. developed myricetin (flavonoid) loaded novel nanogel based on chitosan. This strategy promoted a 2.20-fold increase in oral bioavailability compared to plain myricetin in rats [51]. Khan et al. has designed a nanogel system in an attempt to enhance the solubility of olanzapine (OLZ), an antipsychotic drug, via the crosslinking of Poloxamer-407 and 2-acrylamido-2-methylpropane sulfonic acid (AMPS) with the assistance of methylene bisacrylamide (MBA). The results confirmed that in comparison to the free drug, the solubility of olanzapine in nanogel formulation was improved by up to 38 times [52].
9. Metal Organic Frameworks (MOFs)
Metal-organic frameworks (MOFs), which integrate organic ligands with metal ions or metal complexes via coordinative bonding to form a two-dimensional or three-dimensional network, are very porous and crystalline materials that would provide molecular structural flexibility [53]. Due to their customizable physiochemical characteristics (i.e., surface area, modulable porosity, functional moieties, tunable pore size, and pore volume and flexibility to encapsulate significant active ingredient loadings), MOFs have attracted a lot of consideration as drug delivery carriers in the past 10 years [54]. MOFs are an excellent representation of the ability to merge organic and inorganic chemistry, two areas that are sometimes seen as incompatible [55].
Quercetin (Que) exhibits multifunctional pharmacological properties, which include anti-cancer, anti-hypertensive, and antioxidant activities, but is associated with very poor water solubility. In an attempt to overcome this limitation, Wang et al. loaded Que into γ-cyclodextrin metal-organic frameworks (γ-CD-MOFs) in which they observed the 100-fold enhancement of solubility relative to pure Que [56]. One of the promising solutions by Chen et al. for isosteviol’s (STV) insolubility is the use of the highly porous supramolecular carrier cyclodextrin’s metal-organic framework (CD-MOF). STV’s solubility in water was less than 20 ng/mL at a pH of 1.0 and a pH of 4.5, but it was more soluble at a pH of 6.8 and 129.58 ng/mL; therefore, it exhibits pH dependency. The bioavailability of STV@CD-MOF (1:1) was 8.67 times greater than that of STV, 1.32 times greater than that of STV@CD, and 1.27 times greater than that of STV@CD-MOF (0.5:1) in rats [57]. Similarly, to enhance the solubility of Azilsartan (AZL), an angiotensin II receptor antagonist, He et al. designed a γ-CD metal-organic framework (γ-CD-MOF). AZL was effectively confined in biocompatible versatile γ-CD-MOF high molecular cages, resulting in clusters in the nanometer range, improving solubility. Using this method, when compared to the pure drug, the relative solubility of AZL/CD-MOF increased by 340 times; the bioavailability of AZL increased by 9.7 times after loading into the CD-MOF observed in Sprague-Dawley rats [58].
10. Carbon Nanotubes
Carbon nanotubes (CNTs) are a promising carrier in nanotechnology with peculiar electrical, mechanical, chemical, and optical properties. They are cylinder-shaped, allotropic forms of carbon. On the surfaces of CNTs, functional groups are created via functionalization. These functional groups aid in the enhancement of the contact between the CNTs and the matrix or solvent and produce a homogenous dispersion or cause the solubilization of the CNTs. To avoid aggregation and improve their dispersibility, the surface modification of the CNTs is necessary for better interactions with matrix materials and polymer matrices [59][60]. Due to the high water dispersibility of functionalized CNTs, they can serve as the drug’s nucleating sites for hydrophobic compounds and enhance hydrogen bonding with aqueous media, which facilitates fast dissolution [61]. Therefore, the underlying mechanism by which CNTs improve the solubility of drugs that are weakly water-soluble is known as “functionalized partitioning” [62]. Chen et al. introduced CNTs into hydrophobic drugs (griseofulvin and sulfamethoxazole) during synthesis. The results demonstrated that the carrier enhances the dissolution rate of both pharmaceuticals. For CNTs in griseofulvin (4%), it takes 18 min instead of 66 min. For CNTs in sulfamethoxazole (5.1%), it takes 10 min instead of 67 min to obtain 80% dissolution [63]. Further, Zhu et al. formulated dipyridamole CNTs, as it is a poorly soluble drug, and concluded an increase in drug loading; the form of dipyridamole changed from amorphous to crystalline. Moreover, as drug loading into carriers improved, the release rate of the drug dropped and improvement in dissolution rate was perceived. CNTs have been also shown to be promising carriers for loading dipyridamole [64].
11. Mesoporous Silica
Mesoporous silica has been extensively recognized for possessing the ability to improve solubility by adsorbing and thereby stabilizing APIs in their amorphous state within their porous network [65][66]. Consequently, it has been suggested that mesoporous silica materials (MSMs) be employed as matrices to increase the apparent solubility and dissolution rate of poorly water-soluble drug molecules. Because amorphous silica is a “generally regarded as safe” (GRAS) material, biodegradable by hydrolysis, and easily surface-modifiable to enhance drug loading and subsequent release in the human body, mesoporous silica materials are excellent candidates for drug delivery [67]. The primary benefits of mesoporous silica as drug delivery systems for poorly water-soluble drugs are their pore size, pore morphology, and versatility in altering the surface chemistry; the latter can result in optimized interactions between a drug candidate and the mesoporous silica carrier by modifying the pore surfaces [68]. The fundamental property associated with MSMs in this regard is nevertheless their characteristic pore size, which per the IUPAC definition lies in the mesoporous range (2–50 nm). Namely, Rengajaran et al. were able to derive that molecules residing in pores less than 10 times their size remains in amorphous forms due to not having the space to form crystals [69]. This phenomenon has been utilized in the formulation of orodispersable films (prednisolone) [70], fast dissolving tablets (tamoxifen) [71], and lyophilized tablets (silymarin) [72][73]. In one in vivo study, spherical mesoporous silica nanoparticles (MSNs) were developed by Zhang et al. as an oral drug delivery system to enhance the oral bioavailability of the drug telmisartan (TEL). Model drug permeability tests in the human colon cancer (Caco-2) cell lines showed that MSNs may significantly increase TEL permeability and decrease the rate of drug efflux. The oral bioavailability of TEL-laden ordered MSMs, MSNs, and the commercial drug Micardis were investigated in beagle dogs. They found in their studies that the TEL-loaded MSNs formulation had a relative bioavailability of 154.4 ± 28.4% and the TEL-loaded MSMs formulation had a relative bioavailability of 129.1 ± 15.6% [74]. A similar study was later repeated in a clinical setting by Bukara et al. in which they showed that both the absorption rate and the extent was significantly enhanced for fenofibrate loaded into MSMs vs. a marketed micronized formulation [75]. This study served as the first form of evidence for this relatively novel formulation approach.
This entry is adapted from the peer-reviewed paper 10.3390/life13051099
References
- Chen, J. Preparation of Doxorubicin Liposomes by Remote Loading Method. Methods Mol. Biol. 2023, 2622, 95–101.
- Mufamadi, M.S.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Ndesendo, V.M. A review on composite liposomal technologies for specialized drug delivery. J. Drug Deliv. 2011, 2011, 939851.
- Torchilin, P.V.; Torchilin, V.; Torchilin, V.; Weissig, V. Liposomes: A Practical Approach; Oxford University Press: Oxford, UK, 2003.
- Jin, G.-Z.; Chakraborty, A.; Lee, J.-H.; Knowles, J.C.; Kim, H.-W. Targeting with nanoparticles for the therapeutic treatment of brain diseases. J. Tissue Eng. 2020, 11, 2041731419897460.
- Lee, M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264.
- Chaudhary, S.; Garg, T.; Murthy, R.S.; Rath, G.; Goyal, A.K. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J. Drug Target. 2014, 22, 871–882.
- Rao, M.R.; Babrekar, L.S. Liposomal Drug Delivery for Solubility and Bioavailability Enhancement of Efavirenz. J. Pharm. Sci. 2018, 80, 1115–1124.
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49.
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharmacol. 2017, 8, 261.
- D’Emanuele, A.; Attwood, D. Dendrimer–drug interactions. Adv. Drug Deliv. Rev. 2005, 57, 2147–2162.
- Mignani, S.; El Kazzouli, S.; Bousmina, M.; Majoral, J.P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013, 65, 1316–1330.
- Yellepeddi, V.K.; Ghandehari, H. Poly(amido amine) dendrimers in oral delivery. Tissue Barriers 2016, 4, e1173773.
- Bansal, K.K.; Kakde, D.; Gupta, U.; Jain, N.K. Development and Characterization of Triazine Based Dendrimers for Delivery of Antitumor Agent. J. Nanosci. Nanotechnol. 2010, 10, 8395–8404.
- Patel, J.; Garala, K.; Basu, B.; Raval, M.; Dharamsi, A. Solubility of aceclofenac in polyamidoamine dendrimer solutions. Int. J. Pharm. Investig. 2011, 1, 135–138.
- Gautam, S.P.; Verma, A. PAMAM dendrimers: Novel polymeric nanoarchitectures for solubility enhancement of candesartan cilexetil. J. Pharm. Sci. 2012, 1, 1–4.
- Kulhari, H.; Pooja, D.; Prajapati, S.K.; Chauhan, A.S. Performance evaluation of PAMAM dendrimer based simvastatin formulations. Int. J. Pharm. 2011, 405, 203–209.
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3.
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability Enhancement of Poorly Water-Soluble Drugs via Nanocomposites: Formulation(-)Processing Aspects and Challenges. Pharmaceutics 2018, 10, 86.
- Saddam, H.; Abdul Baquee, A.; Jiban, D. Nanosuspension: A promising drug delivery system for poorly water soluble drug and enhanced bioavailability. Int. J. Pharm. Sci. Res. 2020, 10, 4822–4832.
- Aghrbi, I.; Fülöp, V.; Jakab, G.; Kállai-Szabó, N.; Balogh, E.; Antal, I. Nanosuspension with improved saturated solubility and dissolution rate of cilostazol and effect of solidification on stability. J. Drug Deliv. Sci. Technol. 2021, 61, 102165.
- Rao, M.R.P.; Godbole, R.V.; Borate, S.G.; Mahajan, S.; Gangwal, T. Nanosuspension coated multiparticulates for controlled delivery of albendazole. Drug Dev. Ind. Pharm. 2021, 47, 367–376.
- Vinarov, Z.; Katev, V.; Radeva, D.; Tcholakova, S.; Denkov, N.D. Micellar solubilization of poorly water-soluble drugs: Effect of surfactant and solubilizate molecular structure. Drug Dev. Ind. Pharm. 2018, 44, 677–686.
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238.
- Xu, W.; Ling, P.; Zhang, T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv. 2013, 2013, 340315.
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014, 5, 77.
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Front. Cell Dev. Biol. 2006, 3, 139–162.
- Bansal, K.K.; Ali, A.A.; Rahman, M.; Sjöholm, E.; Wilén, C.-E.; Rosenholm, J.M. Evaluation of solubilizing potential of functional poly (jasmine lactone) micelles for hydrophobic drugs: A comparison with commercially available polymers. Int. J. Polym. Mater. Polym. Biomater. 2022, 1–9.
- Ali, A.; Bhadane, R.; Asl, A.A.; Wilén, C.-E.; Salo-Ahen, O.; Rosenholm, J.M.; Bansal, K.K. Functional block copolymer micelles based on poly (jasmine lactone) for improving the loading efficiency of weakly basic drugs. RSC Adv. 2022, 12, 26763–26775.
- Zhou, Z.; Forbes, R.T.; D’Emanuele, A. Preparation of core-crosslinked linear-dendritic copolymer micelles with enhanced stability and their application for drug solubilisation. Int. J. Pharm. 2017, 523, 260–269.
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313.
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76.
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40.
- Hu, L.; Tang, X.; Cui, F. Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. J. Pharm. Pharmacol. 2004, 56, 1527–1535.
- Khan, S.; Shaharyar, M.; Fazil, M.; Hassan, M.Q.; Baboota, S.; Ali, J. Tacrolimus-loaded nanostructured lipid carriers for oral delivery-in vivo bioavailability enhancement. Eur. J. Pharm. Biopharm. 2016, 109, 149–157.
- Santos, S.; Puna, J.; Gomes, J.J.E. A Brief Review of the Supercritical Antisolvent (SAS) Technique for the Preparation of Nanocatalysts to Be Used in Biodiesel Production. Energies 2022, 15, 9355.
- Abuzar, S.M.; Hyun, S.M.; Kim, J.H.; Park, H.J.; Kim, M.S.; Park, J.S.; Hwang, S.J. Enhancing the solubility and bioavailability of poorly water-soluble drugs using supercritical antisolvent (SAS) process. Int. J. Pharm. 2018, 538, 1–13.
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141.
- Franco, P.; De Marco, I. Supercritical antisolvent process for pharmaceutical applications: A review. J. Process. 2020, 8, 938.
- Park, J.; Cho, W.; Cha, K.H.; Ahn, J.; Han, K.; Hwang, S.J. Solubilization of the poorly water soluble drug, telmisartan, using supercritical anti-solvent (SAS) process. Int. J. Pharm. 2013, 441, 50–55.
- Park, H.J.; Kim, M.S.; Lee, S.; Kim, J.S.; Woo, J.S.; Park, J.S.; Hwang, S.J. Recrystallization of fluconazole using the supercritical antisolvent (SAS) process. Int. J. Pharm. 2007, 328, 152–160.
- Tayeb, H.H.; Felimban, R.; Almaghrabi, S.; Hasaballah, N. Nanoemulsions: Formulation, characterization, biological fate, and potential role against COVID-19 and other viral outbreaks. Colloid Interface Sci. Commun. 2021, 45, 100533.
- Pandey, P.; Gulati, N.; Makhija, M.; Purohit, D.; Dureja, H. Nanoemulsion: A novel drug delivery approach for enhancement of bioavailability. Recent Patents Nanotechnol. 2020, 14, 276–293.
- Kanke, P.K.; Pathan, I.B.; Jadhav, A.; Usman, M.R.M. Formulation and evaluation of febuxostat nanoemulsion for transdermal drug delivery. J. Pharm. BioSci. 2019, 7, 1–7.
- Wik, J.; Bansal, K.K.; Assmuth, T.; Rosling, A.; Rosenholm, J.M. Facile methodology of nanoemulsion preparation using oily polymer for the delivery of poorly soluble drugs. Drug Deliv. Transl. Res. 2020, 10, 1228–1240.
- Pyrhönen, J.; Bansal, K.K.; Bhadane, R.; Wilén, C.-E.; Salo-Ahen, O.M.; Rosenholm, J.M. Molecular Dynamics Prediction Verified by Experimental Evaluation of the Solubility of Different Drugs in Poly (decalactone) for the Fabrication of Polymeric Nanoemulsions. Adv. NanoBiomed Res. 2022, 2, 2100072.
- Kendre, P.; Satav, T.J.P.B. Current trends and concepts in the design and development of nanogel carrier systems. Polym. Bull. 2019, 76, 1595–1617.
- Zhang, Y.; Andrén, O.C.; Nordström, R.; Fan, Y.; Malmsten, M.; Mongkhontreerat, S.; Malkoch, M. Off-Stoichiometric Thiol-Ene Chemistry to Dendritic Nanogel Therapeutics. Adv. Funct. Mater. 2019, 29, 1806693.
- Sharma, A.; Garg, T.; Aman, A.; Panchal, K.; Sharma, R.; Kumar, S.; Markandeywar, T. Nanogel—An advanced drug delivery tool: Current and future. Artif. Cells Nanomed. Biotechnol. 2016, 44, 165–177.
- Kaewruethai, T.; Laomeephol, C.; Pan, Y.; Luckanagul, J.A. Multifunctional Polymeric Nanogels for Biomedical Applications. Gels 2021, 7, 228.
- Soni, G.; Yadav, K.S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm. J. 2016, 24, 133–139.
- Yao, Y.; Xia, M.; Wang, H.; Li, G.; Shen, H.; Ji, G.; Meng, Q.; Xie, Y. Preparation and evaluation of chitosan-based nanogels/gels for oral delivery of myricetin. Eur. J. Pharm. Sci. 2016, 91, 144–153.
- Khan, K.U.; Akhtar, N.; Minhas, M.U. Poloxamer-407-Co-Poly (2-Acrylamido-2-Methylpropane Sulfonic Acid) Cross-linked Nanogels for Solubility Enhancement of Olanzapine: Synthesis, Characterization, and Toxicity Evaluation. AAPS PharmSciTech 2020, 21, 141.
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395.
- Lawson, S.; Newport, K.; Pederniera, N.; Rownaghi, A.A.; Rezaei, F. Curcumin Delivery on Metal-Organic Frameworks: The Effect of the Metal Center on Pharmacokinetics within the M-MOF-74 Family. ACS Appl. Bio Mater. 2021, 4, 3423–3432.
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674.
- Wang, Z.; Ma, Y.; Jiang, Y.; Zhou, F.; Wu, Y.; Jiang, H.; Wang, R.; Xu, Q.; Hua, C. Encapsulating quercetin in cyclodextrin metal-organic frameworks improved its solubility and bioavailability. J. Sci. Food Agric. 2022, 102, 3887–3896.
- Chen, X.; Guo, T.; Zhang, K.; Chen, J.; Wang, C.; Ren, X.; Wang, Q.; Yang, Y.; Liu, C.; Tan, W.; et al. Simultaneous improvement to solubility and bioavailability of active natural compound isosteviol using cyclodextrin metal-organic frameworks. Acta Pharm. Sin. B 2021, 11, 2914–2923.
- He, Y.; Zhang, W.; Guo, T.; Zhang, G.; Qin, W.; Zhang, L.; Wang, C.; Zhu, W.; Yang, M.; Hu, X.; et al. Drug nanoclusters formed in confined nano-cages of CD-MOF: Dramatic enhancement of solubility and bioavailability of azilsartan. Acta Pharm. Sin. B 2019, 9, 97–106.
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744.
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-based nanomaterials for delivery of biologicals and therapeutics: A cutting-edge technology. J. Cancer 2021, 7, 19.
- Gomez-Gualdron, D.A.; Burgos, J.C.; Yu, J.; Balbuena, P.B. Carbon nanotubes: Engineering biomedical applications. Prog. Mol. Biol. Transl. Sci. 2011, 104, 175–245.
- Hasnain, M.S.; Nayak, A.K.; Hasnain, M.S.; Nayak, A.K. Applications of carbon nanotubes. In Carbon Nanotubes Targeteted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 33–36.
- Chen, K.; Mitra, S. Incorporation of functionalized carbon nanotubes into hydrophobic drug crystals for enhancing aqueous dissolution. Colloids Surf. B Biointerfaces 2019, 173, 386–391.
- Zhu, W.; Huang, H.; Dong, Y.; Han, C.; Sui, X.; Jian, B. Multi-walled carbon nanotube-based systems for improving the controlled release of insoluble drug dipyridamole. Exp. Med. 2019, 17, 4610–4616.
- Vialpando, M.; Martens, J.A.; Van den Mooter, G. Potential of ordered mesoporous silica for oral delivery of poorly soluble drugs. Ther. Deliv. 2011, 2, 1079–1091.
- Laine, A.L.; Price, D.; Davis, J.; Roberts, D.; Hudson, R.; Back, K.; Bungay, P.; Flanagan, N. Enhanced oral delivery of celecoxib via the development of a supersaturable amorphous formulation utilising mesoporous silica and co-loaded HPMCAS. Int. J. Pharm. 2016, 512, 118–125.
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347.
- Bremmell, K.E.; Prestidge, C.A. Enhancing oral bioavailability of poorly soluble drugs with mesoporous silica based systems: Opportunities and challenges. Drug Dev. Ind. Pharm. 2019, 45, 349–358.
- Rengarajan, G.; Enke, D.; Steinhart, M.; Beiner, M. Stabilization of the amorphous state of pharmaceuticals in nanopores. J. Mater. Chem. 2008, 18, 2537–2539.
- Sen Karaman, D.; Patrignani, G.; Rosqvist, E.; Smatt, J.H.; Orlowska, A.; Mustafa, R.; Preis, M.; Rosenholm, J.M. Mesoporous silica nanoparticles facilitating the dissolution of poorly soluble drugs in orodispersible films. Eur. J. Pharm. Sci. 2018, 122, 152–159.
- Jadhav, V. Formulation and Evaluation of Mesoporous Silica Nanoparticle Loaded Fast Dissolving Tablet of Tamoxifen. Indian J. Pharm. Sci. 2021, 83, 32–38.
- Ibrahim, A.H.; Rosqvist, E.; Smatt, J.H.; Ibrahim, H.M.; Ismael, H.R.; Afouna, M.I.; Samy, A.M.; Rosenholm, J.M. Formulation and optimization of lyophilized nanosuspension tablets to improve the physicochemical properties and provide immediate release of silymarin. Int. J. Pharm. 2019, 563, 217–227.
- Ibrahim, A.H.; Smått, J.-H.; Govardhanam, N.P.; Ibrahim, H.M.; Ismael, H.R.; Afouna, M.I.; Samy, A.M.; Rosenholm, J.M. Formulation and optimization of drug-loaded mesoporous silica nanoparticle-based tablets to improve the dissolution rate of the poorly water-soluble drug silymarin. Eur. J. Pharm. Sci. 2020, 142, 105103.
- Zhang, Y.; Wang, J.; Bai, X.; Jiang, T.; Zhang, Q.; Wang, S. Mesoporous silica nanoparticles for increasing the oral bioavailability and permeation of poorly water soluble drugs. Mol. Pharm. 2012, 9, 505–513.
- Bukara, K.; Schueller, L.; Rosier, J.; Martens, M.A.; Daems, T.; Verheyden, L.; Eelen, S.; Van Speybroeck, M.; Libanati, C.; Martens, J.A.; et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur. J. Pharm. Biopharm. 2016, 108, 220–225.
This entry is offline, you can click here to edit this entry!
