Patients with heart failure (HF) and associated chronic kidney disease (CKD) are a population less represented in clinical trials; additionally, subjects with more severe estimated glomerular filtration rate reduction are often excluded from large studies. In this setting, most of the data come from post hoc analyses and retrospective studies. Accordingly, in patients with advanced CKD, there are no specific studies evaluating the long-term effects of the traditional drugs commonly administered in HF.
- heart failure
- chronic kidney disease
- estimated glomerular filtration rate
1. Introduction
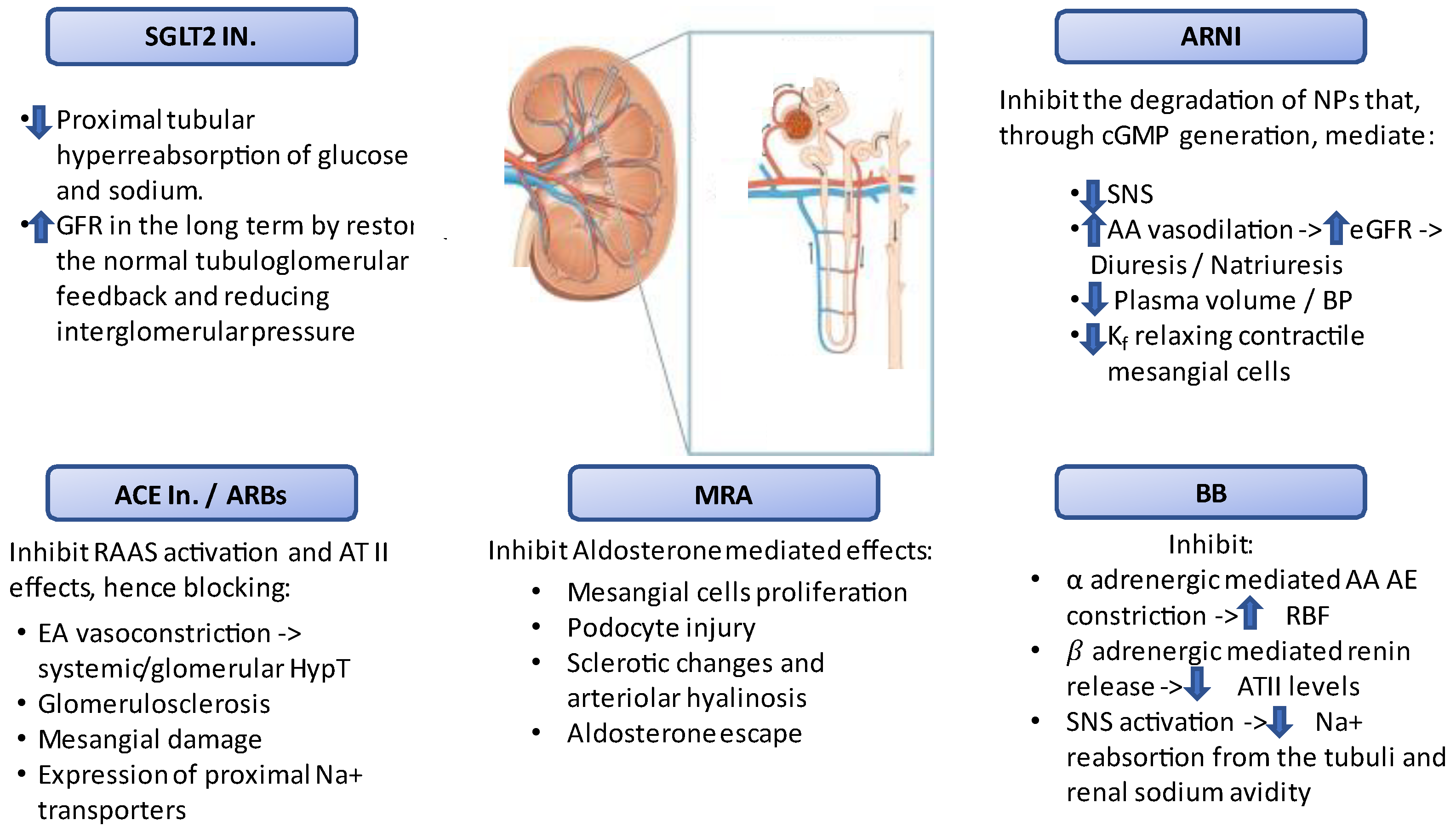
2. Clinical Characteristics of Patients with Chronic Kidney Disease and Heart Failure
| Clinical Scenarios | ||
|---|---|---|
| (1) “Pseudo” WRF | Good renal function at baseline and occurrence of WRF during hospitalization for acute HF, usually secondary to the decongestion therapy. | |
| (2) “True” WRF | WRF due to congestion and hypoperfusion, in which renal deterioration persisted also in the post-discharge period with a higher burden of HF re-hospitalization. | |
| (3) WRF in CKD | WRF could occur in the presence of CKD. This subtype was common in older patients with several comorbidities, where WRF reflected the real deterioration of the renal function, with worse prognostic value. | |
| Laboratory/urine Output Criteria | ||
| eGFR Criteria | Urine output criteria | |
| RIFLE (an acute rise in SCr over 7d) | ||
| Risk | Increased SCr ≥ ×1.5 or eGFR decrease > 25% | UO < 0.5 mL/kg/h × 6 h |
| Injury | Increase in SCr ≥ ×2 or eGFR decrease > 50% | UO < 0.5 mL/kg/h × 12 h |
| Failure | Increase in SCr ≥ ×3 or eGFR decrease > 75% or SCr ≥ 4.0 mg/dL | UO < 0.5 mL/kg/h × 24 h or anuria × 12 h |
| Loss | Persistent ARF = Complete loss of kidney function > 4 wk | |
| ESKD | End stage renal disease (>3 months) | |
| AKIN (an acute rise in SCr within 48 h) | ||
| Stage 1 | Same as RIFLE Risk or increase in SCr ≥ 0.3 mg/dL (≥26.4 μmol/L) | Same as RIFLE Risk |
| Stage 2 | Same as RIFLE Injury | Same as RIFLE Injury |
| Stage 3 | Increase in SCr ≥ ×3 or serum creatinine of ≥4.0 mg/dL with an acute increase of at least 0.5 mg/dL or RRT | Same as RIFLE Failure |
WRF: worsening renal function; CKD: chronic kidney disease; HF: heart failure; AKIN: acute kidney injury network; ARF: acute renal failure; d: days; ESKD: end-stage kidney disease; eGFR: estimated glomerular filtration rate; h: hour; RIFLE: risk of renal failure, injury to the kidney, failure of kidney function, loss of kidney function, and end-stage renal failure; RRT: renal replacement therapy; SCr: serum creatinine; UO: urine output; and wk: weeks.
3. Therapeutic Target and Limitations in Patients with Heart Failure and Chronic Kidney Disease
| Trial; Author; Year | Pts (n) | Design | Main Eligibility Criteria |
Primary Outcome | Mean Follow up (years) |
Renal Function Exclusion |
CKD Groups (eGFR, mL/min/ 1.73 m2) |
Main Findings |
|---|---|---|---|---|---|---|---|---|
| Angiotensin Converting Enzyme inhibitors | ||||||||
| CONSENSUS; 1987; The CONSENSUS Trial Study Group | 253 | Enalapril vs. Pl. | Congested HF, NYHA IV, cardiomegaly on chest X-ray | ACM | 0.5 | Serum creatinine concentration > 3.4 mg/dL | NA | Enalapril significantly reduced ACM in patients with sCr > 1.39 mg/dL compared to pl. (30% vs. 55%) but did not have a significant effect in those with sCr < 1.39 mg/dL. |
| SOLVD treatment; 1991; The SOLVD Investigators [19] | 2569 | Enalapril vs. Pl. | LVEF ≤ 35%, NYHA I–IV (90% NYHA II, III) | ACM | 3.4 | Creatinine > 2 mg/dL | ≥60 (n = 1466) (59, 7%) <60 (n = 1036) (40, 3%) |
Enalapril reduced mortality and hospitalization in SHF patients without significant heterogeneity between those with and without CKD. |
| SOLVD prevention; 1992; The SOLVD Investigators | 4228 | Enalapril vs. Pl. | Receiving digitalis, diuretics, or vasodilators (remainder same as SOLVD treatment trial) | ACM | 3.08 | Creatinine > 2 mg/dL | <45 (n = 450) 10.6% ≥45 <60 (n = 669) 15.8% ≥60 <75 (n = 640) 15.1% >75 (n = 863) 20.4 |
No significant interaction between CKD and treatment |
| SAVE; 1992; Tokmakova et al. [20] | 2331 | Captopril vs. Pl. | Acute myocardial infarction (age 21–80 years) LVEF < 40% |
ACM | 3.5 | Creatinine > 2.5 mg/dL | ≥60 (n = 1562) 67% <60 (n = 769) 33% |
Captopril reduced CV events irrespective of baseline kidney function. CKD was associated with a heightened risk for all major CV events after MI, particularly among subjects with an eGFR < 45 mL/min/1.73 m2. |
| AIRE; 1997; Hall et al. | 2006 | Ramipril vs. Pl. | Acute myocardial infarction (ECG and enzymes) and transient or persistent congestive heart failure after index infarct. Clinical CHF by physical examination or radiography. |
ACM | 1.25 | NA | NA | ACM significantly lower for Ramipril (17%) than pl. (23%). |
| TRACE; 1995; Køber et al. [21] | 1749 | Trandolapril vs. Pl. | Able to tolerate a test dose of 0.5 mg trandolapril adults with acute myocardial infarction 2–6 days prior to trial entry. Echocardiographic ejection fraction < 35% |
ACM | 3 | Creatinine > 2.5 mg/dL | NA | Trandalopril reduced relative risk of death. Trandolapril also reduces the risk of death from CV causes. |
| NETWORK; 1998; The NETWORK investigators |
1532 | Enalapril 2.5 vs. 5 vs. 10 mg BID | Age 18 to 85 years, NYHA II–IV, abnormality of the heart and current treatment for heart failure | ACM, HFH, WHF | 0.5 | Creatinine > 2.3 mg/dL | No relationship between dose of enalapril and clinical outcome in patients with HF. | |
| ATLAS; 1999; Packer et al. | 3174 | Lisinopril high vs. low dose | LVEF ≤ 30 NYHA II–IV |
ACM | 3.8 | Creatinine > 2.5 mg/dL | Creatinine > 1.5 mg/dL 2176 (68.5%) Creatinine < 1.5 mg/dL 998 (31.5%) |
ACM was non-significantly reduced both in patients with and without CKD. |
| Angiotensin Receptor Blockers | ||||||||
| Val-HeFT; 2003; Carson et al. [22] | 5010 | Valsartan vs. Pl. | LVEF < 40%; clinically stable CHF NYHA II–IV; treatment with ACE inhibitors; LVDD > 2.9 cm/bsa | ACM | 1.9 | Creatinine > 2.5 mg/dL | <60 2114 (47%) ≥60 2196 (53%) |
Patients with WRF demonstrated the same benefits with valsartan treatment compared with pl. in the overall population. |
| CHARM added, 2001; McMurray et al. [23] | 2548 | Candesartan vs. Pl. | LVEF ≤ 40%; NYHA II–IV; treatment with ACE inhibitor | CV death or HFH | 3.4 | Creatinine >3 mg/dL | ≥60 67% <60 33% |
The risk for CV death or hospitalization for worsening CHF as well as the risk for ACM increased significantly below an eGFR of 60 mL/min per 1.73 m2. |
| CHARM alternative, 2003; Granger et al. | 2028 | Candesartan vs. Pl. | CHF NYHA II–IV, LVEF ≤ 40%, ACE inhibitors intolerance | CV death or HFH | 2.8 | Creatinine > 3 mg/dL | ≥60 57.4% <60 42.6% |
See above |
| HEEAL; 2009; Konstam et al. | 3846 | High dose vs. Low dose Losartan | LVEF ≤ 40%; NYHA II–IV; ACE inhibitors intolerance | ACM or HFH | 4.7 | Creatinine > 2.5 mg/dL | NA | Losartan 150 mg vs. 50 mg maintained its net clinical benefit and was associated with reduced risk of death or HFH, despite higher rates of WRF and greater rates of eGFR decline. |
| Mineralcorticoid Receptor Antagonist | ||||||||
| RALES; 1999; Kulbertus et al. | 1663 | Spironolactone vs. Pl. | LVEF < 35%; NYHA III–IV; creatinine ≤ 2.5 mmol/L | ACM | 2 | creatinine ≥ 2.5 mg/dL | <60 (n = 792) 47.62% ≥60 (n = 866) 52.07% |
Individuals with reduced baseline eGFR exhibited similar relative risk reductions in all-cause death and the combined. Endpoint of death or hospital stayed for HF as those with normal renal function and greater absolute risk reduction compared with those with a higher baseline eGFR. |
| EMPHASIS-HF, 2001; Zannad et al. [26] | 2737 | Eplerenone vs. Pl. | LVEF ≤ 35%; NYHA II; eGFR ≥ 30 mL/min/1.73 m | CV death or HFH | 1.75 | eGFR < 30 mL/min/1.73 m | <60 (n = 912) 33.32% ≥60 (n = 1821) 66.53% |
Eplerenone, as compared with placebo, reduced both the risk of death and the risk of hospitalization in HFrEF patients with CKD. |
| TOPCAT; 2021; Khumbanj, et al. | 3445 | Spironolactone vs. placebo (n = 3445) | LVEF ≥ 45%; HF hospitalization or elevated NP level; eGFR ≥ 30 mL/min/1.73 m2 or creatinine ≤ 2.5 | CV death or aborted cardiac arrest or hospitalization for HF | 3.3 | eGFR < 30 mL/min/1.73 m or serum creatinine >2.5 mg/dL |
<45 (n = 411) 11.9% 45–60 (n = 533) 15.47% ≥60 (n = 823) 23.88% |
The primary endpoint was similar between the spironolactone and placebo arms. The risk of adverse events was amplified in the lower eGFR categories. These data supported use of spironolactone to treat HFpEF patients with advanced CKD only when close laboratory surveillance was possible. |
This entry is adapted from the peer-reviewed paper 10.3390/jcm11082243
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic. Eur. Heart J. 2021, 42, 4901.
- Onyali, C.B.; Anim-Koranteng, C.; Shah, H.E.; Bhawnani, N.; Ethirajulu, A.; Alkasabera, A.; Mostafa, J.A. Role of Selective Sodium-Glucose Co-Transporter-2 Inhibitors in Managing Cardio-Renal Complications in Type 2 Diabetes Mellitus: Beyond Glycemic Control. Cureus 2021, 13, e17452.
- Ruocco, G.; Palazzuoli, A.; Ter Maaten, J.M. Loop diuretics in acute heart failure: Beyond the decongestive relief for the kidney. Crit. Care 2015, 19, 296.
- Palazzuoli, A.; Ruocco, G.; Pellegrini, M.; Martini, S.; Del Castillo, G.; Beltrami, M.; Franci, B.; Lucani, B.; Nuti, R. Patients with cardiorenal syndrome revealed increased neurohormonal activity, tubular and myocardial damage compared to heart failure patients with preserved renal function. Cardiorenal Med. 2014, 4, 257–268.
- Beltrami, M.; Ruocco, G.; Ibrahim, A.; Lucani, B.; Franci, B.; Nuti, R.; Palazzuoli, A. Different trajectories and significance of B-type natriuretic peptide, congestion and acute kidney injury in patients with heart failure. Intern. Emerg. Med. 2017, 12, 593–603.
- Di Lullo, L.; Bellasi, A.; Barbera, V.; Ronco, C. Cardionephrology and cardiorenal disease in Italy: State of the art. Rev. Cardiovasc. Med. 2021, 22, 563–572.
- Palazzuoli, A.; Beltrami, M.; Nodari, S.; McCullough, P.A.; Ronco, C. Clinical impact of renal dysfunction in heart failure. Rev. Cardiovasc. Med. 2011, 12, 186–199.
- Lofman, I.; Szummer, K.; Evans, M.; Carrero, J.J.; Lund, L.H.; Jernberg, T. Incidence of, associations with and prognostic impact of worsening renal function in heart failure with different ejection fraction categories. Am. J. Cardiol. 2019, 124, 1575–1583.
- Quiroz, R.; Doros, G.; Shaw, P.; Liang, C.S.; Gauthier, D.F.; Sam, F. Comparison of characteristics and outcomes of patients with heart failure preserved ejection fraction versus reduced left ventricular ejection fraction in an urban cohort. Am. J. Cardiol. 2014, 113, 691–696.
- Ather, S.; Chan, W.; Bozkurt, B.; Aguilar, D.; Ramasubbu, K.; Zachariah, A.A.; Wehrens, X.H.; Deswal, A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J. Am. Coll. Cardiol. 2012, 59, 998–1005.
- McAlister, F.A.; Ezekowitz, J.; Tarantini, L.; Squire, I.; Komajda, M.; Bayes-Genis, A.; Gotsman, I.; Whalley, G.; Earle, N.; Poppe, K.K.; et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: Impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ. Heart Fail. 2012, 5, 309–314.
- Streng, K.W.; Nauta, J.F.; Hillege, H.L.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Lang, C.C.; Metra, M.; Ng, L.L.; et al. Non-cardiac comorbidities in heart failure with reduced, mid-range and preserved ejection fraction. Int. J. Cardiol. 2018, 271, 132–139.
- Smith, G.L.; Lichtman, J.H.; Bracken, M.B.; Shlipak, M.G.; Phillips, C.O.; DiCapua, P.; Krumholz, H.M. Renal impairment and outcomes in heart failure: Systematic review and meta-analysis. J. Am. Coll. Cardiol. 2006, 47, 1987–1996.
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469.
- Meta-Analysis Global Group in Chronic Heart Failure. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta-analysis. Eur. Heart J. 2012, 33, 1750–1757.
- Lofman, I.; Szummer, K.; Dahlstrom, U.; Jernberg, T.; Lund, L.H. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1606–1614.
- Ruocco, G.; Palazzuoli, A.; Ter Maaten, J.M. The role of the kidney in acute and chronic heart failure. Heart Fail. Rev. 2020, 25, 107–118.
- Beldhuis, I.E.; Streng, K.W.; van der Meer, P.; Ter Maaten, J.M.; O’Connor, C.M.; Metra, M.; Dittrich, H.C.; Ponikowski, P.; Cotter, G.; Cleland, J.G.F.; et al. Trajectories of Changes in Renal Function in Patients with Acute Heart Failure. J. Card Fail. 2019, 25, 866–874.
- Bowling, C.B.; Sanders, P.W.; Allman, R.M.; Rogers, W.J.; Patel, K.; Aban, I.B.; Rich, M.W.; Pitt, B.; White, M.; Bakris, G.C.; et al. Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: Insights from the SOLVD Treatment trial. Int. J. Cardiol. 2013, 167, 151–156.
- Jose, P.; Skali, H.; Anavekar, N.; Tomson, C.; Krumholz, H.M.; Rouleau, J.L.; Moye, L.; Pfeffer, M.A.; Solomon, S.D. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J. Am. Soc. Nephrol. 2006, 17, 2886–2891.
- Køber, L.; Torp-Pedersen, C.; Carlsen, J.E.; Bagger, H.; Eliasen, P.; Lyngborg, K.; Videbaek, J.; Cole, D.S.; Auclert, L.; Pauly, N.C. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N. Engl. J. Med. 1995, 333, 1670–1676.
- Cohn, J.N.; Tognoni, G.; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 2001, 345, 1667–1675.
- Young, J.B.; Dunlap, M.E.; Pfeffer, M.A.; Probstfield, J.L.; Cohen-Solal, A.; Dietz, R.; Granger, C.B.; Hradec, J.; Kuch, J.; McKelvie, R.S.; et al. Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: Results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004, 110, 2618–2626.
- McCallum, W.; Tighiouart, H.; Ku, E.; Salem, D.; Sarnak, M.J. Acute declines in estimated glomerular filtration rate on enalapril and mortality and cardiovascular outcomes in patients with heart failure with reduced ejection fraction. Kidney Int. 2019, 96, 1185–1194.
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869.
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B.; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21.
