Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Neurotrophins (NTs) are a group of soluble growth factors with analogous structures and functions, identified initially as critical mediators of neuronal survival during development. The relevance of NTs has been confirmed by emerging clinical data showing that impaired NTs levels and functions are involved in the onset of neurological and pulmonary diseases.
- neurotrophins
- neurotrophic factors
- brain
1. Introduction
Neurotrophins (NTs) are a group of soluble growth factors with analogous structures and functions, identified initially as critical mediators of neuronal survival during development. They include nerve growth factor (NGF), the first and best characterised NT, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). These proteins can regulate many aspects of neural functions in the nervous system and airway tree, modulating synaptic function, plasticity, neuronal survival, lung innervation, and inflammatory response, respectively [1][2][3][4].
NTs are produced by neuronal and non-neuronal cells: among the latter ones, in the central nervous system (CNS), in vivo studies confirmed the expression of BDNF by endothelial cells, astrocytes, and oligodendrocytes. Notably, the production of BDNF has been rated 50-fold higher in cerebral endothelial cells than in cortical neurons. However, the neurons present distinct regulated and constitutive secretory pathways, whereas the non-neuronal cells have only the constitutive pathway [5][6].
NTs are synthesised as preproprotein precursors and cleaved intracellularly by metalloproteases into pro-NTs. They are further processed to generate mature NTs, featured by extensive homology and structural similarities. Alternatively, NTs may be secreted in the pro-form and undergo extracellular cleavage into the smaller active peptides [4][7].
2. Neurotrophins: Role and Characteristics
NTs may exert their action through two different mechanisms: a genomic one, which is considered a slow pathway, and a non-genomic mechanism, which has a more rapid effect. By acting in a genomic manner, the NTs may regulate cell growth and survival and signalling events such as ionic fluxes (Figure 1). The term ‘non-genomic’ refers to the effects noted within short time frames and excludes subsequent genomic effects that may occur in response even to a brief stimulation with NTs [7].
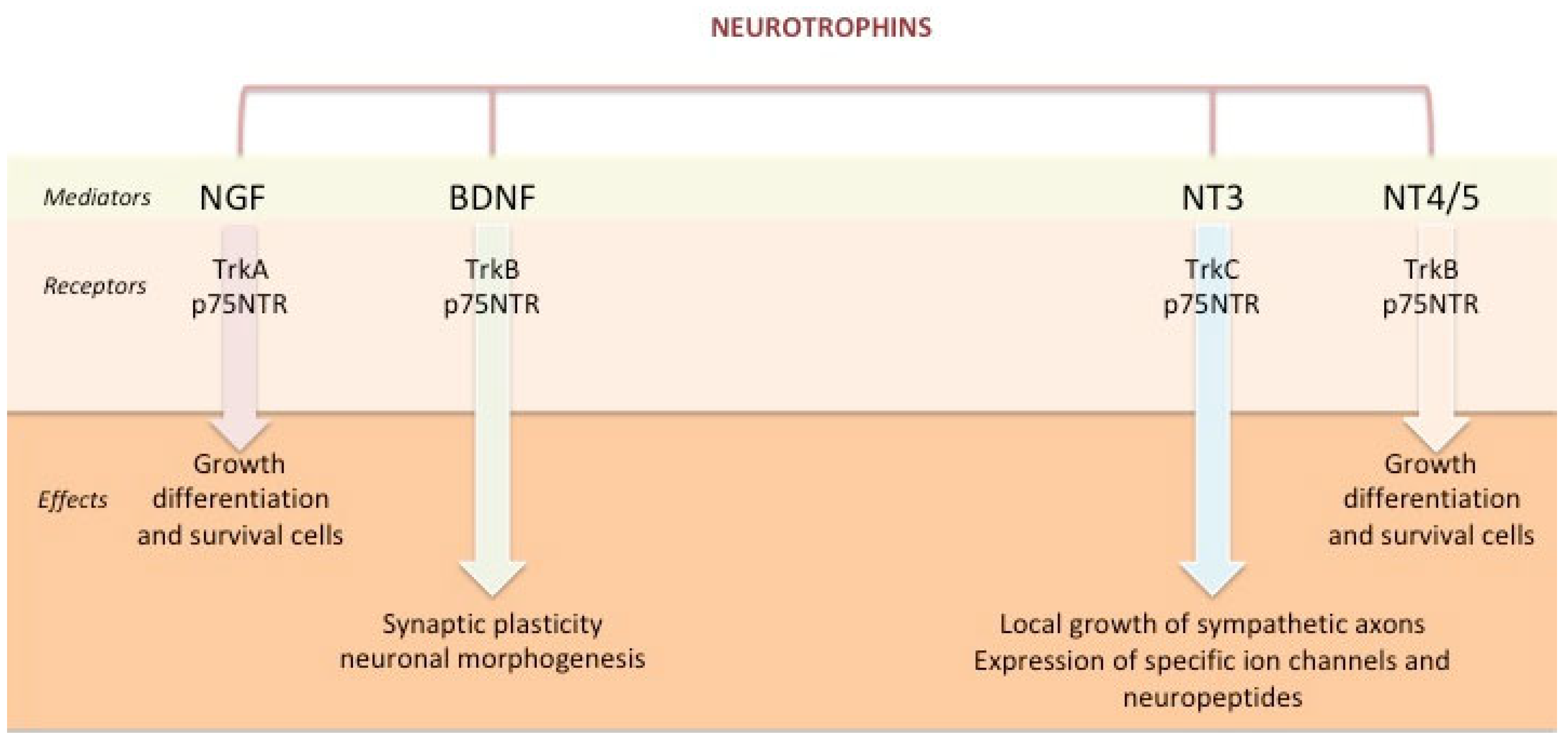
Figure 1. Neurotrophins: Role and effects. Legend: NGF = nerve growth factor; BDNF = brain-derived neurotrophic factor; NT = neurotrophin; Trk = tropomyosin-related kinase P75NTR = P75 neurotrophin receptor.
Two different types of cell surface receptors are involved in the NTs-mediated genomic effects: p75 neurotrophin receptor (p75NTR) and Tropomyosin-related kinase (Trk) receptors [8][9]. Both p75NTR and Trk receptors activate multiple and distinct signalling pathways [3][10]. The effects induced by these receptors are complex since they bind the NTs independently or interact with each other, producing different results [5].
p75NTR is a 75 kDa receptor that binds all NTs with low affinity. It belongs to the tumour necrosis factor (TNF) receptor superfamily and, similar to other family members, contains a death domain that docks with intracellular adapter proteins. The interaction with p75NTR activates a downstream signalling cascade involving transcriptional factors such as NFκB and leading to cellular apoptosis [1][2][11][12]. This process may require the expression of specific adaptor proteins as well as impaired Trk signalling and is the result of the interaction between p75NTR and NTs in their immature form [13][14]. However, its primary function is to act as a coreceptor with Trks to create high-affinity NT receptors [13].
Trk receptors are a group of 140 kDa tyrosine kinases with greater specific affinity for the different NTs, preferentially in their mature forms. They are regulated by p75NTR and require its presence to bind the NTs with high affinity: TrkA was first discovered as the primary signalling receptor for NGF, but in an alternatively spliced form, it can also bind to NT-3 with different effects; TrkB primarily interacts with BDNF and NT-4 and shows a lesser affinity for NT-3; and TrkC preferentially binds to NT-3 [5][13][15]. The NT/Trks binding results in the phosphorylation and pairing of intracellular tyrosine residues. This leads to rapid activation of downstream signalling cascades, such as extracellular-regulated kinase (Ras/MAPK/ERK), phosphoinositide-3 kinase/Akt (protein kinase B), and phospholipase Cγ pathways. These cascades activate cell-specific transcription factors responsible for differentiation and cell survival, apoptosis, and cell growth [15].
Regarding the “non-genomic” effect, NTs have relatively immediate effects and exert their action by binding high-affinity Trk receptors. Furthermore, NTs can directly modulate plasma membrane receptors (e.g., N-methyl-D-aspartate receptors), cationic channels (e.g., Ca2+ influx and voltage-gated Na+ channels), and other key mechanisms to neuronal function [1][2].
3. Neurotrophins and Brain
Since their discovery in 1951, several studies have demonstrated the role of NTs in the developing brain and the mature nervous system. They mediate cell migration and proliferation, synapse formation and function, and regulation of dendritic outgrowth and axonal orientation [16][17][18]. NTs are also involved in motor and cognitive functioning through direct interaction with different neurotransmitter systems: particularly, BDNF participates in the long-term potentiation by up-regulating the N-methyl-D-aspartate (NMDA) receptors, inducing the expression of Gamma-Aminobutyric Acid (GABA) receptor subunit genes, and facilitating the synthesis of choline acetyltransferase, allowing the production and release of acetylcholine (ACh) [18][19][20].
3.1. Neurotrophins and Brain Development
As previously discussed, p75NTR may bind the NTs with lesser affinity or act as a coreceptor with Trks to create high-affinity NT receptors, leading to cell survival or death depending on the ligand [13]. When p75NTR interacts with the immature forms of NTs, it induces proapoptotic signals. It has been demonstrated that the interaction pro-BDNF/p75NTR exerts an opposite action to the antiapoptotic effect of BDNF/TrkB [21]. Finally, p75NTR is also implicated in recycling the NTs and orienting the axon through axon-repulsive agents (e.g., myelin-associated glycoprotein) [17]. During the first trimester of pregnancy (8th–9th gestational weeks), a distinct group of heterogeneous post-migratory cells organizes in the so-called “cortical plate”, containing the neurons that will give rise to the cortical layers 2 to 6. The compartment of cells below the cortical plate constitutes the subplate and expresses markers of synaptic connectivity and axonal outgrowth. At the beginning of the third month of gestation (9th–10th weeks), a group of early-born glutamatergic neurons in the subplate zone expresses the p75NTR and starts to extend subcortical axons [22].
Furthermore, Trk receptors have also been detected in several animal models at early stages of neuronal development: TrkC appears during neurulation in the neural plate of a chicken embryo, participating in neuronal migration. At the same time, TrkB has been identified in the neural tube and especially in the motor neuron progenitors [23]. The presence of NTs receptors since the first trimester of gestation indirectly supports the neurotrophic hypothesis. According to this assumption, in the early stages of development of the nervous system, there is an excess of neurons, some superfluous and inappropriately connected. As a consequence, neuronal survival or death is regulated by NTs produced in their target tissues [24].
Studies on NGF proved that the interaction between TrkA and NGF induces the activation of the Raf pathway, leading to axonal elongation [17][25]. Moreover, NGF/TrkA binding is implicated in neurite outgrowth, development and survival of cholinergic neurons in the CNS, and the survival and maintenance of peripheral neurons [26]. Therefore, during brain development, NGF is considered a critical growth factor for neuronal survival and especially the nociceptive phenotype of neurons [27].
On the other hand, BDNF is widely expressed in the CNS and represents the main regulator of its development and maturation since it is involved in neuronal growth, differentiation, and synaptic and structural plasticity [18]. In fact, BDNF-positive neurons in the frontal lobe increase in size during the fourth month of gestation [28]. Furthermore, it is implicated in establishing neurogenesis, maintaining brain homeostasis, and neuroprotection from the neurotoxic effects of inflammation [26]. It is also responsible, together with NT-3, for the regulation of angiogenesis and vessel maintenance during embryogenesis [17][18].
Conversely, NT-4 is the least studied factor among the NTs. Despite the overlapping distribution and receptor interaction with BDNF in the fetal nervous system, it has been suggested that NT-4 and BDNF may induce growth, differentiation, and cell death through different mechanisms. In addition, it has been proposed a role in the survival of motor and sensory neurons (especially the ones present in the taste buds of the tongue and palate), in the outgrowth of neurite ganglia in the retina, and synapse formation in the hippocampus [29].
There are sparse data in the literature regarding the role of NTs on the human nervous system development during pregnancy. NTs are known to be produced by the placenta and the fetal brain, and some authors hypothesised that especially BDNF and NT-3 participate in the preimplantation stage and early embryonic development [26]. Most of the research focused on BDNF for its involvement in the survival of CNS neurons during pregnancy. Many studies analysed its levels in the maternal blood and amniotic fluid during gestation to better understand its role in neurodevelopment. Indeed, studies on animal models demonstrated that BDNF levels in the maternal blood are consistent with the fetal brain ones [30].
Several hypotheses have been formulated on the synthesis and role of BDNF during gestation. Given that NTs can cross the fetoplacental barrier, Antonakopoulos et al. suggested that maternal BDNF reaches the fetal brain through the placenta and sustains brain maturation [28]. Accordingly, a longitudinal study showed a significant decline in serum BDNF levels in women from the first trimester to the second and from the second to the third. After the partum, serum BDNF levels significantly increase, reaching the previous ranges, thus proving the utilisation of maternal BDNF by the placenta and the fetus [30]. On the other hand, some authors reported increased levels of BDNF during the last trimester of pregnancy compared to the first trimester. This evidence led to the hypothesis of a combined production from the placenta and the fetus, being the BDNF mainly produced by the fetus during the second trimester [26][28]. BDNF levels have also been associated with the fetus’s growth impairment, with higher levels in intrauterine growth restriction and lower ones in maternal type 1 diabetes and nondiabetic macrosomia. Based on these data, it has been proposed that increased BDNF production may represent an adaptive response to an intrauterine adverse environment [26][28]. In addition, prematurity is also associated with reduced BDNF and NT-3 levels; therefore, lower neonatal BDNF levels have been suggested as an early marker of abnormal neurodevelopmental outcomes in preterm infants [18][26].
3.2. Neutrophins and Neurologic Diseases
For their role in neurogenesis, neuronal survival and differentiation, and synaptic function, the involvement of NTs in several neurological diseases is well established, both during the developmental age and adulthood (Table 1) [6][19][31][32][33][34][35][36].
Table 1. NTs/receptors interactions and implications in brain diseases.
| Neurotrophin | Receptor | Brain Region | Effects | Implication in Diseases | References |
|---|---|---|---|---|---|
| BDNF | TrkB | Hippocampus | Promotion of mossy fibers sprouting of the dentate granule cells | ↓ BDNF in deficits of learning and memory ↓ BDNF in ASD ↑ BDNF in seizure activity in temporal lobe epilepsy |
[14][19][29][31][35][37][38][39] |
| TrkB/ P75NTR |
Brainstem and cerebellum | Maturation of GABAergic neurons and development of synapses | ↓ BDNF in RTT syndrome | ||
| NA | HPA axis | Survival and homeostasis of dopaminergic neurons | Controversial data on BDNF in ADHD | ||
| TrkB/ P75NTR |
Frontal cortices and hippocampus | Survival, proliferation, differentiation, and synaptic plasticity | ↓ BDNF in schizophrenia ↓ BDNF in depression |
||
| TrkB | CNS | Survival, proliferation, differentiation, and synaptic plasticity | ↑ BDNF in neonatal blood of children with ASD and ID | ||
| NGF | TrkA | Basal forebrain and prefrontal cortex | Survival of cholinergic neurons and involvement in learning processes and attention systems | ↑ NGF in ADHD | [36] |
| TrkA/ P75NTR |
CNS | Homeostasis of mitochondria | ↑ NGF in ASD | ||
| NT-3 | NA | Cerebellum | Survival of neurons and cerebellar growth | ↑ NT-4 in ASD | [26] |
| NT-4 | TrkB | CNS | Survival, proliferation, differentiation, and synaptic plasticity | ↑ NT-4 in neonatal blood of children with ASD and ID | [29] |
Studies on hippocampal tissue of BDNF-knocked-out mice proved the implication of BDNF in synaptic plasticity and the development of a stable long-term potentiation, affecting learning and memory. Moreover, the BDNF/TrkB activation has been proposed as a mechanism of epileptogenesis promotion: BDNF induces the sprouting of the GABAergic mossy fibre of dentate granule cells, increasing seizure susceptibility. Accordingly, different studies proved that BDNF-knocked-out mice show a reduction in kindling, while transgenic mice that over-express BDNF present more severe seizures in response to kainic acid and spontaneous seizures [19].
In addition, the impairment of the synaptic plasticity of hippocampal GABAergic neurons caused by a reduction in BDNF/TrkB signalling has also been implicated in the development of Autism Spectrum Disorders (ASD) [35]. In the last few decades, several studies have analysed the involvement of NTs in Autism and other neurodevelopmental pathologies. Very recently, Gevezova et al. investigated the role of NGF in ASD and its possible use as a biomarker of mitochondrial function in these patients. The authors propose NGF as a regulator of biogenesis and homeostasis of the mitochondria, supporting its role in the aetiology and progression of ASD and other neurological diseases [36].
Recent studies on patients affected by schizophrenia have also detected a decrease in BDNF levels in CNS and peripheral serum samples. Accordingly, similar data were observed in several animal models of other neuropsychiatric disorders, such as anxiety and memory impairment [14]. Notably, acute and chronic social stress has been related to an alteration in NTs levels such as NGF and BDNF in humans and animals [40]. Recently, research proved a correlation between BDNF levels in the nucleus accumbens and the susceptibility to induced stress (e.g., defeat stimulation), causing social avoidance and anxiety-like and depression-like behaviours [41]. Moreover, even in a mouse model of post-traumatic stress disorder, in which the animals witnessed defeat events, the stress may provoke anxiety-like behaviours related to decreased BDNF levels in the hippocampus and medial prefrontal cortex and increased BDNF levels in the amygdala [42]. This evidence supports the role of the NTs in the stress-induced response mediated by the hypothalamus–pituitary–adrenocortical axis [40][42]. Finally, a reduction in neurotrophic support by BDNF during adulthood has been suggested by some authors in the development of neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and Huntington’s diseases [19].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24087089
References
- Prakash, Y.; Thompson, M.A.; Meuchel, L.; Pabelick, C.M.; Mantilla, C.B.; Zaidi, S.; Martin, R.J. Neurotrophins in lung health and disease. Expert Rev. Respir. Med. 2010, 4, 395–411.
- Rubin, L.; Stabler, C.T.; Schumacher-Klinger, A.; Marcinkiewicz, C.; Lelkes, P.I.; Lazarovici, P. Neurotrophic factors and their receptors in lung development and implications in lung diseases. Cytokine Growth Factor Rev. 2021, 59, 84–94.
- Prakash, Y.; Martin, R.J. Brain-derived neurotrophic factor in the airways. Pharmacol. Ther. 2014, 143, 74–86.
- Bartkowska, K.; Turlejski, K.; Djavadian, R.L. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol. Exp. 2010, 70, 454–467.
- Bothwell, M. NGF, BDNF, NT3, and NT4. Organotypic Models Drug Dev. 2014, 220, 3–15.
- Pejhan, S.; Siu, V.M.; Ang, L.C.; Del Bigio, M.R.; Rastegar, M. Differential brain region-specific expression of MeCP2 and BDNF in Rett Syndrome patients: A distinct grey-white matter variation. Neuropathol. Appl. Neurobiol. 2020, 46, 735–750.
- Purves, D.; Augustine, G.J.; Fitzpatrick, D. Neurotransmitter Synthesis. In Book Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11110/ (accessed on 23 March 2023).
- Skaper, S.D. The Biology of Neurotrophins, Signalling Pathways, and Functional Peptide Mimetics of Neurotrophins and their Receptors. CNS Neurol. Disord. Drug Targets 2008, 7, 46–62.
- Underwood, C.K.; Coulson, E.J. The p75 neurotrophin receptor. Int. J. Biochem. Cell Biol. 2008, 40, 1664–1668.
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564.
- Barbacid, M. Structural and functional properties of the TRK family of neurotrophin receptors. Ann. N. Y. Acad. Sci. 1995, 766, 442–458.
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309.
- Hempstead, B.L. The many faces of p75NTR. Curr. Opin. Neurobiol. 2002, 12, 260–267.
- Manchia, M.; Isayeva, U.; Collu, R.; Primavera, D.; Deriu, L.; Caboni, E.; Iaselli, M.N.; Sundas, D.; Tusconi, M.; Pinna, F.; et al. Converging Evidence Points to BDNF as Biomarker of Depressive Symptoms in Schizophrenia-Spectrum Disorders. Brain Sci. 2022, 12, 1666.
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642.
- Levi-Montalcini, R. The nerve growth factor. Ann. N. Y. Acad. Sci. 2006, 118, 149–170.
- Segal, R.A. Selectivity in neurotrophin signaling: Theme and Variations. Annu. Rev. Neurosci. 2003, 26, 299–330.
- Krey, F.C.; Stocchero, B.A.; Creutzberg, K.C.; Heberle, B.A.; Tractenberg, S.G.; Xiang, L.; Wei, W.; Kluwe-Schiavon, B.; Viola, T.W. Neurotrophic Factor Levels in Preterm Infants: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 643576.
- Hu, Y.; Russek, S.J. BDNF and the diseased nervous system: A delicate balance between adaptive and pathological processes of gene regulation. J. Neurochem. 2008, 105, 1–17.
- Yao, Q.; Zaidi, S.I.; Haxhiu, M.A.; Martin, R.J. Neonatal Lung and Airway Injury: A Role for Neurotrophins. Semin. Perinatol. 2006, 30, 156–162.
- Zagrebelsky, M.; Tacke, C.; Korte, M. BDNF signaling during the lifetime of dendritic spines. Cell Tissue Res. 2020, 382, 185–199.
- Bystron, I.; Blakemore, C.; Rakic, P. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 2008, 9, 110–122.
- Bernd, P. The Role of Neurotrophins During Early Development. Gene Expr. 2008, 14, 241–250.
- Davies, A.M. The emerging generality of the neurotrophic hypothesis. Trends Neurosci. 1988, 11, 243–244.
- Markus, A.; Zhong, J.; Snider, W.D. Raf and Akt Mediate Distinct Aspects of Sensory Axon Growth. Neuron 2002, 35, 65–76.
- Sahay, A.; Kale, A.; Joshi, S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides 2020, 83, 102075.
- Lewin, G.R. Neurotrophins and the specification of neuronal phenotype. Philos. Trans. R. Soc. B Biol. Sci. 1996, 351, 405–411.
- Antonakopoulos, N.; Iliodromiti, Z.; Mastorakos, G.; Iavazzo, C.; Valsamakis, G.; Salakos, N.; Papageorghiou, A.; Margeli, A.; Kalantaridou, S.; Creatsas, G.; et al. Association between Brain-Derived Neurotrophic Factor (BDNF) Levels in 2nd Trimester Amniotic Fluid and Fetal Development. Mediat. Inflamm. 2018, 2018, 8476217.
- Benn, K.; Passos, M.; Jayaram, A.; Harris, M.; Bongiovanni, A.M.; Skupski, D.; Witkin, S.S. Association Between Neurotrophin 4 and Long-Chain Polyunsaturated Fatty Acid Levels in Mid-Trimester Amniotic Fluid. Reprod. Sci. 2014, 21, 1395–1400.
- Christian, L.M.; Mitchell, A.M.; Gillespie, S.L.; Palettas, M. Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: Associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology 2016, 74, 69–76.
- Gumus, C.; Yazici, I.P.; Yazici, K.U.; Ustundag, B. Increased Serum Brain-derived Neurotrophic Factor, Nerve Growth Factor, Glial-derived Neurotrophic Factor and Galanin Levels in Children with Attention Deficit Hyperactivity Disorder, and the Effect of 10 Weeks Methylphenidate Treatment. Clin. Psychopharmacol. Neurosci. 2022, 20, 635–648.
- Li, W.; Pozzo-Miller, L. BDNF deregulation in Rett syndrome. Neuropharmacology 2014, 76, 737–746.
- Nelson, K.B.; Grether, J.K.; Croen, L.A.; Dambrosia, J.M.; Dickens, B.F.; Jelliffe, L.L.; Hansen, R.L.; Phillips, T.M. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann. Neurol. 2001, 49, 597–606.
- Sajdel-Sulkowska, E.M.; Xu, M.; Koibuchi, N. Increase in Cerebellar Neurotrophin-3 and Oxidative Stress Markers in Autism. Cerebellum 2009, 8, 366–372.
- Sgritta, M.; Vignoli, B.; Pimpinella, D.; Griguoli, M.; Santi, S.; Bialowas, A.; Wiera, G.; Zacchi, P.; Malerba, F.; Marchetti, C.; et al. Impaired synaptic plasticity in an animal model of autism exhibiting early hippocampal GABAergic-BDNF/TrkB signaling alterations. iScience 2022, 26, 105728.
- Gevezova, M.; Minchev, D.; Pacheva, I.; Todorova, T.; Yordanova, R.; Timova, E.; Ivanov, I.; Sarafian, V. Association of NGF and Mitochondrial Respiration with Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 11917.
- Camuso, S.; La Rosa, P.; Fiorenza, M.T.; Canterini, S. Pleiotropic effects of BDNF on the cerebellum and hippocampus: Implications for neurodevelopmental disorders. Neurobiol. Dis. 2022, 163, 105606.
- Francis, K.; Dougali, A.; Sideri, K.; Kroupis, C.; Vasdekis, V.; Dima, K.; Douzenis, A. Brain-derived neurotrophic factor (BDNF) in children with ASD and their parents: A 3-year follow-up. Acta Psychiatr. Scand. 2018, 137, 433–441.
- Bryn, V.; Halvorsen, B.; Ueland, T.; Isaksen, J.; Kolkova, K.; Ravn, K.; Skjeldal, O. Brain derived neurotrophic factor (BDNF) and autism spectrum disorders (ASD) in childhood. Eur. J. Paediatr. Neurol. EJPN 2015, 19, 411–414.
- Alleva, E.; Santucci, D. Psychosocial vs. “physical” stress situations in rodents and humans: Role of neurotrophins. Physiol. Behav. 2001, 73, 313–320.
- Miao, Z.; Wang, Y.; Sun, Z. The Relationships Between Stress, Mental Disorders, and Epigenetic Regulation of BDNF. Int. J. Mol. Sci. 2020, 21, 1375.
- Miao, Z.; Mao, F.; Liang, J.; Szyf, M.; Wang, Y.; Sun, Z.S. Anxiety-Related Behaviours Associated with microRNA-206-3p and BDNF Expression in Pregnant Female Mice Following Psychological Social Stress. Mol. Neurobiol. 2018, 55, 1097–1111.
This entry is offline, you can click here to edit this entry!
