Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hyaluronan (HA) is a naturally occurring non-sulfated glycosaminoglycan (GAG), cell-surface-associated biopolymer, and is the crucial component of tissue extracellular matrix (ECM). HA is a regular polymer of disaccharides composed of glucuronic acid and N-acetylglucosamine linked via alternating β-1,4 and β-1,3 glycosidic bonds.
- hyaluronan (HA)
- reactive oxygen species (ROS)
- reactive nitrogen species (RNS)
- hyaluronan degradation
- signaling
- homeostasis
- disease progression
1. Introduction
Hyaluronan (HA) is a naturally occurring non-sulfated glycosaminoglycan (GAG), cell-surface-associated biopolymer, and is the crucial component of tissue extracellular matrix (ECM). The chemical and structural characterization of GAGs is still a matter of intensive development [1]. HA is a regular polymer of disaccharides composed of glucuronic acid and N-acetylglucosamine linked via alternating β-1,4 and β-1,3 glycosidic bonds (Figure 1).

Figure 1. Chemical structure of two repeating units of HA; (β-D-GlcNAc 1→3 β-D-GlcA 1→4)n; 3-D representations of the low energy conformation of a tetrasaccharide unit, extracted from a longer segment shown in an extended two-fold helical conformation.
This GAG is widely distributed in vertebrate tissues and fluids, displaying remarkable physicochemical properties and biological effects. However, the occurrence of HA in tissues varies with; for instance, the following levels reported: human navel cords (4.10 mg/mL), human joint synovial fluid (1.50–3.60 mg/mL), vitreous humor (0.14–0.34 mg/g), brain (0.035–0.115 mg/g) [2], and human dermis and epidermis (0.20–0.50 and 0.10 mg/g, respectively). On average, HA turnover in vertebrate tissues equals 5 g per day and is provided by biosynthesis and enzymatic degradation [3]. Meanwhile, the turnover of HA in the blood flow reaches 30–100 mg daily.
2. HA Structure and Physicochemical Properties
2.1. Physicochemical Properties
In healthy individuals, HA comprises about 10,000 (with variations) disaccharide units, which correspond to up to 40,000 kDa, and the chain can reach a contour length of several micrometers. HA is an elastoviscous component in synovial fluid that provides a smooth, gliding surface for joints to articulate. Such features are expressed in rheological properties, as HA exhibits a so-called Newtonian behavior at a low shear rate and a marked decrease in viscosity as the shear-rate increases. The HA chains tend to entangle at low concentrations, showing viscoelastic behavior, i.e., while they act as a viscous liquid at low frequencies, they show elastic behavior at a higher frequency. Other fundamental rheological properties (viscosity, static compression, elastic modulus in shear stress, and elastic modulus in compression) confer HA-based hydrogel with the properties that make it suitable for use as fillers in aesthetic medicine to shape the face or to treat the signs of facial aging. In the eye’s vitreous humour, HA supports and maintains a network of collagen fibers that protect the ocular tissues and maintain a clear visual path between the lens and retina. In solid tissues, the content of HA varies widely. Tissues such as cartilage and skin have extensive extracellular matrices in which HA binds and organizes proteoglycans, providing a highly hydrated network to resist tissue compression. Likewise, HA has a crucial role in the brain since this organ lacks the collagenous and elastic networks prevalent in other tissues. Indeed, HA and proteoglycan networks provide an alternative supportive matrix in brain tissue. A key role in the regulation of physiological functions as well as pathological processes has been attributed to HA deposited in the brain [4].
2.2. HA Turnover
Three HA synthase enzymes (HAS1, HAS2, HAS3) localized in the plasma membrane synthesize and export high-molecular weight HA chains to the extracellular environment [5]. Newly synthesized HA chains in healthy tissue can be as large as 6000 kDa. At the cell surface, HA interacts noncovalently with the integral membrane receptor protein CD44 and other receptors through different binding modes that, in unison, define an interaction fingerprint. The conformational flexibility of HA chains facilitates such multivalent binding with proteins based on complementary surface densities in contrast to mere affinity. The multiplicity of interactions creates a complex peri-cellular matrix network that frequently undergoes further dynamic re-modeling and acts as a physiological protection of cells. HA has a high turnover rate at the cellular and tissue levels, mainly due to the enzymatic hydrolysis of hyaluronidases (HYALs), of which HYAL1 and HYAL2 are considered major HA-degrading enzymes in somatic tissue [6]. Indeed, HYAL2 localized to the cell surface is stabilized with a glycophoshatidyloinositol (GPI) anchor or is located in lysosomes, cleaving high molecular weight HA (HMW HA) to smaller, approximately 20 kDa fragments. Upon the internalization and transport of the fragments to lysosomes, further degradation to mainly tetrasaccharides is perpetrated by HYAL1 [6].
HMW HA is bound to receptor CD44 on the cell surface, often in the vicinity of HYAL2. HYAL2 can cleave HA chains of up to 20 kDa, corresponding to a chain of approximately 50 disaccharide residues. Such fragments are then internalized and transported to lysosomes.
Mammalian hyaluronidase, HYAL1, is an endo-β-N-acetyl-hexosaminidases (EC 3.2.1.35; glycosyl hydrolase family) that hydrolyzes the β1→4 glycosidic bond of HA into various oligosaccharide lengths, the shortest of which are tetrasaccharides. For stereochemical reasons, the β1→3 glycosidic bond is resistant to enzymic cleavage. The crystal structure reveals a molecule composed of two closely associated domains: a catalytic domain that adopts a distorted [β/α]8 barrel resembling that of bee venom hyaluronidase and a novel, EGF-like domain, characteristic of involvement in protein−protein interactions and regulatory processes [7]. By catalyzing the hydrolysis of hyaluronan, hyaluronidase lowers the viscosity of hyaluronan, thereby increasing tissue permeability.
Recently, HYBID (hyaluronan binding protein involved in hyaluronan depolymerization), alias KIAA1199/CEMIP, has also been shown to depolymerize HA into small- and intermediate-sized fragments, thereby contributing to HA degradation [8]. Indeed, as discussed by Spataro et al., HYBID depolymerizes HA that has been internalized via clathrin-coated vesicles into early endosomes. Subsequently, the degraded HA fragments are excreted into the extracellular space [9].
2.3. ECM Organization
In native tissues, the noncovalently assembled HA network interacts with another GAG: chondroitin sulfate, which is incorporated into proteoglycans such as versican, neurocan and aggrecan [10]. The HA-based matrix further acts as a protective target for reactive oxygen and nitrogen species (ROS/RNS) generated during inflammation and limits penetration of these species to the cell membrane [11]. However, excessive cleavage/degradation of HA and release of bioactive fragments facilitate pathologies, including inflammation and cancer [12]. Effects other than the ECM organization, such as immunomodulation and various cellular processes, occur. Environmental cues such as tissue injury or infection change downstream signaling functionalities of HA.
HA is susceptible to degradation by hydroxyl radicals, peroxynitrite, and hypochlorite anion, all of which can be created in vivo and are increased during inflammation [13]. Unlike native HA, such fragments of HA have diversified effects on inflammation, cancer, fibrosis, angiogenesis, and the autoimmune response. Inflammation is associated with a potential reduction in HA molecular mass and the resulting production of bioactive “danger signal” fragments throughout cell signaling pathways which depend on the status of the HA component and the peri-cellular matrix [14]. The degradation of HA results in the presence of low molecular weight (LMW) HA fragments, which disrupt the clustering of CD44 receptors at the cell surface while increasing the availability for interactions with other receptor proteins such as TLR4, TLR2, and RHAMM [15].
2.4. Mechanisms for HA Degradation by Reactive Oxygen Species (ROS)
In vivo, degradation of HA may arise from hydroxyl radicals, peroxynitrite, and hypochlorite anion and is likely to be increased in the context of inflammation. There exists rich literature describing the mechanisms leading to the production of reactive oxygen species (ROS) or reactive nitrogen species (RNS) in biological systems [13], as well as attempts at mathematical and computational modeling [16]. ROS/RNS can be classified as either free radicals containing one unpaired electron or nonradicals. The former generally includes superoxide radical (O2•−), hydroxyl radical (●OH), NO radical (●NO). peroxyl (ROO●), and alkoxyl radicals (RO●). The latter contains hydrogen peroxide (H2O2), hypochlorous acid (HClO), peroxynitrite (ONOO−), ozone (O3), singlet oxygen (1O2), aldehydes, and organic peroxides [17].
Superoxide anion radical (O2•−): The reduction of the oxygen molecule is the reaction by which animal cells produce metabolic energy (O2 + 4e− + 4H+ = 2H2O). Along this reaction, several sub-cellular structures reduce O2 molecules producing the superoxide anion radical O2•−, which in an aqueous (acidic) milieu forms per hydroxyl radical ●O2H. O2•−is formed in neutrophils, monocytes, macrophages, and eosinophils due to the action of NADPH oxidase enzyme, also called respiratory burst oxidase. NADPH oxidase, a highly regulated enzyme complex composed of several proteins, reduces oxygen to a superoxide anion radical at the expense of NADPH [18]. Hydroxyl Radical ●OH, the hydroxyl radical, is the most reactive ROS. The degradation of HA by hydroxyl radicals and its protection by free radical scavengers are well documented. The source of free radicals in biological systems is termed the Fenton reaction, which implies the participation of a transition metal cation (e.g., Fe2+):
[(Fe2+ + H2O2 → Fe3+ + OH− + ●OH]
In this reaction, Fe3+ can be reduced back to Fe2+ [19].
The hydroxyl radical can abstract hydrogen atoms at all ring C-H bonds except C-2 of N-acetyl hexosamine. The abstraction of hydrogen atoms generates carbon-center radicals. The radicals at carbons, which form glycosidic bonds, undergo a β-scission reaction resulting in the breakdown of the HA chains [20] (Figure 2). Under physiological (healthy) conditions, the iron ions are always firmly bound. In blood, they circulate associated with protein transferrin, and in cells, they are stored and linked to the protein ferritin. Nevertheless, cells observe an increase in the so-called “labile iron pool” under stress conditions. The source of superoxide anion in biological systems are activated PMNs (polymorphonuclear leukocytes).
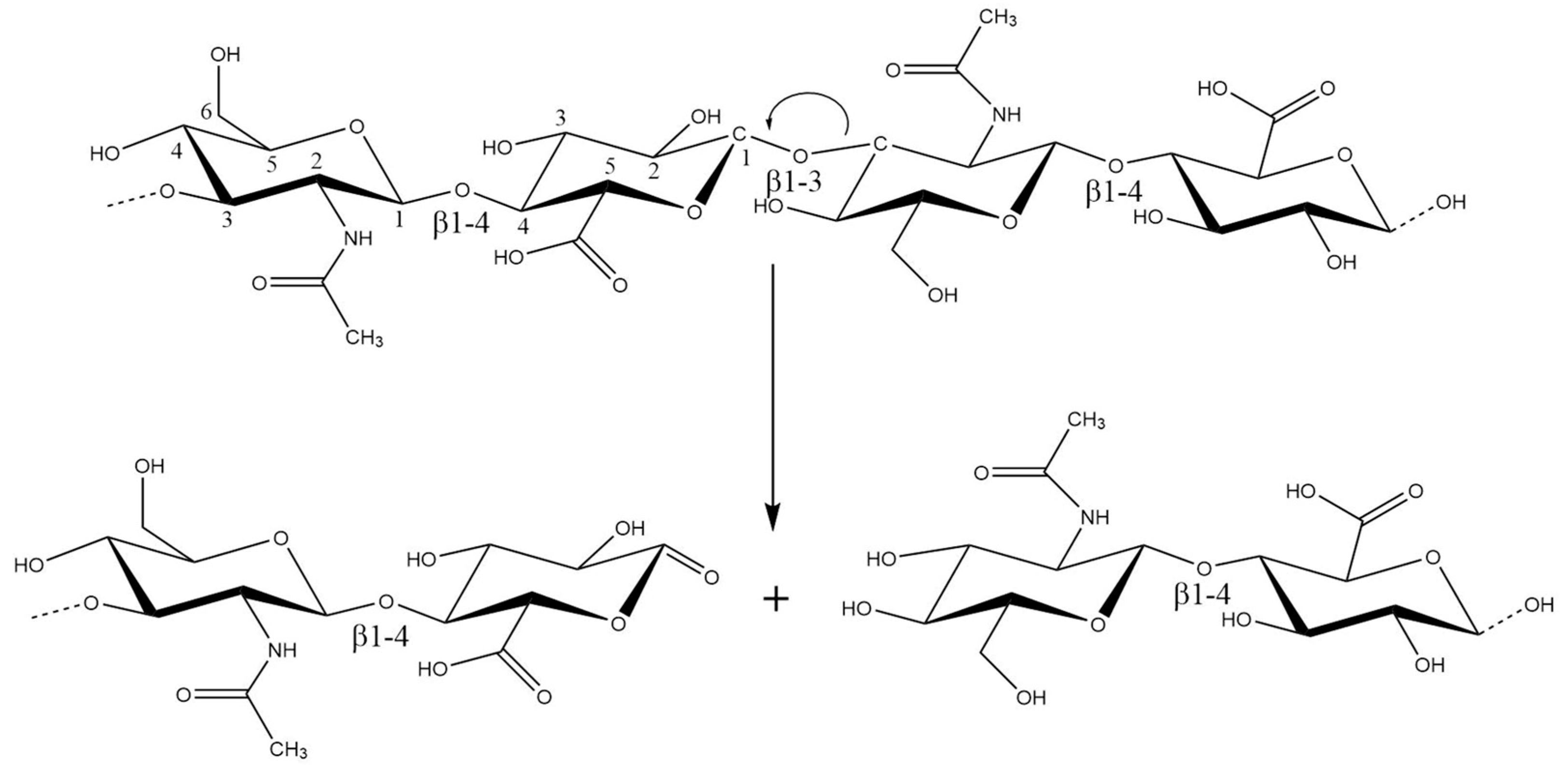
Figure 2. The radicals at carbons, which form glycosidic bonds, undergo a β-scission reaction resulting in the breakdown of the HA chains at C1 of the GlcA residue.
Nitrogen Monoxide (●NO): The bioactive free radical nitrogen monoxide (●NO) is produced in various cells/tissues by the enzyme NO synthase. O2•−and ●NO are the precursors of different reactive oxygen species (ROS), including hydrogen peroxide, peroxynitrite, and hypochlorous acid.
Hydroxyl Peroxide: H2O2 is produced from two superoxide anion radical species (O2•−+ O2•−+ 2H+ → H2O2 + O2). In vivo, the family of superoxide dismutases (SOD) catalyzes such a reaction (the cytosolic SOD form contains Cu and Zn, and the mitochondrial form contains Mn).
Hypochlorous acid (HOCl) and Hypochlorite (OCl−): Myeloperoxidase enzymes of PMNs can generate hypochlorite ions (H2O2 + Cl−OCl− + H2O). Methionine blocks this reaction. Hypochlorite anions may function by participating in the production of hydroxyl radicals. N-acetyl residues react with hypochlorite to generate polymer-derived N-chloro derivatives. The decomposition of these derivatives gives rise to nitrogen-centered radicals. They undergo rapid intramolecular abstraction reactions to give carbon-centered radicals at C-2 on the amino sugar rings via a 1,2-hydrogen atom shift [21] and/or at C-4 on the neighboring glycosidic residues (via 1,5-hydrogen atom shifts) [22]. These products cleave glycosidic bonds through β-scission [21].
Peroxynitrite (ONOO−): Peroxynitrite can be generated by the reaction of nitric oxide, produced by the nitric oxide synthase enzymes (NOS), with superoxide anion, released by activated polymorphonuclear leukocytes (PMN) (O2•−+ ●NO → ONOO−), which yields the formation of two radicals: (ONOO− + H+ → ONOOH → ONO− + ●OH). An increased expression of NOS is common in inflammatory disease processes. Peroxynitrite degrades HA via a hydroxyl radical-like mechanism [23].
ROS-generated HA fragments may not have the same biological effects as those created by enzymatic cleavage. Therefore, several factors must be considered to characterize ROS-induced alterations to GAGs in vivo [17][24]. The first factor is the GAGs’ proximity to the adjacent ROS/RNS production sites, as these highly reactive species have a relatively short life span. Furthermore, the concentration and levels of antioxidation need to be sufficiently high and low, respectively. ROS-mediated HA degradation occurs randomly and generates products with a polydisperse size. Not only are glycosidic linkages cleaved, but there is also the possibility of other structural changes, including ring opening and different conformational characteristics [25], which would affect their downstream reactions. As a result of their depolymerization effects, ROS/RNS could serve as the substrates for the following enzymatic cleavage, thereby facilitating further HA degradation. One possible explanation is that the partial degradation of HA would overcome steric factors which prevent the interaction between the hydrolases and their highly polymerized substrates.
This entry is adapted from the peer-reviewed paper 10.3390/antiox12040824
References
- Perez, S.; Makshakova, O.; Angulo, J.; Bedini, E.; Bisio, A.; de Paz, J.L.; Fadda, E.; Guerini, M.; Hricovini, L.; Hricovini, M.; et al. Glycosaminoglycans: What Remains to be Deciphered. J. Am. Chem. Soc. Au 2023.
- Laurent, U.B.; Tengblad, A. Determination of hyaluronate in biological samples by a specific radioassay technique. Anal. Biochem. 1980, 109, 386–394.
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33.
- Perkins, K.L.; Arranz, A.M.; Yamaguchi, Y.; Hrabetova, S. Brain extracellular space, hyaluronan, and the prevention of epileptic seizures. Rev. Neurosci. 2017, 28, 869–892.
- Weigel, P.H. Systemic Glycosaminoglycan Clearance by HARE/Stabilin-2 Activates Intracellular Signaling. Cells 2020, 9, 2366.
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839.
- Chao, K.L.; Muthukumar, L.; Herzberg, O. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry 2007, 46, 6911–6920.
- Spataro, S.; Guerra, C.; Cavalli, A.; Sgrignani, J.; Sleeman, J.; Poulain, L.; Boland, A.; Scapozza, L.; Moll, S.; Prunotto, M. CEMIP (HYBID, KIAA1199): Structure, function and expression in health and disease. FEBS J. 2022.
- Yoshida, H.; Nagaoka, A.; Kusaka-Kikushima, A.; Tobiishi, M.; Kawabata, K.; Sayo, T.; Sakai, S.; Sugiyama, Y.; Enomoto, H.; Okada, Y.; et al. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc. Natl. Acad. Sci. USA 2013, 110, 5612–5617.
- Monslow, J.; Govindaraju, P.; Pure, E. Hyaluronan—A functional and structural sweet spot in the tissue microenvironment. Front. Immunol. 2015, 6, 231.
- Gao, L.; Lipowsky, H.H. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc. Res. 2010, 80, 394–401.
- Dogne, S.; Flamion, B. Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am. J. Pathol. 2020, 190, 768–780.
- Cowman, M.K. Hyaluronan and Hyaluronan Fragments. Adv. Carbohydr. Chem. Biochem. 2017, 74, 1–59.
- Kavasi, R.M.; Berdiaki, A.; Spyridaki, I.; Corsini, E.; Tsatsakis, A.; Tzanakakis, G.; Nikitovic, D. HA metabolism in skin homeostasis and inflammatory disease. Food Chem. Toxicol. 2017, 101, 128–138.
- Nikitovic, D.; Tzardi, M.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N. Cancer microenvironment and inflammation: Role of hyaluronan. Front. Immunol. 2015, 6, 169.
- Kavdia, M. Mathematical and computational models of oxidative and nitrosative stress. Crit. Rev. Biomed. Eng. 2011, 39, 461–472.
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650.
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free. Radic. Biol. Med. 2009, 47, 344–356.
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74.
- Rees, M.D.; Kennett, E.C.; Whitelock, J.M.; Davies, M.J. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic. Biol. Med. 2008, 44, 1973–2001.
- Rees, M.D.; Davies, M.J. Heparan sulfate degradation via reductive homolysis of its N-chloro derivatives. J. Am. Chem. Soc. 2006, 128, 3085–3097.
- Rees, M.D.; Hawkins, C.L.; Davies, M.J. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. Biochem. J. 2004, 381, 175–184.
- Kennett, E.C.; Davies, M.J. Degradation of matrix glycosaminoglycans by peroxynitrite/peroxynitrous acid: Evidence for a hydroxyl-radical-like mechanism. Free Radic. Biol. Med. 2007, 42, 1278–1289.
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286.
- McNeil, J.D.; Wiebkin, O.W.; Betts, W.H.; Cleland, L.G. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann. Rheum. Dis. 1985, 44, 780–789.
This entry is offline, you can click here to edit this entry!
