Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
The ideal chemical desalination approach is undertaken (i) without specialist training, (ii) on a typical agricultural holding (1 to 100 ha−1), and (iii) using existing water tanks and/or impoundments. It reduces the overall feed water required to produce x m3 of water (relative to reverse osmosis). It produces minimal or no waste products that require disposal.
- crop yields
- desalination polymers
- Ostwald ripening
1. Introduction
The primary goal [1] of precision agriculture (PA) is to increase efficiency and productivity, while reducing input costs and increasing environmental sustainability [2,3,4,5]. PA aims to optimize the use of resources (e.g., saline irrigation water) [2,3,4], maximize crop [6,7] and livestock yields (through the use of desalination or partial desalination of irrigation water) [8,9,10], and raise the quality of some high-value crop [11,12,13] and livestock products [14,15] (through the desalination or partial desalination of irrigation water [16,17]). PA strategies are promoted by both national and supra-national organizations (e.g., United Nations, Sustainable Development Goals, the European Green Deal, and the EU Farm-to-Fork Strategy). These strategic objectives must respond to both anthropogenic and natural environmental changes (e.g., climatic variation, anthropogenic over exploitation of water resources, desertification resulting from anthropogenic activities, etc.). These changes can be progressive, or very rapid.
2. Benefits of Chemical Desalination
The ideal chemical desalination approach is undertaken (i) without specialist training, (ii) on a typical agricultural holding (1 to 100 ha−1), and (iii) using existing water tanks and/or impoundments. It reduces the overall feed water required to produce x m3 of water (relative to reverse osmosis). It produces minimal or no waste products that require disposal. The delivered desalinated (or partially desalinated) irrigation water cost is a fraction of the cost of providing desalinated water using reverse osmosis (RO) [19,20,21,22] or another physical process (e.g., evaporation [23,24,25], cryo-desalination [26]).
The only desalination routes with the potential to achieve all of these objectives involve chemical desalination [18]. Chemical desalination involves one or more of adsorption (on functionalized surfaces) or chemical separation approaches. The adsorption approaches that have been investigated include:
- (i)
-
The use of crown ether desalination (patent US2011/0147314A1 [26,27]. Crown ether technology is currently focused on the recovery of Li+ ions from water. The same technology can also be used to selectively recover Na+, K+, and Li+ ions from a water body [28,29,30]. It is being evaluated for use in the recovery of Cs+ and Mg2+ ions [31,32,33]. The ion recovery process from the absorbent is energy intensive (US2011/0147314A1). This technology tethers the Na+ ion to the absorbent site and forces the Cl− ion to become a spectator ion. Polymer desalination can reverse the adsorption site charges to tether the Cl− ions and maintain the Na+ ion as a spectator ion;
- (ii)
-
Functionalization of the surface of a particle with negative charged or positive charged sites. Negatively charged sites attract Na+ ions and positively charged sites attract Cl− ions. The use of a charged particle in water remediation has been the focus of substantive patent activity (e.g., JP5405454B2; RU2463256C2; US9617175B2). The application of this adsorption technology to water desalination is addressed in patents US8636906B2 and FR2983191A1 [34];
- (iii)
-
The use of hydrophilic polymers. These polymers actively adsorb water but not Na+ ions and Cl− ions. The desalination requires recovery and dehydration of the hydrated hydrophilic polymers to release desalinated water. This approach produces a waste brine and is not considered further;
- (iv)
-
The use of hydrophobic polymers, which preferentially adsorb Na+ and Cl− ions from water was first outlined in patent US9617175B2. This approach was largely ignored in the academic literature until 2022 [18,34]. The discovery, in 2013 (GB2520775A), that these polymers abstract Na+ and Cl− ions from water and sequester them within dead-end pores (Equation (1)) has since been confirmed by patent US10919784B2 and academic publications [18,34]. These polymers combine chemical adsorption with chemical separation [18,34].
Saline Water = Reduced Salinity Water + Salinity contained in polymer pores,
Chemical separation can be operated, using membranes, or particles. Hydrophilic membranes, e.g., US10179842B2, use a functionalized membrane to separate water from saline water [35]. This process produces partially desalinated water and a concentrated brine [35]. Hydrophobic membranes, e.g., US10179842B2, are used to remove ions from water. The removed ions (Na+ and Cl− ions) are concentrated within the membranes.
Hollow, entrained, hydrophobic and hydrogel spheres can be used to scavenge and sequester Na+ and Cl− ions from a water body [18]. The spheres can be created by templating (e.g., ES2908075T3; ES2891098T3) or they can be produced using a sol–gel precipitation approach, e.g., US20090061226A1 [18]. The sol–gel spheres (Figure 1) are constructed from rod-like, polymer crystallites, which radiate from the sphere’s center [36]. The spheres grow by a process termed Ostwald ripening [37]. In this process, the crystallite located in the sphere’s center dissolves to release motile polymer ions. These ions are replaced by water or another fluid [37]. The motile polymer ions then migrate to the outer surface of the sphere (Figure 1). They are incorporated within the diffuse, hydrated outer layer of the sphere (Figure 1). This surface contains the growing edge of the polymer crystallite [37]. The Ostwald Ripening process results in the sphere diameter and sphere core volume increasing in size with time [37]. The outer, diffuse, hydrated layer of the spheres (Figure 1) is highly functionalized. It contains both positive charged and negative charged adsorption sites. These sites allow the growing crystallites, within the diffuse layer, to adsorb ions from the water [38,39] (Figure 1). These ions are then incorporated within the crystallite structure as additional hydroxy oxides accrete onto the end of the crystallites [38].
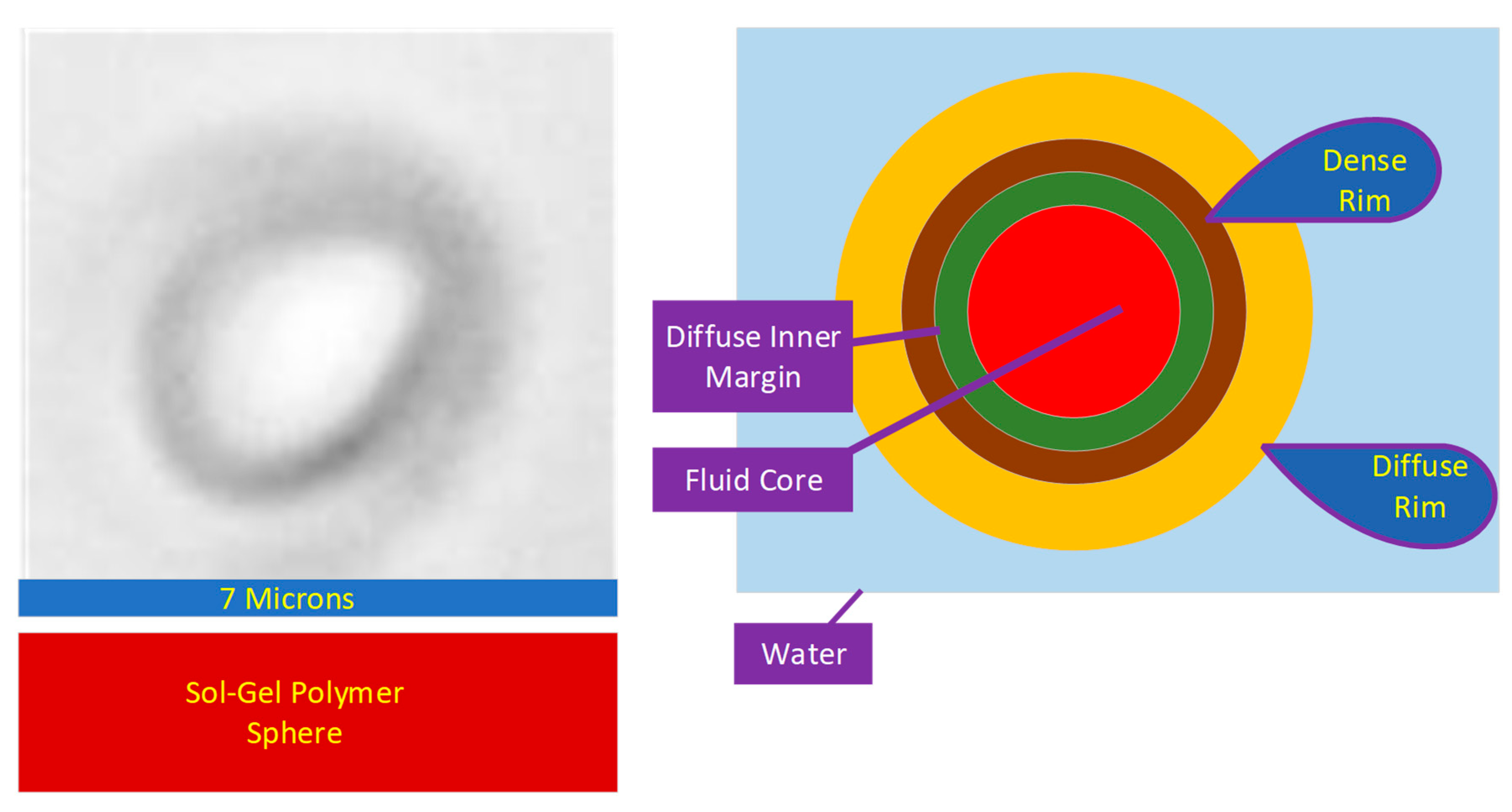
Figure 1. Hydrophobic, metal polymer, hydrogel sphere.
The crystallite packing within the rim increases (and hydration decreases) towards the interior of the sphere (Figure 1). This creates a dense rim of polymer surrounded by a diffuse rim of polymer. A typical diffuse rim may be 500–800 nm thick, whereas the typical dense rim may be 100–800 nm thick.
The Ostwald ripening process results in the dense rim surrounding a diffuse inner margin (Figure 1). Active dissolution of the crystallites occurs within the diffuse inner margin. The typical diffuse margin thickness is within the range 300–800 nm. The diffuse inner margin surrounds a fluid core which expands with time.
All the ions captured in the diffuse rim are eventually transferred to the sphere’s core [39]. The polymer surface (in the diffuse rim) contains both –[H+], and –[OH−] molecular end elements. Removal of –[H+], creates a –[−ve] site, which can be used to adsorb Na+ ions. Similarly, removal of –[OH−] creates a –[+ve] site, which can be used to adsorb Cl− ions. Supra-hydrophobic structures are created [40], by incorporating (into the polymer formation) one or more of ZnO (Zn(OH)2), MO2 (e.g., TiO2, MnO2), clays (e.g., Ca-montmorillonite), feldspars (e.g., K-feldspar), polysiloxanes (-Si-O-Si- groups), carbon (n-C0, -C-C-), non-polar materials containing CH3/CH2 groups, and polymers with combined chemistry. These supra-hydrophobic polymers are characterized [39,40,41], by having a rough surface. This surface is created through the incorporation of one or more metals or metal oxides. They are selected from the transition group metals in the periodic table groups III to XII (e.g., Fe, Mn, Zn) (Figure 1).
Desalination using hollow, entrained, hydrophobic, hydrogel particles (Figure 1) makes it possible to (i) reduce the feed water input for irrigation (using sea water and reverse osmosis (RO)) from about five units of water [42] to one unit of water [18]; (ii) reduce the associated waste brine volume for disposal into the environment (when compared with RO) from four units of water [42,43] to zero units of water [18]; and (iii) reduce the energy required to operate the process from a theoretical level of about 0.9 kWh m−3 (actual level of 2.4–5 kWh m−3) using reverse osmosis [44] to an actual level of <0.05 kWh m−3 (for pumps delivering and removing the water) [18].
This entry is adapted from the peer-reviewed paper 10.3390/su15097063
This entry is offline, you can click here to edit this entry!
