Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Alzheimer’s disease (AD) is the most common form of dementia. It increases the risk of other serious diseases and causes a huge impact on individuals, families, and socioeconomics. AD is a complex multifactorial disease, and current pharmacological therapies are largely based on the inhibition of enzymes involved in the pathogenesis of AD.
- Alzheimer’s disease
- enzyme inhibitors
- microbial source
1. Introduction
Alzheimer’s disease (AD) is a multifactorial disease featured by the deposition of amyloid-beta (Aβ) plaques and neurofibrillary tangles in the brain, leading to the death of neuronal cells and memory loss [1][2]. AD increases the risk of other serious diseases and has a great impact on individuals, families, and socioeconomics [3]. Currently, there are about 50 million AD patients worldwide, and this number is predicted to double every five years; it is expected to increase to 152 million by 2050 [2][4]. AD is a multifactorial disease with a complex pathophysiology, and to date, the exact etiology has not been elucidated, making the disease difficult to treat [1][2]. To date, there is no cure for AD; instead, there are treatments that improve the symptoms and conditions of the disease [5][6]. The development of compounds can prevent or treat AD by targeting several pathogenic mechanisms [7][8]. According to AD pathogenesis, current pharmacological therapies are mainly based on the inhibition of target enzymes causing AD [9]. However, these traditional drugs only affect a single target, helping to reduce symptoms and disease progression while causing many side effects. Therefore, efforts to find new potential inhibitors are attracting increasing scientific interest.
2. Overview of Enzyme-Associated Pathogenesis Mechanisms of Alzheimer’s Disease
Many target enzymes related to the pathogenesis mechanisms of AD have been recorded (Figure 1). Cholinergic neurotransmission has been shown to be intimately involved in several key psychological processes, such as memory [10]. The cholinergic hypothesis is the earliest hypothesis for AD, which proposes that the cause of the disease is an impaired synthesis of the acetylcholine neurotransmitter induced by acetylcholinesterase (AchE). The enzyme butyrylcholinesterase (BuChE), similar to AchE, also plays an important role in the progression of AD [11][12][13]. One of the main reasons for the resistance of AD to AChE inhibitors is that BuChE acts as a substitute for the loss of AChE in the neurons of patients with AD. Thus, BuChE continues the activity of AChE in cases where AChE is insufficient or inhibited. Therefore, several studies have evaluated the inhibitory activity of both of these enzymes, simultaneously targeting multi-targeted inhibitors [14]. Although other relevant pathophysiological mechanisms have been further investigated in recent years, treatments that improve cholinergic function remain important in the management of patients with AD [15]. Of the five drugs approved by the Food and Drug Administration (FDA) for AD therapy, four are acetylcholinesterase inhibitors (AchEI), and the other is memantine, an N-methyl-D-aspartic acid receptor antagonist. However, these drugs only help reduce the symptoms of dementia while causing many side effects. To date, several new drug applications have been developed to exploit other new targets of the disease. However, effective therapeutic agents to correct the disease have not yet been found. Besides cholinergic reconstitution, researchers are also looking for other AD targets [16]; two major factors in the development of AD are identified as involving insoluble Aβ peptides and abnormal Tau proteins [17].
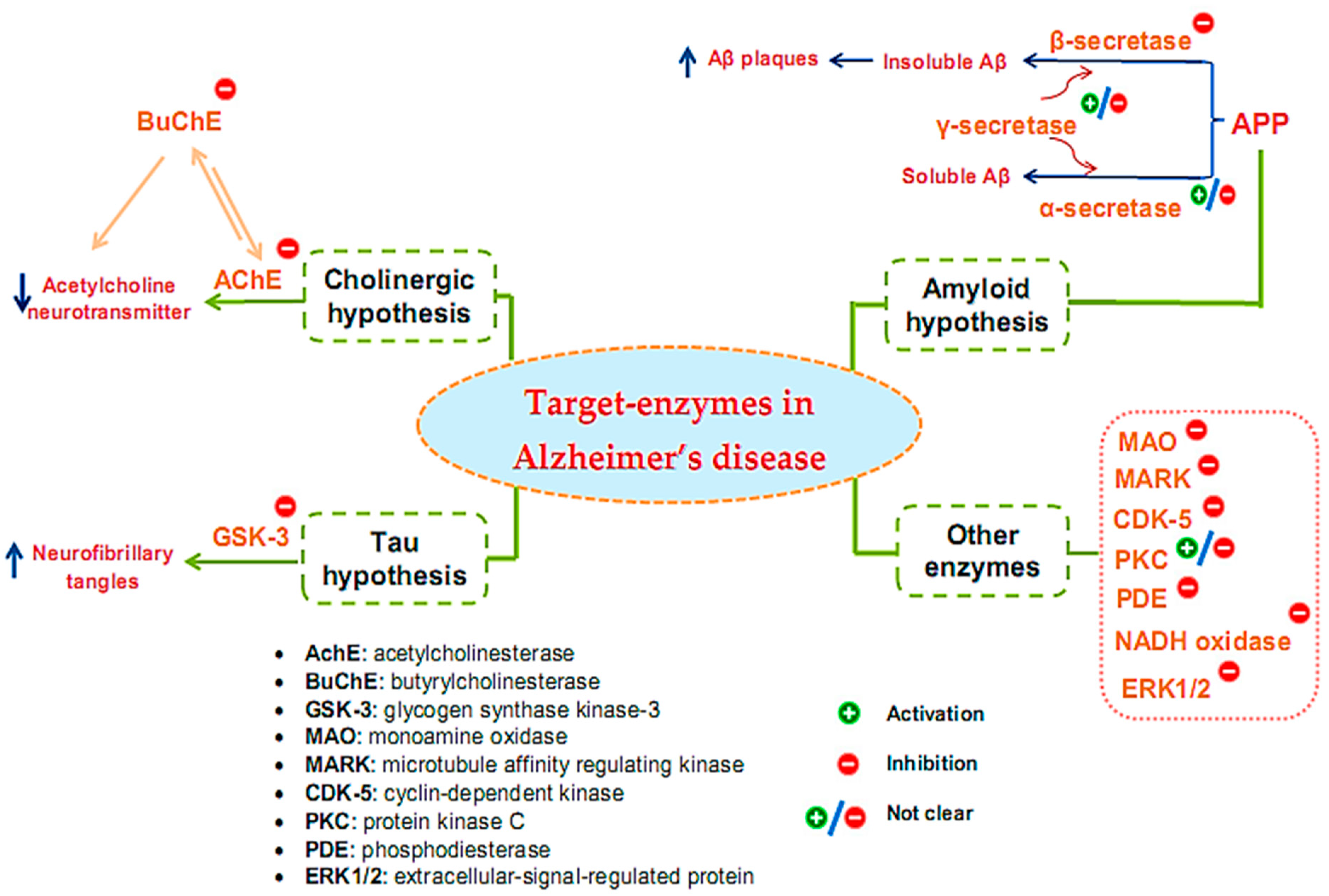
Figure 1. Target enzymes related to the pathogenesis of Alzheimer’s disease.
The amyloid hypothesis suggests the clinical symptoms of the disease are related to the accumulation of insoluble Aβ peptides due to altered transmembrane amyloid precursor protein (APP) processing. Normal APP processing is achieved by cleavage of the precursor APP by α-secretase, followed by γ-secretase to form soluble Aβ. In pathological cases, precursor APP is cleaved by β-secretase and γ-secretase, leading to the formation of insoluble Aβ, contributing to synaptic damage [9]. Thus, many strategies have been tested for the treatment of AD based on this hypothesis by inhibiting β- and γ-secretase, which are responsible for generating Aβ, regulating Aβ incorporation, or enhancing Aβ elimination [18]. Enzyme-related therapies based on this hypothesis involve the inhibition of β- and γ-secretase while also activating α-secretase activity. However, the inhibition of γ-secretase can cause undesirable side effects because γ-secretase has many vital physiological functions. At present, whether the function of γ-secretase in Aβ production can be specifically inhibited without interfering with other important functions of this protease remains unclear [19]. Thus, β-secretase inhibitor candidates are of particular interest to develop, and the majority of AD treatment studies targeting secretase inhibition have focused on this enzyme [20][21][22].
The Tau protein hypothesis is one of the most important hypotheses of AD related to the formation of fibrillary tangles because of the formation of fibrin plexuses by over-phosphorylated Tau proteins, in which glycogen synthase kinase-3 (GSK-3) is the major kinase responsible for phosphorylating Tau proteins [9]. The elevated phosphorylation can be controlled through the inhibition of the enzyme GSK3 [23]. Much effort has been made in the discovery and development of GSK-3 inhibitors, as this is an area of research that has been actively explored by academic centers and pharmaceutical companies. Although numerous GSK-3 inhibitors have been developed, no GSK-3 inhibitor has been placed in the market due to various concerns regarding the non-selective-target activity causing serious side effects. Thus, despite being a potential AD therapeutic target, analytical evaluations using a specific GSK-3 inhibitor still need to be clarified in-depth [24].
In addition to the above hypotheses, other enzyme targets for AD are also of great interest. For instance, monoamine oxidase (MAO), an activated enzyme, plays an important role in the pathogenesis of AD, including the formation of amyloid plaques from Aβ production and accumulation and the formation of neurofibrillary tangles and cognitive impairment due to cholinergic destruction of neurons and disturbances of the cholinergic system [25]. Therefore, MAO inhibitors (MAOIs) can be considered promising therapeutic agents for AD. While the first-generation MAOIs are indistinguishable, second-generation MAOIs preferentially inhibit MAO-A or MAO-B [26]. Clinical trials involving MAO-inactivating drugs have been conducted; nonetheless, MAOIs have many drug interactions that can produce some undesirable side effects [27]. Similar to γ-secretase, the enzyme protein kinase C (PKC) also encountered some inconsistencies in its handling when early studies suggested the pharmacological activation of PKC as a target for the treatment of AD [28][29]. However, the prolonged activation of PKC is also associated with AD pathology; hence, the activation or inhibition of this enzyme for the treatment of AD also needs further consideration and evaluation [30]. Some other target enzymes, such as cyclin-dependent kinase (CDK-5), microtubule affinity regulating kinase (MARK), phosphodiesterase (PDE), and NADH oxidase, are also reported to be related to AD [31]. CDK-5 is a proline-directed serine-threonine protein kinase [32]; it plays a vital role in the physiological development of the central nervous system and phosphorylates several relevant substrates. CDK-5 is activated by its neuron-specific and membrane-localized (p35 and p39) or respective truncated forms (p25 and p29). Enhanced CDK5 activity leads to abnormal hyperphosphorylation or enhanced amyloid production, causing the neurodegenerative pathology of AD [33][34][35]. MARK is a kinase that plays initiating role in Tau abnormality phosphorylation [36]. PDEs are an enzyme family that hydrolyzes the 3′-phosphodiester links in cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) in signal-transduction pathways for the generation of 5′-cyclic nucleotides. PDEs are essential for controlling cell functioning and adjusting the levels of cAMP and cGMP. Abnormal cAMP signaling is related to AD [37]. NADPH oxidase is the main enzyme causing the creation of damaging free radicals that lead to oxidative stress—a general cause in the pathology of neurodegenerative disorders such as AD [38]. Research has shown that the activity of ERK1/2 (the extracellular-signal-regulated protein kinase of the mitogen-activated protein kinase family) is involved in Tau phosphorylation in AD [39]. Therefore, ERK1/2 is tightly implicated in AD pathogenesis, and it has also become a promising therapeutic target. Currently, no effective therapeutic agents are targeting ERK1/2 for the treatment of AD; thus, related studies are still ongoing [40].
Although various kinds of enzymes were discovered involving AD pathology, studies have mainly focused on the inhibition of enzymes commonly hypothesized to participate in the pathogenesis of AD [8]. While other enzymes have not been studied much, until now, five commercial drugs were issued, and of those, two are of natural origin [41]. Four of the drugs are AChE inhibitors (donepezil, rivastigmine, galantamine, and tacrine), and the other is an NMDA receptor antagonist (memantine) [42]. However, these drugs only support treatment to help reduce the symptoms of dementia and also cause many side effects. In 2021, the FDA expedited the approval of an intravenous injection: aducanumab (AduhelmTM) [42]. Drugs in this category may halt clinical degeneration to improve cognitive function and impact individuals with AD [42]. Additionally, studies to find drugs with effective pharmacology in the treatment of AD continue to be carried out. Recently, there were around 868 drugs in different trial stages. However, only 273 drugs are being actively developed by biotech or pharma companies [2].
This entry is adapted from the peer-reviewed paper 10.3390/ph16040580
References
- Cacabelos, R. Pharmacogenomics of Alzheimer’s and Parkinson’s diseases. Neurosci. Lett. 2020, 726, 133807.
- Hajjo, R.; Sabbah, D.A.; Abusara, O.H.; Al Bawab, A.Q. A review of the recent advances in Alzheimer’s disease research and the utilization of network biology approaches for prioritizing diagnostics and therapeutics. Diagnostics 2022, 12, 2975.
- Kaushik, V.; Smith, S.T.; Mikobi, E.; Raji, M.A. Acetylcholinesterase inhibitors: Beneficial effects on comorbidities in patients with Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 73–85.
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590.
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments in Alzheimer’s disease: An update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397.
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446.
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789.
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Sig. Transduct. Target Ther. 2019, 4, 29.
- McGirr, S.; Venegas, C.; Swaminathan, A. Alzheimer’s disease: A brief review. J. Exp. Neurol. 2020, 1, 89–98.
- Chen, X.Q.; Mobley, W.C. Exploring the pathogenesis of Alzheimer disease in basal forebrain cholinergic neurons: Converging insights from alternative hypotheses. Front. Neurosci. 2019, 13, 446.
- Zhou, S.; Huang, G. The biological activities of butyrylcholinesterase inhibitors. Biomed. Pharmacother. 2022, 146, 112556.
- Miličević, A.; Šinko, G. Evaluation of the key structural features of various butyrylcholinesterase inhibitors using simple molecular descriptors. Molecules 2022, 27, 6894.
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh, S.T.; Somogyi, M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. Prim. Care Companion CNS Disord. 2013, 15, PCC.12r01412.
- Adeowo, F.Y.; Ejalonibu, M.A.; Elrashedy, A.A.; Lawal, M.M.; Kumalo, H.M. Multi-target approach for Alzheimer’s disease treatment: Computational biomolecular modeling of cholinesterase enzymes with a novel 4-N-phenylaminoquinoline derivative reveal promising potentials. J. Biomol. Struct. Dyn. 2021, 39, 3825–3841.
- Harald, H.; Marsel, M.; Claudio, C.; Martin, R.F.; Ezio, G.; George, T.G.; Ara, S.K.; Andrea, V.; Enrica, C.; Peter, J.S.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933.
- Uddin, M.S.; Kabir, M.T.; Jeandet, P.; Mathew, B.; Ashraf, G.M.; Perveen, A.; Bin-Jumah, M.N.; Mousa, S.A.; Abdel-Daim, M.M. Novel anti-alzheimer’s therapeutic molecules targeting amyloid precursor protein processing. Oxid. Med. Cell Longev. 2020, 2020, 7039138.
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423.
- Kim, N.; Lee, H.J. Target enzymes considered for the treatment of Alzheimer’s disease and Parkinson’s disease. Biomed. Res. Int. 2020, 2020, 2010728.
- Ghosh, A.K.; Brindisi, M.; Tang, J. Developing β-secretase inhibitors for treatment of Alzheimer’s disease. J. Neurochem. 2012, 120, 71–83.
- Kwak, H.M.; Jeon, S.Y.; Sohng, B.H.; Kim, J.G.; Lee, J.M.; Lee, K.B.; Jeong, H.H.; Hur, J.M.; Kang, Y.H.; Song, K.S. Beta-secretase (BACE1) inhibitors from pomegranate (Punica granatum) husk. Arch. Pharm. Res. 2005, 28, 1328–1332.
- Naushad, M.; Durairajan, S.S.K.; Bera, A.K.; Senapati, S.; Li, M. Natural compounds with anti-BACE1 activity as promising therapeutic drugs for treating Alzheimer’s disease. Planta Med. 2019, 85, 1316–1325.
- Kazuya, M. Chemical diversity of β-secretase inhibitors from natural resources. Nat. Prod. Commun. 2019, 14, 1934578X19894819.
- Maqbool, M.; Mobashir, M.; Hoda, N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 107, 63–81.
- Khan, I.; Tantray, M.A.; Alam, M.S.; Hamid, H. Natural and synthetic bioactive inhibitors of glycogen synthase kinase. Eur. J. Med. Chem. 2017, 125, 464–477.
- Shoaib, M.; Nasimul, H. A comprehensive review of monoamine oxidase inhibitors as anti-Alzheimer’s disease agents: A review. Eur. J. Med. Chem. 2020, 206, 112787.
- Ron, W.; Robert, H.; Marianne, G.H.; Timothy, E.; Susan, W. Antidepressants. In Rosen’s Emergency Medicine: Concepts and Clinical Practice, 10th ed.; Ron, M.W., Robert, S.H., Marianne, G.H., Eds.; Elsevier: Philadelphia, PA, USA, 2018.
- Cai, Z. Monoamine oxidase inhibitors: Promising therapeutic agents for Alzheimer’s disease (review). Mol. Med. Rep. 2014, 9, 1533–1541.
- Jacobsen, J.S.; Spruyt, M.A.; Brown, A.M.; Sahasrabudhe, S.R.; Blume, A.J.; Vitek, M.P.; Muenkel, H.A.; Sonnenberg, R.J. The release of Alzheimer’s disease beta amyloid peptide Is reduced by phorbol treatment. J. Biol. Chem. 1994, 269, 8376–8382.
- Hung, A.Y.; Haass, C.; Nitsch, R.M.; Qiu, W.Q.; Citron, M.; Wurtman, R.J.; Growdon, J.H.; Selkoe, D.J. Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J. Biol. Chem. 1993, 268, 22959–22962.
- Lordén, G.; Newton, A.C. Conventional protein kinase C in the brain: Repurposing cancer drugs for neurodegenerative treatment. Neuronal Signal. 2021, 5, NS20210036.
- Alam, J.; Sharma, L. Potential enzymatic targets in Alzheimer’s: A comprehensive review. Curr. Drug Targets 2019, 20, 316–339.
- Liu, S.L.; Wang, C.; Jiang, T.; Tan, L.; Xing, A.; Yu, J.T. The role of Cdk5 in Alzheimer’s disease. Mol. Neurobiol. 2016, 53, 4328–4342.
- Kimura, T.; Ishiguro, K.; Hisanaga, S. Physiological and pathological phosphorylation of tau by Cdk5. Front. Mol. Neurosci. 2014, 7, 65.
- Liu, F.; Su, Y.; Li, B.; Zhou, Y.; Ryder, J.; Gonzalez, D.P.; May, P.C.; Ni, B. Regulation of amyloid precursor protein (APP) phosphorylation and processing by p35/Cdk5 and p25/Cdk5. FEBS Lett. 2003, 547, 193–196.
- Wen, Y.; Yu, W.H.; Maloney, B.; Bailey, J.; Ma, J.; Marie, I.; Maurin, T.; Wang, L.; Figueroa, H.; Herman, M.; et al. Transcriptional regulation of beta-secretase by p25/cdk5 leads to enhanced amyloidogenic processing. Neuron 2008, 57, 680–690.
- Toshiya, O.; Taro, S.; Akiko, A.; Sawako, S.; Koichi, M.I.; Kanae, A. Microtubule affinity–regulating kinase 4 with an Alzheimer’s disease-related mutation promotes tau accumulation and exacerbates neurodegeneration. J. Biol. Chem. 2020, 295, 17138–17147.
- Sheng, J.; Zhang, S.; Wu, L.; Kumar, G.; Liao, Y.; Fan, H. Inhibition of phosphodiesterase: A novel therapeutic target for the treatment of mild cognitive impairment and Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1019187.
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Krishnan, M.D.; Ratna, K.V.; Darrell, W.B. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7.
- Mohammad, R.K.; Keyvan, Y.; Ayda, E.; Morteza, G.B. The role of ERK1/2 pathway in the pathophysiology of Alzheimer’s disease: An overview and update on new developments. Cell. Mol. Neurobiol. 2023, 43, 177–191.
- Muraleva, N.A.; Kolosova, N.G.; Stefanova, N.A. MEK1/2-ERK pathway alterations as a therapeutic target in sporadic Alzheimer’s disease: A study in senescence-accelerated OXYS rats. Antioxidants 2021, 10, 1058.
- Atanasova, M.; Dimitrov, I.; Ivanov, S.; Georgiev, B.; Berkov, S.; Zheleva, D.D.; Doytchinova, I. Virtual screening and hit selection of natural compounds as acetylcholinesterase inhibitors. Molecules 2022, 27, 3139.
- Alhazmi, H.A.; Albratty, M. An update on the novel and approved drugs for Alzheimer disease. Saudi Pharm. J. 2022, 30, 1755–1764.
This entry is offline, you can click here to edit this entry!
