Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Ceramics
Severe plastic deformation (SPD) through the high-pressure torsion (HPT) process has been effectively used in recent years to develop novel active catalysts for CO2 conversion. HPT as an SPD method has been used since 1935 until now for grain refinement and the production of nanostructured materials. In addition to grain refinement, the introduction of various defects, such as vacancies and dislocations, is another feature of HPT, which resulta in the improvement of the functionality of materials proceeded by this method.
- functional properties
- ultrafine-grained (UFG) materials
- nanostructured materials
1. Introduction
Nowadays, environmental crises, especially global warming caused by CO2 emission from burning fossil fuels and humankind activities, are considered one of the most significant challenges in the world. Reduction of CO2 to reactive CO gas or useful components and fuels, such as CH4 and CH3OH, using photocatalysts is one of the clean and new strategies, which is developing rapidly [1,2,3]. In photocatalytic CO2 conversion, excited electrons transfer from the valence band to the conduction band of the photocatalysts by solar irradiation and contribute to the reduction of CO2 to form desirable products, as shown in Figure 1a [3]. To perform these reduction reactions, some thermodynamic and kinetic conditions should be provided. From the viewpoint of thermodynamics, the standard potential of the reduction and oxidation reactions should be between the valence band and the conduction band of the photocatalyst [3,4]. On the other hand, from the kinetic viewpoint, the electrons should absorb the light, transfer to the conduction band, migrate to the surface of the photocatalyst, and take part in the reactions before combining with the holes [3,4]. To satisfy these kinetic and thermodynamic conditions, a photocatalyst should have some features, including high light absorbance, appropriate band structure, low recombination rate of electrons and holes, easy migration of charge carriers, and high surface affinity to adsorb CO2 with abundant active sites [3,4]. A combination of these thermodynamic and kinetic factors determines the speed of the reactions and the type of final products in photocatalysis.
Semiconductors, such as TiO2 [5,6,7], g-C3N4 [8,9], and BiVO4 [10,11,12], are typical photocatalysts that have been engineered by various strategies to enhance the catalytic efficiency for CO2 conversion. Doping with impurities, such as nitrogen, phosphorous, copper, and palladium [13,14,15]; defect engineering [16,17]; strain engineering [18,19]; mesoporous structure production [20]; and heterojunction introduction [21,22] are some of the most promising strategies that have been used so far to improve the optical properties and catalytic activity of various photocatalysts. Among these strategies, doping with impurities is the most investigated and feasible method, but impurities can increase the recombination rate of electrons and holes [13,14,15]. Therefore, finding new strategies to improve the photocatalytic activity and suppress the recombination rate of electrons and holes is a key issue. In this regard, severe plastic deformation (SPD) through the high-pressure torsion (HPT) method, which is mainly used for nanostructuring of metallic materials, has been used as a new tool to develop active photocatalysts for water splitting [23,24,25,26,27,28,29,30], dye degradation [31,32,33,34], and especially CO2 conversion [35,36,37,38]. This method not only does not increase the recombination rate of electrons and holes but also effectively suppresses it and improves some other optical properties. The SPD method has also been used effectively to synthesize new families of catalysts, such as high-pressure photocatalysts and high-entropy photocatalysts [23,27].
2. Influence of HPT on Photocatalytic CO2 Conversion
HPT as an SPD method has been used since 1935 until now for grain refinement and the production of nanostructured materials. In addition to grain refinement, the introduction of various defects, such as vacancies and dislocations, is another feature of HPT, which resulta in the improvement of the functionality of materials proceeded by this method [39,40]. In the HPT method, both large shear strain and high pressure (in the range of several gigapascals) are simultaneously utilized to process or synthesize various ranges of materials [39,40]. Strain and pressure are applied to the material (disc or ring shape) using two anvils that rotate with respect to each other, as shown in Figure 1b [41]. Due to the high processing pressure in HPT, it is applicable to hard and less ductile materials, such as high-melting temperature metals (hafnium [42], molybdenum [43], and tungsten [44]), amorphous glasses [45,46], silicon-based semiconductors [47,48], and even ultrahard diamond [49,50]. Another advantage of HPT is its capacity to induce ultra-SPD (i.e., shear strains over 1000 for mechanical alloying) [51]. The inducing ultra-SPD [51] together with fast dynamic diffusion [52,53] introduces the HPT method as a unique path to mechanically synthesize new materials even from immiscible systems [54,55]. Due to these unique features of HPT, the method was even used for the process and synthesis of hard and brittle ceramics, but the number of publications on ceramics is quite limited despite the high potential of these materials for various applications [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. Published studies regarding ceramics processed or synthesized by HPT are presented in Table 1, although there are other classic publications on HPT processing of ceramics mainly by physicists and geologists [40].
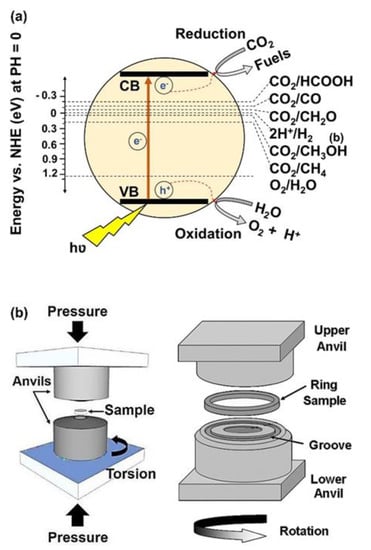
Figure 1. Schematic illustration of (a) photocatalytic CO2 conversion and (b) high-pressure torsion [41].
Table 1. Summary of major publications about ceramics treated by high-pressure torsion and their major properties and applications.
| Materials | Investigated Properties and Applications | Reference |
|---|---|---|
| Various Materials | Impact of pressure and strain on allotropy | Bridgman (1935) [56] |
| α-Al2O3 | Microstructure and mechanical properties | Edalati et al. (2010) [57] |
| ZrO2 | Allotropic phase transformations | Edalati et al. (2011) [58] |
| CuO | Dielectric properties | Makhnev et al. (2011) [59] |
| CuO, Y3Fe5O12, FeBO3 | Optical properties and electronic structure | Gizhevskii et al. (2011) [60] |
| ZrO2 | Phase transformation | Delogu et al. (2012) [61] |
| Cu2O, CuO | Middle infrared absorption and X-ray absorption | Mostovshchikova et al. (2012) [62] |
| CuO, Y3Fe5O12, FeBO3 | Optical properties | Telegin et al. (2012) [63] |
| BaTiO3 | Optical and dielectric properties | Edalati et al. (2015) [64] |
| TiO2-II | Photocatalytic activity for hydrogen production | Razavi-Khosroshahi et al. (2016) [23] |
| Various Materials | Review on HPT | Edalati et al. (2016) [40] |
| TiO2 | Plastic strain and phase transformation | Razavi-Khosroshahi et al. (2016) [65] |
| Y2O3 | Optical properties | Razavi-Khosroshahi et al. (2016) [66] |
| YBa2Cu3Oy | Microstructural investigation | Kuznetsova et al. (2017) [67] |
| BN | Coupled elastoplasticity and plastic strain-induced phase transformation | Feng et al. (2017) [68] |
| ZnO | Photocatalytic activity for dye degradation | Razavi-Khosroshahi et al. (2017) [26] |
| Fe3O4 | Lithium-ion batteries | Qian et al. (2018) [69] |
| ZnO | Plastic flow and microstructural instabilities | Qi et al. (2018) [70] |
| Fe71.2Cr22.7Mn1.3N4.8 | Microstructural features | Shabashov et al. (2018) [71] |
| BN | Modeling of plastic flow and strain-induced phase transformation | Feng et al. (2019) [72] |
| TiO2-II | Electrocatalysis for hydrogen generation | Edalati et al. (2019) [73] |
| γ-Al2O3 | Photocatalytic activity for dye degradation | Edalati et al. (2019) [27] |
| Various Oxides | Review on HPT of oxides | Edalati et al. (2019) [74] |
| MgO | Photocatalytic activity for dye degradation | Fujita et al. (2020) [28] |
| ZrO2 | Photocatalytic activity for hydrogen production | Wang et al. (2020) [26] |
| SiO2 | Photocatalytic activity for dye degradation | Wang et al. (2020) [34] |
| CsTaO3, LiTaO3 | Photocatalytic activity for hydrogen production | Edalati et al. (2020) [24] |
| GaN-ZnO | Photocatalytic activity for hydrogen production | Edalati et al. (2020) [25] |
| Fe53.3Ni26.5B20.2, Co28.2Fe38.9Cr15.4Si0.3B17.2 | Microstructure and mechanical properties | Permyakova et al. (2020) [75] |
| TiHfZrNbTaO11 | Photocatalytic activity for hydrogen production | Edalati et al. (2020) [27] |
| TiO2-ZnO | Photocatalytic activity for hydrogen production | Hidalgo-Jimeneza et al. (2020) [28] |
| Bi2O3 | Enhanced photocurrent generation | Fujita et al. (2020) [76] |
| TiO2-II | Visible-light photocurrent generation | Wang et al. (2020) [77] |
| TiO2-II | Photocatalytic activity for CO2 conversion | Akrami et al. (2021) [30] |
| TiZrHfNbTaO6N3 | Photocatalytic activity for hydrogen production | Edalati et al. (2021) [29] |
| SiO2, VO2 | Phase transformation | Edalati et al. (2021) [78] |
| TiO2 | Grain coarsening and phase transformation | Edalati et al. (2021) [79] |
| ZnO | Bandgap narrowing | Qi et al. (2021) [80] |
| BiVO4 | Photocatalytic activity for CO2 conversion | Akrami et al. (2022) [29] |
| TiHfZrNbTaO11 | Photocatalytic activity for CO2 conversion | Akrami et al. (2022) [31] |
| TiZrNbTaWO12 | Photocatalytic activity for oxygen production | Edalati et al. (2022) [30] |
| TiZrHfNbTaO6N3 | Photocatalytic activity for CO2 conversion | Akrami et al. (2022) [32] |
As given in Table 1, the recent usage of HPT to process and synthesize ceramics for photocatalysis, especially photocatalytic CO2 conversion, has shown a high potential of this method for the enhancement of photocatalytic activity [35,36,37,38]. The HPT method effectively leads to increased efficiency by narrowing the bandgap, increasing the light absorbance, aligning the band structure, introducing the interphases and active sites for chemical adsorption and reaction, and accelerating the charge carrier migration [35,36,37,38]. While the HPT method can control all these features simultaneously by simple mechanical treatment, chemical methods are not usually able to improve all these features at the same time. The main drawbacks of the HPT method are the small quantity of the sample and the low specific surface area of the catalyst due to the high pressure and strain utilized. However, upscaling the HPT method and increasing the specific surface area by a post-HPT treatment are issues that can be addressed in the future. The improvement of features of photocatalysts by HPT has been achieved using four main strategies, including simultaneous strain and oxygen vacancy engineering, the introduction of high-pressure phases, the formation of defective high-entropy phases, and the production of low-bandgap high-entropy oxynitride phases. The responsibility of each mentioned strategy to improve the photocatalytic CO2 conversion activity is discussed in detail as follows. It should be noted that all photocatalytic CO2 conversion experiments on HPT-processed catalysts were performed in an aqueous liquid phase inside a quartz photoreactor with a continuous flow of CO into the liquid phase and NaHCO3 as the sacrificial agent.
2.1. Simultaneous Strain and Oxygen Vacancy Engineering
Oxygen vacancy engineering is an effective method that has been used to improve photocatalytic CO2 conversion. Oxygen vacancies increase the photocatalytic efficiency by increasing the light absorbance, accelerating the charge carrier separation, and enhancing the surface reactions [35,36]. Oxygen vacancies on the surface of the photocatalysts act as active sites to trap the electrons for various ranges of reduction reactions. It was also observed that oxygen vacancies have a significant role in adsorbing and activating the CO2 molecules and increasing the local electronic density [35,36].
BiVO4 is one of the common photocatalysts utilized for photocatalytic CO2 conversion, but it suffers from a high recombination rate of electrons and holes and an inappropriate conduction band position [35]. Different strategies have been used to solve these problems, but in all of them, impurity atoms or a second phase are added to this material [35]. The HPT method was used to solve the problems of BiVO4 for photocatalytic CO2 conversion by simultaneous engineering of strain and oxygen vacancies without the addition of impurities. BiVO4 was processed by HPT for N = 0.25, 1, and 4 turns to investigate the impact of strain on photocatalytic properties and efficiency. Increasing the lattice strain and decreasing the crystallite size by increasing the HPT turns is shown in Figure 2a. The occurrence of lattice strain was also confirmed by Raman peak shift to lower wavenumbers, as shown in Figure 2b. It was also observed that the concentration of oxygen vacancies increases in BiVO4 by increasing the applied shear strain. Figure 2c illustrates the oxygen vacancy concentration, calculated by X-ray photoelectron spectroscopy (XPS), against the number of HPT turns, confirming that the concentration of vacancies increases by increasing the applied shear strain. Furthermore, strain and vacancy engineering led to an increase in light absorbance (Figure 2d) and a slight narrowing of the bandgap from 2.4 eV for the initial powder to 2.1 eV for the sample proceeded by HPT for N = 4 turns [35].
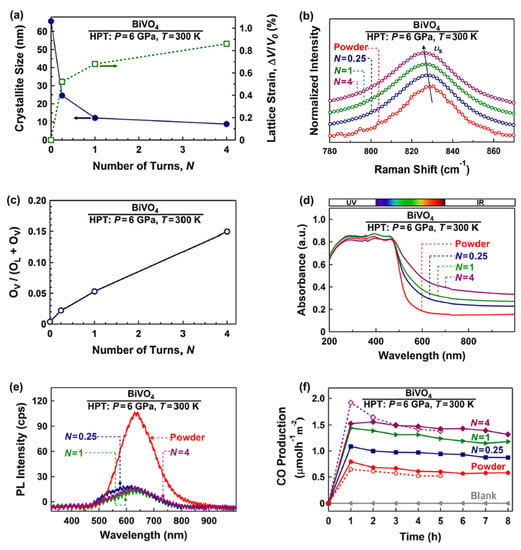
Figure 2. Improvement of light absorbance, suppression of recombination, and enhancement of photocatalytic CO2 conversion for BiVO4 by simultaneous strain and oxygen vacancy engineering using high-pressure torsion (HPT). (a) Crystallite size and volumetric strain versus the number of HPT turns (N), (b) Raman spectroscopy of initial and HPT-processed samples (inset: the appearance of samples), (c) oxygen vacancy concentration versus the number of HPT turns calculated by X-ray photoelectron spectroscopy, (d) UV–VIS spectroscopy, (e) photoluminescence spectra, and (f) photocatalytic CO production rate versus time for initial powder and sample proceeded by HPT for N = 0.25, 1, and 4 turns [35].
Simultaneous strain and oxygen vacancy engineering could significantly solve the problem of BiVO4 in terms of the high recombination rate of electrons and holes, as shown in Figure 2e. This figure demonstrates that the HPT method decreases the photoluminescence intensity, which is a piece of evidence for the suppression of recombination. Finally, this strategy was successful in improving the photocatalytic activity of BiVO4, as shown in Figure 2f. The CO production rate from CO2 photoreduction was effectively increased by increasing the number of HPT turns. This study was the first successful work that used simultaneous strain and oxygen vacancy engineering to improve the photocatalytic activity of BiVO4 without using impurities, suggesting SPD as a new path to improve the optical and electronic structure of photocatalysts for CO2 conversion [35].
2.2. Introducing High-Pressure Phases
The formation of high-pressure phases is one of the HPT effects that can occur for some ceramics, such as TiO2 [65], ZrO2 [58], ZnO [26], SiO2 [34], VO2 [78], Y2O3 [66], BaTiO3 [64], Al2O3 [27], and BN [68]. It was observed that these high-pressure phases contain defects, such as oxygen vacancies and dislocations, and have nanosized grains, which makes them attractive for photocatalytic applications. TiO2 with the anatase and rutile crystal structures is one of the most active photocatalysts for CO2 conversion. As shown in Figure 3a, in addition to anatase and rutile, TiO2 has a high-pressure TiO2-II (columbite) phase with the orthorhombic structure. Despite many studies on TiO2 photocatalysts, there was not any research work on photocatalytic CO2 conversion on the TiO2-II phase until 2021. Groups of current authors stabilized the TiO2-II phase by the HPT method and investigated it for photocatalytic CO2 conversion [36]. To decrease the fraction of oxygen vacancies in the bulk, which can act as recombination centers, an HPT-processed sample was further treated by annealing [36]. The formation of high-pressure TiO2-II was proved by X-ray diffraction (XRD), Raman spectroscopy, and transmission electron microscopy (TEM). Raman spectra along with the appearance of samples are shown in Figure 3b. New Raman peaks at wavenumbers 171, 283, 316, 340, 357, 428, 533, and 572 cm−1 correspond to the TiO2-II phase. The changes in the color of the sample from white to dark green after HPT processing and from dark green to white after annealing indicate that large fractions of oxygen vacancies are formed after HPT processing, while some of them are annihilated after annealing, a fact that was also proved by various characterization techniques [36].
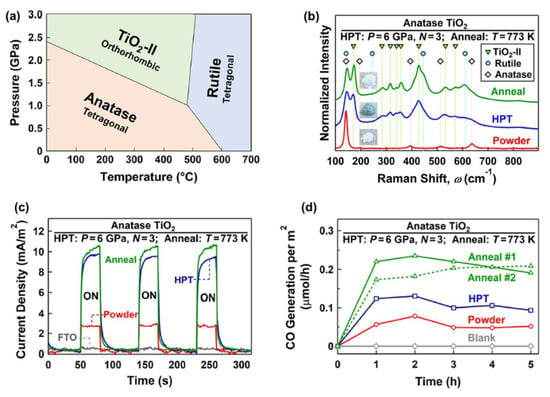
Figure 3. Improved charge carrier migration and photocatalytic CO2 conversion by introducing the high-pressure TiO2-II phase. (a) Pressure–temperature phase diagram of TiO2. (b) Raman spectra, (c) photocurrent spectra, and (d) photocatalytic CO production rate versus time for TiO2 before and after high-pressure torsion processing and after annealing [36].
The light absorbance of the TiO2-II phase produced by HPT processing was higher, and it had a narrower optical bandgap of 2.5 eV compared with anatase (3 eV), although the bandgap slightly increased to 2.7 eV after annealing [36]. Introducing the high-pressure TiO2-II phase using HPT suppressed the recombination rate of electrons and holes. It also had a positive impact on photocurrent generation, as shown in Figure 3c so that the annealed sample had the highest current density, suggesting the improvement of charge carrier separations by introducing the high-pressure TiO2-II phase. The potential of this new phase for CO2 adsorption was measured by attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy. It was observed that the annealed sample had the highest potential for CO2 adsorption, which can help with photocatalytic CO2 conversion. Finally, this new phase showed a higher potential for photocatalytic CO production compared with the anatase phase, as shown in Figure 3d. The introduction of the TiO2-II phase with an optimized fraction of oxygen vacancies significantly improved the activity so that the annealed sample had the highest efficiency for CO2-to-CO conversion. The formation of anatase–columbite interphases can also contribute to the high activity of the HPT-processed sample by increasing the electron–hole separation and migration. In conclusion, high-pressure phases show great potential to be used as photocatalysts, and SPD can be used to stabilize these high-pressure phases under ambient conditions [36].
2.3. Formation of Defective High-Entropy Phases
Introducing high-entropy ceramics as new materials with five or more principal elements opened a new path in the field of materials science to produce materials with high functionality for various applications [81,82]. High configurational entropy caused by a large number of elements in these materials leads to decreasing the Gibbs free energy and improving the phase stability. High-entropy ceramics have been utilized for various applications, and in many cases, they have shown better efficiencies than conventional materials [81,82]. Li-ion batteries [83], catalysts [84], dielectrics [85], magnetic components [86], thermal barrier coating [87], and so on are some of the applications of these materials. The high potential of high-entropy ceramics for various applications is attributed to their high stability, cocktail effect, lattice distortion, inherent defects, and valence electron distribution [81,82]. Despite the high functionality of these materials, their application for photocatalytic CO2 conversion was not investigated until a study was conducted by the current authors in 2022 [37].
The HPT method, followed by oxidation, was used to fabricate a defective high-entropy oxide (HEO) with the composition of TiZrNbHfTaO11 and dual crystal structure of monoclinic and orthorhombic [37]. The selection of elements for this high-entropy ceramic was conducted by considering the d0 electronic structure of cations that have shown high potential for photocatalysis. The oxidation states of anionic and cationic elements and their uniform distribution were proved by XPS and energy-dispersive X-ray spectroscopy (EDS), respectively. The microstructure of the oxide is shown in Figure 4a using scanning electron microscopy (SEM) and in Figure 4b using high-resolution TEM. In addition to a nanocrystalline dual-phase structure, the material exhibited the presence of various defects, such as vacancies and dislocations, as shown in Figure 4b. The formation of oxygen vacancies in this material was examined by electron paramagnetic resonance (EPR) spectroscopy. These oxygen vacancies can act as shallow traps between the valence band and the conduction band for easier charge carrier separation, as shown in Figure 4c [37].
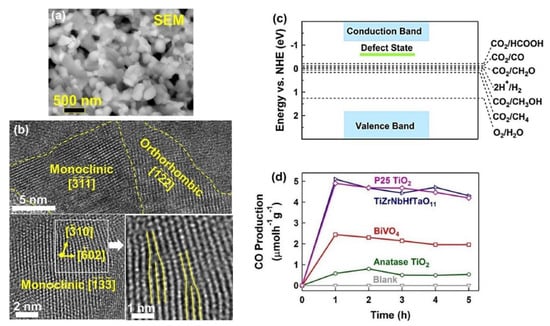
Figure 4. High photocatalytic CO2 conversion on defective high-entropy oxide TiZrNbHfTaO11 synthesized by high-pressure torsion. Microstructure of TiZrNbHfTaO11 by (a) scanning electron microscopy and (b) high-resolution transmission electron microscopy. (c) Electronic band structure of TiZrNbHfTaO11. (d) Photocatalytic CO production rate on TiZrNbHfTaO11 versus time compared with P25 TiO2, BiVO4, and anatase TiO2 [37].
TiZrNbHfTaO11 had a higher light absorbance and lower bandgap compared with the binary oxides, including TiO2, ZnO, Nb2O5, HfO3, and Ta2O5 [37]. This HEO could successfully generate photocurrent, which indicates its potential for easy separation of electrons and holes to improve photocatalytic activity. TiZrNbHfTaO11 showed higher activity for photocatalytic CO production compared with BiVO4 and TiO2 as two typical photocatalysts, as shown in Figure 4d. Additionally, it had the same photocatalytic activity as P25 TiO2 as a benchmark photocatalyst. The high activity of this HEO for photocatalytic CO2 conversion was attributed to the presence of defects, such as oxygen vacancies and dislocations; interphases; and high light absorbance. This study reported the first application of high-entropy ceramics for photocatalytic CO2 conversion and introduced a new way to design and synthesize highly efficient high-entropy photocatalysts by SPD processing [37].
2.4. Synthesis of Low-Bandgap High-Entropy Oxynitrides
Metal oxides are the most conventional photocatalysts for CO2 conversion but suffer from a large bandgap. On the other hand, metal nitrides have a low bandgap but suffer from low stability compared with metal oxides. Metal oxynitrides are rather new materials that can solve the problems of metal oxides and nitrides in terms of large bandgap and low stability, respectively [88]. Although oxynitrides have been used for photocatalytic water splitting in many research works, their application for photocatalytic CO2 conversion has been limitedly investigated mainly due to their limited chemical stability. The concept of high-entropy materials with high stability is one strategy used to produce high-entropy oxynitrides with low bandgap and high stability for CO2 photoreduction [38].
A high-entropy oxynitride (HEON) with the composition of TiZrNbHfTaO6N3 was fabricated by the HPT method, followed by oxidation and nitriding, and its photocatalytic performance was compared with a corresponding HEO TiZrNbHfTaO6 and P25 TiO2 benchmark photocatalyst [38]. This HEON had dual phases with face-centered cubic (FCC) and monoclinic structures with uniform distribution of elements. This HEON material had much higher light absorbance compared with P25 TiO2 and relevant HEO, as shown in Figure 5a. It showed a superior low bandgap of 1.6 eV as one of the lowest bandgaps reported in the literature for oxynitride photocatalysts. The improved electronic band structure of this HEON compared with P25 TiO2 and HEO is shown in Figure 5b. The recombination rate of electrons and holes in HEON was low so that its photoluminescence intensity was negligible compared with P25 TiO2 and HEO (Figure 5c). The shape of photocurrent spectra shown in Figure 5d also confirmed the low recombination rate of electrons and holes in this HEON compared with the HEO and P25 TiO2 catalysts. The potential of this HEON for CO2 adsorption was measured by diffuse reflectance infrared Fourier transform (DRIFT) spectrometry, which showed the higher physical adsorption and chemisorption (in the form of carbonate) of CO2 on this HEON compared with P25 TiO2 and HEO (Figure 5e).
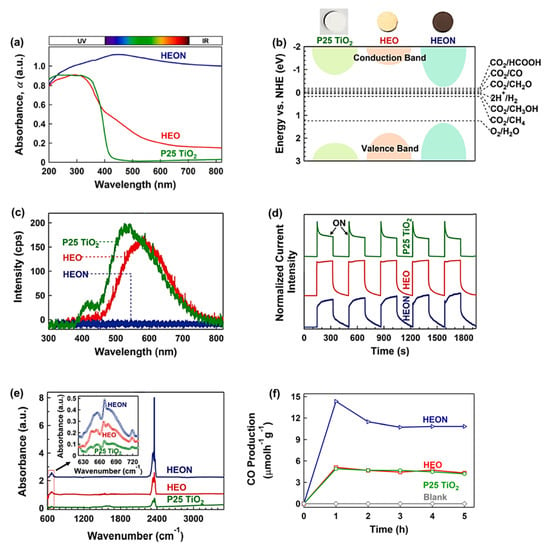
Figure 5. High light absorbance, appropriate band structure, suppressed recombination, significant CO2 adsorption, and high photocatalytic CO2 conversion for high-entropy oxynitride (HEON) TiZrNbHfTaO6N 3. (a) UV–VIS light absorbance spectra, (b) electronic band structure together with the appearance of samples, (c) photoluminescence spectra, (d) photocurrent density versus time, (e) diffuse reflectance infrared Fourier transform spectra (peaks at 665 and 2350 cm−1 represent chemisorption and physisorption of CO2), and (f) photocatalytic CO production rate versus time for TiZrNbHfTaO6N3 compared with P25 TiO2 and high-entropy oxide (HEO) TiZrNbHfTaO11 [38].
This HEON successfully converted CO2 to CO with extremely high efficiency even compared with the P25 TiO2 benchmark photocatalyst, as shown in Figure 5f. Although HEON could adsorb the light in both visible and infrared regions of light, it could not convert CO2 in these regions within the detection limits of the gas chromatograph. The stability of HEON was examined by conducting a long-term photocatalytic test for 20 h after storage of the sample in the air for 6 months. The photocatalytic activity of the material was not degraded, and X-ray diffraction analysis confirmed that the crystal structure of HEON did not change after 6-month storage and the long-time photocatalytic reaction. In conclusion, the low-bandgap HEON catalysts synthesized by SPD can be considered a new family of highly efficient photocatalysts for CO2 conversion [38].
This entry is adapted from the peer-reviewed paper 10.3390/ma16031081
This entry is offline, you can click here to edit this entry!
