Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
Osteosarcopenic adiposity (OSA) syndrome denotes the confluence of bone, muscle, and adipose tissue deterioration. Being a complex entity, numerous uncertainties about OSA still exist, despite the extensive research on the topic.
- osteosarcopenic adiposity
- osteosarcopenic obesity
- nutrients
1. Introduction
Osteosarcopenic adiposity (OSA) syndrome, originally coined as osteosarcopenic obesity (OSO), is the most advanced stage on the spectrum of body composition disorders. Its first identification and proof of concept were established in 2014 [1]. Briefly, OSA is a condition with simultaneous deterioration of bone (osteopenia/osteoporosis) and muscle (sarcopenia/dynapenia) with increased presence of body fat (adipose tissue). Body fat or adiposity may be manifested as an apparent overweight/obesity, or as a redistributed fat around organ tissues (visceral) and/or as an infiltrated fat (ectopic) into bone, muscle, and other organs [2]. The original term osteosarcopenic obesity was adjusted to osteosarcopenic adiposity [2] to better reflect the heterogeneity of adipose tissue: subcutaneous, visceral, and ectopic. Each produces different molecules that might render positive or negative health consequences. In the context of OSA, it is the excess of adiposity that leads to physiological and endocrine disturbances. Additionally, obesity, in general terms, typically refers to being overweight, most often defined by high body mass index (BMI), despite that the inadequacies of BMI for characterizing the body composition phenotypes and its paradoxical relations to morbidity/mortality have been widely recognized [2,3,4,5].
OSA concept was substantiated based on several established constructs characteristic for each of the three body composition tissues (bone, muscle, fat) [1,2], including (1) common precursors in the mesenchymal stem cells (osteoblasts, myocytes, adipocytes) and their deregulation leading to osteopenia/osteoporosis, sarcopenia/dynapenia, and compromised adipose tissue; (2) hormonal interactions among three tissues (e.g., osteocalcin and sclerostin released from bone cells; myostatins and troponins released from muscle cells; estrogens and adipokines released from adipocytes); (3) common etiologies of the impairment of each tissue; from lifestyle (overnutrition/malnutrition, lack of physical activity), to low-grade chronic inflammation, to changes in hormonal levels, to neuromuscular dysfunction; (4) aging and all accompanied inevitable changes; (5) easily observed deterioration in body composition phenotypes with the ensuing medical diagnoses [1,2,6,7,8,9]. Figure 1 depicts the OSA/OSO conceptualization as a body tissue triad.
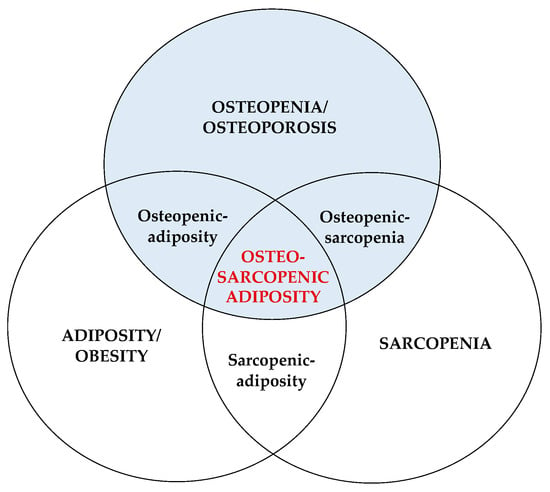
Figure 1. Osteosarcopenic adiposity/obesity and its components. Adapted from Ilich et al. [1].
Since its conception, OSA has been studied across the world in diverse populations and with different methods/techniques and cutoff criteria used for its identifications. As a result, no unique methodology has been identified, and a wide range of prevalence rates has been reported. So far, it has been associated with functional disabilities, increased frailty and risk of falls, systemic metabolic deregulation, and multiple chronic conditions [2,7]. Additionally, based on the characteristics of the syndrome, OSA may be associated with poor overall nutrition or inadequate intake of certain nutrients, as well as with a low engagement in physical activities of any kind [10,11]. However, no in-depth evaluation of such studies has been performed other than a small meta-analysis analyzing four studies that examined resistance training intervention effects on body composition and physical function in elderly OSA participants [12].
Keeping in mind the complex phenotype of OSA syndrome, we recommended earlier the nutritional and physical activity approaches for each of the conditions, as well as for the whole syndrome [7,8,10,11,13]. We also provided the algorithm for the nutritional and exercise treatment principles [7]. These were based on the already existing recommendations for each individual condition: osteoporosis, sarcopenia, and obesity. Namely, among nutrients, the intake of calcium, magnesium, vitamin D, and fiber, fitting the official recommendations [14], was enforced, while a higher-than-recommended intake of protein and omega-3 polyunsaturated fatty acids was suggested [13]. Regarding physical activity, we recommended a comprehensive exercise program to include weight bearing, aerobic, and strength training of moderate intensity for about 30–60 min 5 times/week [13]. All these recommendations were based on theoretical assumptions and scientific evidence extrapolated from other conditions. Therefore, it was necessary to perform a literature search and evaluate the original studies relating nutrition and physical activity with OSA syndrome. This will subsequently enable medical professionals to provide firsthand recommendations and management for OSA grounded on evidence-based data and clinical research.
2. Dietary Intake, Nutritional Status, and OSA
Table S1 presents the characteristics and results of studies with dietary intake or nutritional status as independent/exposure variables related to OSA/OSO. Of the eleven studies in this section, eight were either cross-sectional or observational, examining the relationship between OSA and the nutritional status of the participants or their overall dietary intake and/or intake of specific nutrients [15,16,17,18,19,20,21,22]. Three of them also evaluated engagement in physical activity or a sedentary lifestyle [18,19,21]. In addition, one study was the 6-month randomized clinical trial investigating the effects of weight loss and dairy foods and/or calcium/vitamin D supplements on body composition [23], followed by the subsequent secondary analysis evaluating the cardiometabolic risk factors as the outcomes of the same intervention [24]. The only prospective study followed the participants for 5 and 10 years, examining the influence of energy-adjusted dietary inflammatory index (E-DII) on body composition and fracture rates [25]. The total number of evaluated participants was n = 19,151 (at baseline), with n = 12,143 (63.4%) women. The average age of the participants was 66.3 years, ranging from 50 to 95.2 years, with women being slightly older than men. The highest prevalence of OSA/OSO (when reported separately in women and men) was 91.9% in women [19] and 53.3% in men [17]. The lowest prevalence in women and men (reported as combined) was 6.4% [21]. The studies were conducted on five continents: Europe (Croatia) [16,17], the U.S. [23,24], Asia (South Korea) [15,18,19,20,22], South America (Brazil) [21], and Australia [25]. See Table S1.
The important findings from these studies are described below and are listed in Table S1. Briefly, Cvijetic et al.’s [16] study was conducted in several nursing homes, revealing that more than 1/3 of participants were at risk of developing malnutrition and ~6% were already malnourished (based on the Mini Nutritional Assessment), but there was no difference in the risk of malnutrition or malnourishment between those with or without OSA. This study was conducted during the COVID-19 pandemic and also revealed no difference in OSA prevalence or nutritional status in those who had COVID and those who had not to have a disease. However, a lower phase angle (indicating lower cell integrity and muscle quality) and a lower bone mass, while a higher intramuscular adipose tissue, were all significantly associated with the presence of OSA. Another, although smaller, study conducted in a nursing home utilized 24-hour dietary recalls for the assessment of participants’ dietary intake. The results showed a lower intake of protein, omega-3 polyunsaturated fatty acids, fiber, calcium, magnesium, potassium, and vitamins D and K—all below both European and U.S. recommendations [14,26] in all participants. However, those with OSA had significantly higher extracellular water, indicating a heightened inflammatory state in that group.
A six-month randomized clinical trial of weight loss employing a 25% reduction in energy intake complemented with either 4–5 servings of low-fat dairy foods or calcium-plus-vitamin D supplements (1500 mg/day and 600 IU/day, respectively) or placebo (control group) revealed improvement in all body composition components [23]. All participants lost weight, but those in the dairy group experienced higher loss in fat and a smaller loss in lean mass compared to the control group or the group taking supplements. The group with calcium/vitamin D supplements showed the best improvements in BMD in several skeletal sites compared to dairy or control groups [23]. Additionally, the subsequent secondary analysis in these participants (same 6-month intervention) showed improvement in blood pressure and numerous other cardiometabolic risk factors with weight loss in all participants, but significantly better in dairy and/or calcium/vitamin D supplements groups [24] (Table S1). Since the evaluation of cardiometabolic outcomes was not a goal of this review, they are not discussed further but just noted as possible additional benefits to body composition and/or OSA.
The only prospective study in this section followed the participant for 5 and 10 years, investigating the influence of E-DII on body composition outcomes in a population of Australian women and men (n = 1098 at baseline) [25]. The results revealed that the consumption of a pro-inflammatory diet (higher E-DII scores) increased the incidence of fractures over 10 years in men but not in women, despite being associated with reductions in the lumbar spine and total hip BMD in both sexes. In addition, higher E-DII scores were significantly associated with higher fall risk and lower appendicular lean mass in men but not in women.
Each of the following five studies [15,18,19,20,22] utilized the Korea National Health and Nutrition Examination Survey (KNHANES) databases (from different years) to assess the relationship between several nutrients or foods [18,19,20] or Dietary Inflammatory Index (DII) [22], or Diet Quality Index International (DQI-I) [15] with OSA/OSO.
Among these, three studies [18,19,20] were published by the same group of researchers who used the KNHANES from 2008–2011, 2008–2009, and 2008–2010, respectively, thus having the advantage of large sample size (see Table S1). Choi et al., 2021 [18] reported that lower intake of calcium was significantly associated with both osteosarcopenia and OSA, while physical activity was the lowest among the participants with OSA. In Choi et al.’s study [19], protein intake below the Korean recommendation (~0.9 g/kg body weight) was associated with higher odds of developing OSO in men. Additionally, intake of plant-based protein in men with OSO was higher than in men without (possibly indicating lower protein quality), while physical activity was significantly lower in men with OSO. No significant associations were noted in women, of whom 91.9% were identified as having OSO. Bae et al. [20] examined the intake of fruits, particularly those rich in vitamin C and potassium, in women only. They found that a lower intake of potassium and vitamin C and/or a lower intake of fruits was significantly associated with OSO. Kim et al. and Park et al. [15,22] used KHANES from 2009–2011 and 2008–2010, respectively. The latter found that the higher DII scores (denoting a higher proinflammatory diet) were significantly associated with the OSO phenotype [22], while the former study examining the DQI-I (higher scores denote better food quality), reported a significantly better body composition with higher DQI-I scores [15]. A small study conducted in Brazilian community dwelling women and men reported a significant association of lower protein intake (g/kg/weight, but not as a percentage of energy) in participants with OSO (women and men combined), while none of the other nutrients were significantly different among groups. The participants with OSO also had significantly lower grip strength and preferred a more sedentary lifestyle [21].
3. Serum Nutritional Biomarkers and OSA/OSO
A set of four evaluated studies reported the relationship between nutritional serum biomarkers, namely serum ferritin and 25(OH)D, and OSO [27,28,29,30] presented in Table S2. One study was derived from a Korean cohort of the Kangbuk Samsung Health Study [27], one from China’s community dwelling women and men [28], and two from the KNHANES databases [29,30]. The total number of evaluated participants was n = 39,227, with n = 25,427 (64.8%) women. The average age of the participants was 63.1 years (women 62.9 and men 65.4 years). The highest prevalence of OSO was 40.1% and 28.1% in women and men, respectively [29]. The lowest prevalence was 6.4% and 9.4% in women and men, respectively [27] (Table S2).
Chung et al. [27] examined sex-specific serum ferritin concentration in relation to OSO, reporting that women with the highest ferritin tertiles had the highest OSO prevalence. Additionally, higher serum ferritin concentrations were significantly associated with other adverse body composition impairments in women but not in men. The following three studies [28,29,30] investigated the association of serum 25(OH)D (calcidiol) with OSO phenotype, comparing it with other impairments, namely, osteopenic obesity, sarcopenic obesity, and obesity-only, revealing its supporting role in the pathogenesis of the conditions. Ma Y. et al. [28] examined a large sample of Chinese citizens dividing them into groups based on the body composition impairments and tertiles of serum 25(OH)D. They found that the serum 25(OH)D deficiency was associated with a greater likelihood of having OSO. The results also revealed the independent negative dose-response associations of 25(OH)D with OSO and other impaired body composition components. The two studies [29,30] showed, respectively, that vitamin D deficiency/inadequacy was significantly higher in both women and men with OSO compared to other groups and that higher 25(OH)D was associated with significantly lower odds of having adverse body composition features, especially OSO. Additionally, Kim, Y.M. et al. [29] reported that both women and men in the OSO group engaged in the lowest physical activity compared with those belonging to other groups. Please see Table S2.
4. Physical Activity and OSA
In Table S3, eight interventional studies [31,32,33,34,35,36,37,38] with resistance training are presented. Among these studies, only one included both women and men and a combination of resistance training with aerobic exercise [36]. The total number of evaluated participants was n = 182, with the majority being women. The age ranged from 60 to 85 years, and all recruited participants were identified as having OSA/OSO as per inclusion criteria (except Cunha et al. [32]). Three of the studies were respectively conducted on community dwelling individuals in Taiwan [31], Main China [36], and Brazil [32]. The other five papers were published by the same group of researchers from Iran [33,34,35,37,38], where the results from the same participants engaged in the same 12-week interventional resistance training regimen with the elastic band were analyzed but reported different outcome variables affecting women with OSA/OSO (Table S3).
Overall, each of the papers reported some benefits, including improvements in body fat, muscle mass components (even BMD), some functional performance measures and some biomarkers (see Table S3). More specifically, Lee et al. and Shen et al. [31,36] reported an increase in BMD, and the former was the only study with a follow-up at 6 months, showing none of the reported benefits were sustained. The study by Cunha et al. [32] is unique as they investigated the response to two resistance training regimens (1-set vs. 3-sets) vs. the control group and found a dose response with higher activity (3 sets induced greater improvement) and both sets induced greater improvement compared to the control group. The authors did not identify OSO as such but created composite Z-scores (derived from average of the muscular strength, skeletal muscle mass (SMM), % body fat, and BMD) and noted a significant improvement after the exercise.
A research group from Iran published several papers with the results derived from the same interventional study with elastic band resistance training in n = 63 women (n = 32, intervention and n = 31, control), all having OSA/OSO as per inclusion criteria [33,34,35,37,38]. The results from their earliest paper revealed a significant increase in handgrip strength, timed chair-rise test, muscle quality, slight improvement in OSO composite Z-score, as well as an increase in estradiol and a decrease in leptin [35]. In one of these papers [33], serum microRNA (miR-133 and miR-206) changes correlated with changes in FRAX scores, serum 25(OH)D, and alkaline phosphatase. Banitalebi et al. [34] reported improved composite cardiometabolic risk factors (e.g., lipid-accumulation product, triglyceride-glucose-BMI index, visceral adiposity index, atherogenic index of plasma, Framingham risk score). Hashemi et al. [37] (from the same group) reported a significant decrease in serum microR-146, total cholesterol, and low-density lipoproteins (LDL) and an increase in high-density lipoproteins (HDL), while Kazemipour et al. [38] reported a significant increase in insulin growth factor (IGF-2), and fibroblast growth factor (FGF-2). However, this intervention did not result in any significant improvement of the body composition components (e.g., BMD, BMI, body fat, skeletal muscle index) or serum biomarkers, such as triglycerides and C-reactive protein (Table S3).
This entry is adapted from the peer-reviewed paper 10.3390/nu15071619
This entry is offline, you can click here to edit this entry!
