With rapidly increasing environmental pollution, there is an urgent need for the development of fast, low-cost, and effective sensing devices for the detection of various organic and inorganic substances. Silver nanoparticles (AgNPs) are well known for their superior optoelectronic and physicochemical properties, and have, therefore, attracted a great deal of interest in the sensor arena. The introduction of AgNPs onto the surface of two-dimensional (2D) structures, incorporation into conductive polymers, or within three-dimensional (3D) nanohybrid architectures is a common strategy to fabricate novel platforms with improved chemical and physical properties for analyte sensing. In the first section of this review, the main wet chemical reduction approaches for the successful synthesis of functional AgNPs for electrochemical sensing applications are discussed. Then, a brief section on the sensing principles of voltammetric and amperometric sensors is given. The current utilization of silver nanoparticles and silver-based composite nanomaterials for the fabrication of voltammetric and amperometric sensors as novel platforms for the detection of environmental pollutants in water matrices is summarized. Finally, the current challenges and future directions for the nanosilver-based electrochemical sensing of environmental pollutants are outlined.
- silver nanoparticles
- chemical reduction
- voltammetric sensors
- amperometric sensors
- environmental analysis
1. Introduction
2. Application of Silver Nanoparticles in Voltammetric and Amperometric Sensors
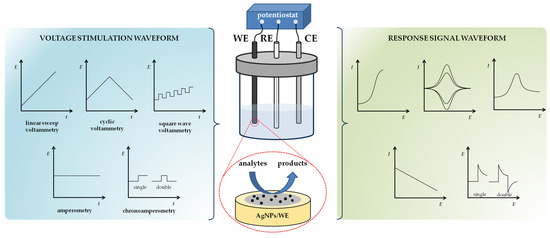
2.1. Working Principles of Voltammetric and Amperometric Sensing Techniques
2.2. Electrochemical Sensors for Detection of Heavy Metal Ions
2.2.1. Electrochemical Sensors for Divalent HMs
3.2.2. Electrochemical Sensors for Trivalent and Hexavalent HMs
3.3. Electrochemical Sensors for Nitrogen-Containing Inorganic Species
3.3.1. Electrochemical Sensors for Nitrite (NO2−) and Ammonium (NH4+) Detection
3.3.2. Electrochemical Sensors for Nitrate (NO3−) and Ammonia (NH3) Detection
3.4. Electrochemical Sensors for Phenolic Compounds
3.5. Electrochemical Sensors for Pharmaceuticals
3.6. Electrochemical Sensors for Nitroaromatics
3.7. Electrochemical Sensors for Natural and Synthetic Estrogens
4. Discussion
4.1. Electrochemical Sensor Technology
Electrochemical sensor technology has become an important aspect of modern analytical chemistry, and great efforts are being made to develop novel and cost-effective sensing platforms that provide a fast, accurate, and repeatable response to the analyte of interest. Compared to ubiquitous colorimetric sensors, the main advantage of electrochemical detection is the ability to measure in turbid samples [18]. Moreover, working electrodes can also be made in a planar and/or flexible mode [67,113,143,148], i.e., they can be easily miniaturized and integrated into more hierarchical devices [186]. Electrochemical sensors can be used in various fields, including pharmaceuticals, medicine, chemistry, synthesis, materials engineering, and biotechnology [137,187,188]. In addition, electrochemical sensors based on disc (3D) or planar (2D) electrodes decorated with AgNPs have proven to be simple but reliable analytical tools for the rapid and selective detection of emerging water pollutants (i.e., heavy metal ions, nitrogen-containing inorganic species, phenolic compounds, nitroaromatics, pharmaceuticals, and natural and synthetic hormones), which is highlighted in this review.
4.2. Wet Chemical Synthesis of AgNPs for Sensing Applications
For the fabrication of highly selective and sensitive voltammetric and amperometric electrochemical sensors, the usage of silver nanomaterials with precise particle size, shape, crystalline facets, and morphology is essential. Hence, controlled synthesis is a paramount for achieving excellent electrocatalytic activity for practical sensing application. AgNPs are usually synthesized using a chemical reduction from soluble silver(I) precursors (mostly AgNO3), and many of these synthetic processes are carried out under harsh reaction conditions applying toxic hydrazine [45] or borohydride [113,163,166] reducing agents and/or (volatile) organic solvents [149]. Although there has been more recent focus towards green synthesis [31,38,189], in 64 of the 86 electrochemical sensors presented in this review the reduction of silver(I) to zero-valent form was assisted by electric current or inorganic reducing agents. It is important to emphasize that if the chemical reduction process using strong inorganic agents is precisely controlled, i.e., if no hazardous chemicals are released into the environment during the synthesis [44], such an approach does not necessarily contradict to the principles of green synthesis. Moreover, AgNPs of smaller size and cleaner particle shapes can be produced with strong reducing agents: (i) nanoball-shaped AgNPs (d = 5–6 nm) via the polyol process [184], (ii) spherical crystals (mean diameter of 10.6 nm) obtained via borohydride reduction [163], or (iii) globular particles with a diameter of ~20 nm via ethanolamine reduction [135]. On the other hand, biosynthesized AgNPs generally exhibit irregular morphologies, are polydisperse, and often form larger particle agglomerates [67,129,171]. Nevertheless, even strong reducing agents can render AgNPs of various morphologies (quasi-spherical, cubic, twinned structure, and triangles) and larger size distributions when deposited on highly wrinkled and folded graphene nanosheets [155], or when the Ag(I) precursor is previously encapsulated with a massive chelating ligand [156], while a precisely controlled green approach can produce remarkably small particles (d = 5 nm) with high catalytic efficiency [168]. AgNPs of precise size and shape can also be formed and fine-tuned using electric current [79,80,119,182]. In electrochemical sensors based on electrodeposited silver nanomaterial [81,148], the addition of silver material was found to play a key role in the trace-level quantification of both trivalent and hexavalent chromium through formation and stabilization of functional bimetallic metal oxides [81], and in the formation of dual-region WE for simultaneous detection of inorganic nitrogen-containing species (nitrate/ammonia) through a signal current channel [148].
4.3. Electroanalytical Techniques for Characterization of AgNPs-Modified Electrodes and Analyte Quantification
In general, cyclic voltymmetry and impedance measurements are used to study the changes in the interfacial phenomena between the sensor body and solution when different electrode coating materials are used. In other words, CV and the EIS technique are indispensable tools to observe in detail the stepwise evolution of the electrochemical sensor fabrication pathway. To implement both techniques the usage of a ferri/ferrocyanide redox probe is needed, and measurements are usually performed in aqueous 0.1 M KCl [103,133,141,171,176] or in a phosphate buffer solution [159,166]. With the introduction of AgNPs as electrode modifier material, both the increase of current peak and the reduction in peak separation are noticeable with cyclic voltammetry [184]. The AgNPs modification of the bare electrode is also evident in electrochemical impedance spectroscopy, where the value of electron transfer resistance, which corresponds to the semicircle diameter of the Nyquist diagram, is significantly lower compared to the bare electrode (Figure 10). Furthermore, the diffusion-limited process is represented by the linear portion of the Nyquist plot at lower frequencies, while the process is related to the part of the diagram at higher frequencies. In summary, the specific role of the nanosilver material in the exchange of the electron transfer rate between the electroactive species (redox couple) and the modified sensing surface is attributed to the enhancement of the maximum peak current (CV analysis) and the diminution of the impedance (EIS analysis).
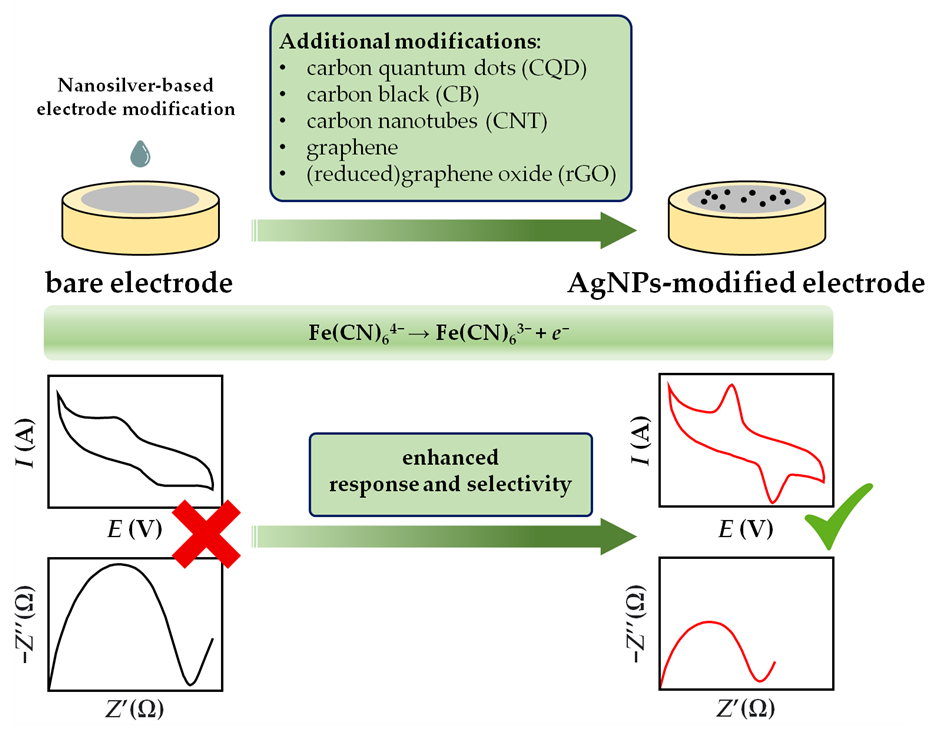
Figure 10. Schematic illustration of AgNPs electrochemical signal boost through the changes in current (CV) and impedance (EIS) responses on bare and nanosilver-modified electrodes.
Voltammetry and amperometry are particularly attractive techniques for the analysis of complex sample matrices such as various environmental waters (groundwater, lakes, rivers, streams, seawater, etc.) The choice of voltammetric technique depends primarily on the location of the sample and the expected concentration of the analyte. Although CV can be used to determine analyte concentration [65,97,138,148], DPV or SWV methods have been shown to be better for quantitative analysis. SWV is known for its superior sensitivity and measurement speed compared to DPV technique [68]. For greater sensitivity, analyte preconcentration can be performed by adsorptive square wave stripping voltammetry [69,177].
4.4. Mechanisms of AgNPs-based Voltammetric and Amperometric Sensors
The detection mechanism of nanosilver-based voltammetric and amperometric sensors for water pollutant monitoring summarized in this review is based on several different mechanisms: (i) AgNPs aggregation; (ii) AgNPs displacement; (iii) AgNPs electronic conductive channels enhancement/inhibition via selective site recognition; and (iv) OR reaction of inorganic/organic water pollutants.
(i) Aggregation of AgNPs triggered by the addition of the analyte is a common optical sensing approach that can be converted to voltammetric [98] and amperometric sensors [101]. This strategy is feasible usually in the form of dual- [98,99] or multisensing [102,106,111,122] platforms, with optical, fluorescent, and electrochemical response towards the detection of HMs. In contrast to the colorimetric assay, electrochemical aggregation of functional silver material results in voltammetric/amperometric signal amplification or inhibition, depending on the chemistry of the nanoparticle stabilizer and the target analyte. For the aggregation-induced sensing mechanism, the role of biogenic synthesized AgNPs is highlighted because biogenic molecules are rich in electron-donor groups that successfully act as analyte-chelating ligands. AgNPs synthesized using fungus Agaricus bisporus [98] and the Mimosa diplotricha leaf extract [99] were found to be potent sensors towards hazardous mercury, boosting the DPV responses through strong stabilizer complexation. Green AgNPs also play a prominent role as multisensor probes for mercury [102], cadmium [106], arsenic [120], and chromium [122].
(ii) Electrochemical sensors based on the displacement of functional nanomaterials are considered a novel tool for highly selective and sensitive detection of analytes. In two examples of the reviewed scientific papers, the mechanism of mercury detection was associated with the removal of the surface-bound stabilizer (calixarene moiety), leaving the AgNPs uncapped [101], or with galvanic displacement of silver by mercury [104]. As thermodynamically unstable species, the bare nanoparticles tend to aggregate into larger clusters, or form a amalgam [101]. This leads to signal inhibition, which is visible in the optical mode by a reduction in the SPR band in the UV-Vis spectrum, and in the amperometric mode by a reduction in current output. In the galvanic displacement method, the AgNPs interact with divalent mercury ions in the solution and convert the zero-valent silver into ionic silver species, resulting in loss of stripping signal. Since only small amounts of the analyte are required to displace measurable amounts of silver, this sensor can quantify inorganic mercury in the picomolar range.
(iii) Aggravation or enhancement of the electron transfer pathway of AgNPs-modified WE leads to the amplification or decrement of voltammetric performance. Two voltammetric platforms based on MIPs supported by AgNPs-decorated GCE have been developed for highly selective and sensitive detection of natural estrogen in environmental and drinking water with picomolar and femtomolar detection limits [182,183]. The detection of the analyte is catalyzed by the AgNPs (signal amplification or inhibition), while the analyte capture and selective recognition were performed by the MIPs. In the presence of E2, more imprinted cavities of the poly(p-aminophenol) (MIP-pAPh) were occupied, resulting in direct amplification of the current response by AgNPs signal amplification [182]. The reverse sensing mechanism was proposed for the AgNP/GO/MIP (poly-imidazole; PImi) functional platform [183]. In addition to the imidazole moiety (p-type-electron acceptor), additional functional sites of GO (n-type electron donor) were available for analyte binding, which blocked the diffusion of the ferri/ferrocyanide redox probe at the electrode/solution interface, reducing the SWV current responses.
(iv) Voltammetric and amperometric sensors, which detect inorganic/organic pollutants based on their OR response, are the most represented electrochemical sensor class. AgNPs increase the active surface area of the bare and/or modified electrode, facilitate electron transfer, and serve as active catalytic sites for oxidation/reduction of the aquatic pollutant. It has been reported that the reaction rate constant (k) is proportional to the total effective surface area of the nanosilver material [190]. Therefore, the homogeneity of particle size and shape of AgNPs anchored on sensing platforms is of utmost importance for catalytic efficiency. The adsorption of the analyte (the first step in the sensing mechanism) is more promoted on small particles with larger effective surface area, which facilitates the OR process of electroactive species on the modified electrodes [135]. The interactions between the AgNPs, electrode support and/or other electrode modifiers, and the target analyte, depends on the nature of the surface-active species. On the one hand, stabilizers can be interpreted as a barrier between the AgNPs and the electrode material, controlling the electron transfer kinetics and, thus, affecting the analytical performance of the sensor, especially in terms of achieving lower detection limits [103]. Therefore, clean and uncovered active surface sites of nanoclusters are preferred for catalytic and sensing applications [154]. However, since unprotected AgNPs tend to aggregate, the presence of a stabilizer is extremely important. The catalytic activity of green-capped AgNPs can be altered by the size of the stabilizer (bulky ligands or small molecules) [66,166], and by the adsorption of intermediary molecules (formed during the sensing mechanism) onto the biostabilizers, as was the case in the amperometric quantification of nitrobenzene [172]. The determination of nitrites based on their irreversible electrochemical oxidation via two-electron transfer was reported for various AgNPs-functionalized electrodes, i.e., nanospheres decorated GCE [129], core-shell Au@Ag structure anchored over carboxylated graphene/GCE [86], AgNPs-(r)GO platform [130,131], AgNPs/rGO nanohybrid in conjunction with PPy [133] and PANI [134] conducting polymers, AgNPs/ZnO nanocomposite [136], and AgNPs/HNT/MoS2 platform [139]. In contrast, one-electron transfer was demonstrated in nitrite sensing with a nanosilver-polymer composite (chitosan, PEDOT:PSS) [142], and also with an AgNPs-MWCNTs hybrid platform [74]. AgNP/SWCNT/CPE was used to detect DNL by the irreversible oxidation of phenolic groups to (semi)quinone species [84]. The overall chemical–electrochemical mechanism involves a transfer of two protons and two electrons, but the final product depends on the applied potential and the electrode modifier used. Oxidation products of E3, obtained using GCE coated with AgNP/CNB [184] and AgNPs-rGO [185], correspond to ketone derivatives. For ultrasensitive detection of AM and AT drugs, an oxidation mechanism involving rapid electron transfer from the analytes through the COOH-CNTs/Ag/NH2-CNTs ternary sandwich architecture to the GCE was proposed [177]. The overall electrochemical oxidation (single SWASV profile) of the electroactive center 1,4-dihydropyridine (AM) occurs through a two-electron/two-proton process, whereas the pyrrole center of AT exhibits two-electron/one-proton transfer. The reversible oxidation of CC and HQ phenolic compounds to o- and p-benzokinone species via a two-electron/two-proton transfer was mediated by the cobalt active site of the AgNPs/TACoPc/PANI ternary composite using the DPV technique [153] and with AgNPs/Fe3O4-rGO hybrid material [87]. Quantitative determination of BPA via electrooxidation to 2,2-bis(4-phenylquinone) was successfully performed with an AgNPs-rGO composite on a ITO substrate [67], and with an AgNPs-rGO/PLL-coated GCE [45]. Conversely, several phenolic compounds were detected using the reduction mechanism. The nitro groups of 4-nitrophenol [154,156,158], 2,6-dinitrophenol [161], 4-nitrotoluene [174], nitrofurantoin [176], and nitrobenzene [170–172] were irreversibly converted into hydroxylamine or aminophenol groups by an electrochemical mechanism involving four protons and four electrons. Moreover, nitrobenzene was detected on a silicate sol–gel matrix GCE functionalized with AgAu alloy, depending on the electrochemical reduction of the nitro group involving six protons and six electrons [83]. Gold electrode coated with AgNPs [146,149], in conjunction with the SWV technique, proved to be a highly efficient combination for the detection of nitrates in seawater due to their two-electron reduction to nitrites. In addition, nitrate was detected on a Cu(II)-terephthalate decorated SPCE via a Cu2+/Cu+ redox couple [46]. The adsorption of nitrates is promoted on the Cu(I) active site by copper–oxygen coordination interactions, which is further supported by the charge transferability of MOFs and the enhanced transfer of two electrons to the catalytic AgNP sites.
4.5. Design of AgNPs-based Voltammetric and Amperometric Sensors for Detection of Aquatic Pollutants
The choice of bare electrode substrate (supporting material) is of paramount importance in manufacturing process of electrochemical sensors. The common denominator in the use of pure metal electrodes (Au or Pt), or bare carbon-based electrodes (GCE, GPE, PGE, CPE, SP(C)E, etc.), is the difficult electron transfer between the electroactive analyte molecule and the sensing electrode materials. This leads to low selectivity towards the analyte (the redox reaction is possible at potentials substantially higher than thermodynamic potentials), and inability to distinguish the target molecule from interfering species in real matrices. Therefore, modification of bare electrodes with (hybrid) nanomaterials, especially noble metallic nanoparticles, offers a unique combination of excellent catalytic and sensory properties [65,75,191]. Silver nanoparticles outperform gold and platinum nanomaterials as a low-cost and efficient electrocatalysts for practical sensing applications. Moreover, the synergistic effects of bimetallic [69,86,164], AgNPs/metal oxide composites [109,136] can be also used to promote and/or enhance catalytic sensing.
Due to its diverse structural and morphological forms, carbon is remarkably important and one of the mostly widely used electrode materials [192]. In 54% of the voltammperometric sensors presented in this review, GCE was used as the core material. The main advantages of GCE are its resistance to acidic and alkaline environments and the improved adhesion of the AgNPs to the glassy carbon material [193]. Only a few examples of GCE modified exclusively with AgNPs for the detection of nitrites [65,75,129], nitrobenzene [171,172], and 4-nitrophenol [154] have been found; the vast majority of GCE-based electrochemical sensors reviewed in this manuscript are modified with composite silver (nano)materials. One of the frequently used strategies in the development of novel sensor surfaces is the anchoring of AgNPs on the surface of 2D nanomaterials, mostly graphene-derived, due to their large surface area, high chemical stability, and mechanical strength [9,194]. The GO platform is rich in oxygen-containing functional groups, which greatly improves its hydrophilicity and functionality. Therefore, GO is easily dispersed in both water and organic solvents to obtain a homogeneous dispersion, and can be easily applied to electrode substrates by simple immersion or drop casting technique [130,135]. In addition, GO has an enhanced ability to capture target molecules due to its rich edge oxygen chemistry, a property that has been used to develop a nitrite sensing platform [130]. To increase the sensitivity of the sensor, the strategy of combining AgNPs with a more conductive reduced GO form (restoration of sp2 hybrid carbon networks) was developed [45,87,155,185]. Thus, (r)GO serves as an outstanding platform for the immobilization of various electroactive species through covalent or noncovalent bonds. In addition to (r)GO sheets, other 2D layered structures with semiconducting properties such as activated C3N4 [141,170,181], MOFs [46,114], and transition metal dichalogenides [168] have also emerged as prospective candidates for improved catalyst supports for simultaneous voltammetric detection of HMs, selective oxidation of nitrite, amperometric nitrate detection, and selective dopamine quantification, respectively. 3D MWCNTs, especially functionalized by acid treatment, possess excellent thermal and electrical conductivity, which, combined with their large surface area, significantly improves the catalytic properties for the oxidation of ammonium and nitrite species [74,138,144], and the detection of phenolic compounds [80,152] in real water matrices. In only one scientific paper, SWCNTs were used in conjunction with AgNPs as a sensing surface for the quantification of the synthetic estrogen dienestrol in river water [84]. Another approach to increase the sensitivity is to use the CPs in the nanosilver-based sensing hierarchy in the form of an AgNPs@p-1,8-DAN layer [73] or an AgNPs/CB/PEDOT:PSS film [163] built over disc GCE, or ternary hybrid structures of nanosilver on the (r)GO platform with PPy [133] or PANI [134]. Due to their (high) conductivity, redox reversibility, long-term environmental stability, high solution process ability, and simple synthetic procedures with controllable thickness on the sensing electrode, CPs are often employed in voltammperometric sensor design. Synthetic MIPs based on GCE sensing platforms are the best alternative to circumvent the stability and cost issues associated with biological receptors traditionally used for the detection of (bio)molecules [187]. Since the size, shape, and orientation of the recognition sites of the cavities imprinted in MIP directly reflect the properties of the analyte, the molecular imprint technology is more than efficient for the development of highly selective sensors. MIPs in combination with AgNPs and AgNPs/GO composites have proven to be exquisite sensing platforms—picomolar and femtomolar LOD values have been achieved for the detection of the endocrine-disrupting 17-β-estradiol in real water matrices [182,183].
In addition to GCE, another robust carbon-based 3D electrode—CPE—can be quickly fabricated, modified with various (nano)materials, and its surface is easy to clean. Although their sensitivity is (usually) lower than that of GCEs, CPEs decorated with AgNPs have proven to be a reliable tool for amperometric detection of synthetic estrogen (nM LOD) [84], amperometric quantification of nitrites in aquatic solutions (μM LOD) [139], selective voltammetric reduction of p-nitroaniline in wastewater (nM LOD) [175], and electrochemical sensing of lead ions in tap water and wastewater specimens (μM LOD) [108]. Another simple and inexpensive form of a carbon-based electrode, with advantages such as high mechanical strength, good quality, stability, and reproducibility, is the PGE [195]. This user-friendly electrode can be used as disposable electrode, eliminating the time-consuming cleaning of solid electrodes. A PGE sensing probe modified with an AgNP-ZnO nanocomposite has been successfully used for the determination of nitrites in lake water and pickled water samples [136], and one decorated with biosynthesized AgNPs was selective towards divalent mercury [97].
Noble metal electrodes, such as gold and platinum material, find application in a variety of electrochemical processes due to their high electrical conductivity, mechanical stability, and chemical resistance. Nevertheless, only a few electrochemical sensors based on nanosilver-decorated noble metal electrodes have been presented in this review. Gold (Au) disc electrodes predominate in the development of voltammetric sensors for the detection of arsenic in river water and lake water [50,119,120], but are also suitable as substrates for the selective and sensitive detection of nitrates in seawater matrices [146,149]. Platinum (Pt) electrodes, decorated with AgNPs biosynthesized utilizing Agaricus bisporus mushroom extract [98] and Mimosa diplotricha plant leaf extract [99], were found to be an excellent solution for the selective and sensitive detection of toxic Hg(II) in lake water, tap water, and river water specimens, respectively. In addition, a Pt disc electrode decorated with bark green AgNPs (Moringa oleifera bark extract) was utilized to detect hazardous Cu(II) from electroplating plant effluents [111], while AgNPs produced with garlic extract were used for the quantification of Cd(II) in lakes [106].
Planar screen-printed (carbon) electrodes are suitable for on-site analyses because they are built on the same substrate with a three-electrode setup (WE, RE, and CE). Planar SPE surfaces can be modified in the same way as disc electrodes by using a: (i) hybrid of AgNPs and Cu(II)–terephthalate MOFs for the quantification of nitrate in drinking water [46], (ii) silver nanoseed/carbon nanofiber modifier for simultaneous detection of Cu(II) and Pb(II) in groundwater matrices [113], (iii) a curcumin-stabilised AgNPs-FeCo2O4 nanosheets-rGO complex architecture for the detection of 4-NP in industrial wastewater [159], (iv) a ternary Ag/Co3O4/chitosan composite for the removal of 4-nitrophenol in sewage [160], (v) bimetallic silver-gold-oxide nanoparticles for simultaneous determination of trivalent and hexavalent chromium in tap water [81]. In addition to carbon-based planar surfaces, AgNPs-decorated glass [142], ITO substrates [67,148], and FTO glass substrates [47] were successfully employed for the selective detection of nitrite, nitrate, BPA, and hazardous copper, respectively.
4.6. Summary and Future Perspective
In summary, the use of silver nanomaterial-decorated electrodes as voltammperometric sensors offers several advantages based on the electroanalysis output: (i) efficient catalysis; (ii) rapid mass transfer of the target analyte from the bulk solution to the sensing surface and vice versa; (iii) large sensor surface area; and (iv) the ability to precisely tune the electrode microenvironment. Although electrochemical sensing technologies may achieve quantification of analytes even at femtomolar levels, they still lack quality assurance and reliability for long-term measurements in complex water matrices.
Therefore, to ensure an advanced and self-sufficient next generation of nanosilver-based voltammetric/amperometric probes, future research must focus on the: (i) the development of low-cost and submersible sensor platforms for real-time detection of pollutants in water matrices; (ii) fabrication of lighter weight and small-sized devices—miniaturization and planar (all-printed all-solid state) electrode design; (iii) construction of wireless and portable sensors for measurements at remote locations with minor facilities; (iv) sensitivity and selectivity augmentation overcoming interference and fouling challenges; and (v) development of self-sustained devices.
5. Conclusions and Outlooks
Electrochemical sensing in combination with nanosilver-based materials and various electroanalytical techniques offers attractive opportunities to meet the requirements for accurate detection of emerging water pollutants. The high conductivity of AgNPs facilitates rapid charge transfer during detection of the target species, while superior catalytic efficiency can be easily tuned by changing the particle size, shape, and temperature of the reaction mixture. Compared to other metal-based nanoparticles, AgNPs have relatively lower cost and toxicity, ensuring their great potential for practical applications. Modification of electrode substrates with silver hybrid architectures by introducing 2D carbon (graphene and its derivatives, carbon black, carbon cloth), 3D carbon nanomaterials (quantum dots, carbon nanotubes), and MOFs or CPs as support materials forms adventurous sensing surfaces for detection of various analytes. Molecular imprinting is by far the most sensitive targeting technology, enabling rapid and ultrasensitive quantification of analytes by fine-tuning the recognition sites in the MIP cavities.
So far, different synthesis strategies have been developed to prepare Ag-based nanocomposite materials decorated WE, from simple immersion and drop casting to in situ reduction method. By combining the advantages of the different synthesis methods and the various modification techniques, high-performance detection is possible for practical in-field applications. Although numerous studies on the synthesis and environmental applications of AgNPs-based electrochemical sensors have been conducted recently, the development of highly selective and sensitive platforms with the possibility of miniaturization and integration into portable devices for real-time measurements and sustainable use of water resources remains a challenge.
This entry is adapted from the peer-reviewed paper 10.3390/s23073692
