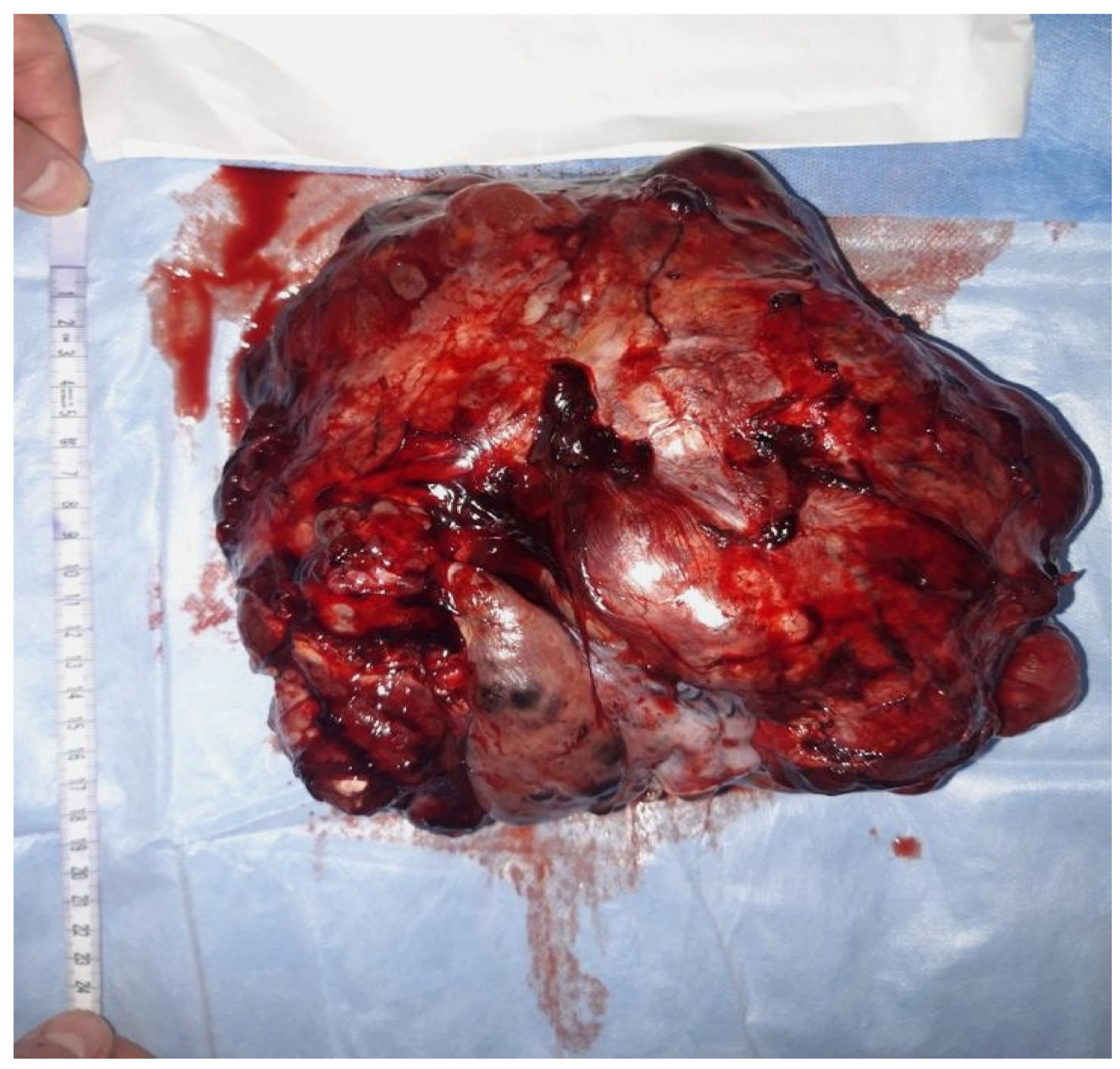
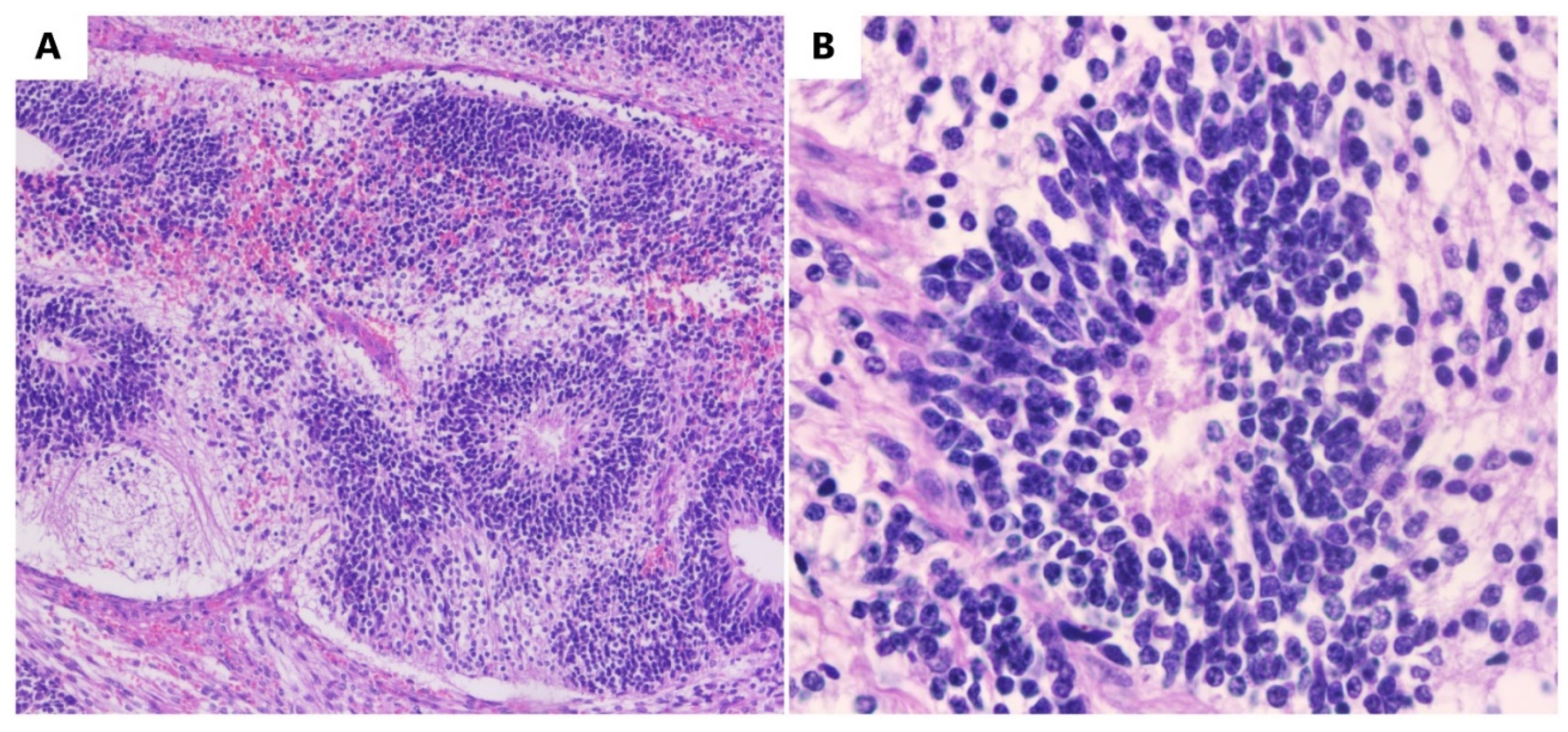
An immature teratoma is a germinal malignant tumor that is composed of three germ cell layers (the ectoderm, endoderm, and mesoderm), and it is histologically characterized by immature tissue, most frequently, neuroepithelial tissue. The most frequently affected individuals are adolescents and young adults in their reproductive age.
- immature teratoma
- germ cell
- oocyte
1. Introduction
An immature teratoma is the second most frequent, after dysgerminoma, among malignant germinal tumors [1]. An immature teratoma is the only neoplasm with germ cells that are histologically graded depending on the immature neural elements, and this is a prognostic factor for overall survival [2]. This grading system is called the Norris grading system [3]. Immature teratomas represent <1% of ovarian cancers, occurring more frequently in young women [4]. Statistically, its incidence is 2.3 per 100,000 patients [5]. It represents 35.6% of all germ tumors in young women.
2. Pathogenesis
3. Methods of Diagnosis
- (A)
-
Signs and symptoms
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13091516
References
- Chai, Y.; Woo, C.G.; Kim, J.Y.; Kim, C.J.; Khang, S.K.; Kim, J.; Park, I.A.; Kim, E.N.; Kim, K.R. Diagnostic Significance of Cellular Neuroglial Tissue in Ovarian Immature Teratoma. J. Pathol. Transl. Med. 2017, 51, 49–55.
- Alwazzan, A.B.; Popowich, S.; Dean, E.; Robinson, C.; Lotocki, R.; Altman, A.D. Pure Immature Teratoma of the Ovary in Adults: Thirty-Year Experience of a Single Tertiary Care Center. Int. J. Gynecol. Cancer 2015, 25, 1616–1622.
- Li, X.; Zhu, D.; Lv, L.I.; Yu, J. An uncommon recurrence of an immature teratoma: A case report. Oncol. Lett. 2016, 11, 2453–2456.
- Wang, M.; Jiang, S.; Zhang, Y.; Jiang, C.; Xia, F.; Lyu, W.; Ma, X. The application of 18F-FDG PET/CT in ovarian immature teratomas when pathological examination results contradict clinical observations: A case report. Medicine 2017, 96, e9171.
- Shinkai, T.; Masumoto, K.; Chiba, F.; Shirane, K.; Tanaka, Y.; Aiyoshi, T.; Sasaki, T.; Ono, K.; Gotoh, C.; Urita, Y.; et al. Pediatric ovarian immature teratoma: Histological grading and clinical characteristics. J. Pediatr. Surg. 2020, 55, 707–710.
- Balakrishnan, A.; Chaillet, J.R. Role of the inositol polyphosphate-4-phosphatase type II Inpp4b in the generation of ovarian teratomas. Dev. Biol. 2013, 373, 118–129.
- Stolnicu, S.; Szekely, E.; Molnar, C.; Molnar, C.V.; Barsan, I.; D’Alfonso, V.; Moldovan, C.; Zheng, G.; Ronnett, B.M.; Soslow, R.A. Mature and Immature Solid Teratomas Involving Uterine Corpus, Cervix, and Ovary. Int. J. Gynecol. Pathol. 2017, 36, 222–227.
- Kato, N.; Kamataki, A.; Kurotaki, H. Mature and immature ovarian teratomas share methylation profiles of imprinted genes: A MS-MLPA analysis. Virchows Arch. 2023, 482, 561–566.
- Heskett, M.B.; Sanborn, J.Z.; Boniface, C.; Goode, B.; Chapman, J.; Garg, K.; Rabban, J.T.; Zaloudek, C.; Benz, S.C.; Spellman, P.T.; et al. Multiregion exome sequencing of ovarian immature teratomas reveals 2N near-diploid genomes, paucity of somatic mutations, and extensive allelic imbalances shared across mature, immature, and disseminated components. Mod. Pathol. 2020, 33, 1193–1206.
- Talukdar, S.; Kumar, S.; Bhatla, N.; Mathur, S.; Thulkar, S.; Kumar, L. Neo-adjuvant chemotherapy in the treatment of advanced malignant germ cell tumors of ovary. Gynecol. Oncol. 2014, 132, 28–32.
- Khanna, S.; Srivastava, V.; Saroj, S.; Mishra, S.P.; Gupta, S.K. An unusual presentation of ovarian teratoma: A case report. Case Rep. Emerg. Med. 2012, 2012, 845198.
- Saleh, M.; Bhosale, P.; Menias, C.O.; Ramalingam, P.; Jensen, C.; Iyer, R.; Ganeshan, D. Ovarian teratomas: Clinical features, imaging findings and management. Abdom. Radiol. 2021, 46, 2293–2307.
- Al-Hazaimeh, M.; Jaradat, M.; El-Sadoni, M.; Smadi, T.; Shannaq, R.; Bani Hani, O.; Alhesa, A.; Abu Shahin, N.; Saleh, T. An Uncommon Recurrent Metastasis of Ovarian Immature Teratoma to the Small Bowel. Case Rep. Oncol. 2021, 14, 1834–1840.
- Hasdemir, P.S.; Guvenal, T.; Menekse, S.; Solmaz, U.; Kandiloglu, A.R.; Koyuncu, F.; Ayhan, A. Ovarian Immature Teratoma Detected During Pregnancy. Med. Sci. Discov. 2016, 3, 1–6.
- Reddihalli, P.V.; Subbian, A.; Umadevi, K.; Rathod, P.S.; Krishnappa, S.; Nanaiah, S.P.; Bafna, U.D. Immature teratoma of ovary--outcome following primary and secondary surgery: Study of a single institution cohort. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 192, 17–21.
- Zhao, Y.; Xu, T.; Bu, X.; Yuan, D.; Wu, Y.; Qian, H. Immature teratoma arising from uterine corpus in an 11-year-old girl: Case report and review of the literature. J. Obstet. Gynaecol. Res. 2021, 47, 452–455.
- Gkrozou, F.; Tsonis, O.; Vatopoulou, A.; Galaziou, G.; Paschopoulos, M. Ovarian Teratomas in Children and Adolescents: Our Own Experience and Review of Literature. Children 2022, 9, 1571.
- Kaijser, J.; Bourne, T.; Valentin, L.; Sayasneh, A.; Van Holsbeke, C.; Vergote, I.; Testa, A.C.; Franchi, D.; Van Calster, B.; Timmerman, D. Improving strategies for diagnosing ovarian cancer: A summary of the International Ovarian Tumor Analysis (IOTA) studies. Ultrasound Obstet. Gynecol. 2013, 41, 9–20.
- Pashankar, F.; Hale, J.P.; Dang, H.; Krailo, M.; Brady, W.E.; Rodriguez-Galindo, C.; Nicholson, J.C.; Murray, M.J.; Bilmire, D.F.; Stoneham, S.; et al. Is adjuvant chemotherapy indicated in ovarian immature teratomas? A combined data analysis from the Malignant Germ Cell Tumor International Collaborative. Cancer 2016, 122, 230–237.
- Ghosh, A.; Ghartimagar, D.; Thapa, S.; Sathian, B.; Narasimhan, R.; Talwar, O.P. Ovarian Tumors: Pattern of Histomorphological Types-A 10 Years Study in a Tertiary Referral Center and Review of Literature. Kathmandu Univ. Med. J. 2016, 14, 153–158.
- Nasioudis, D.; Chapman-Davis, E.; Frey, M.K.; Caputo, T.A.; Holcomb, K. Management and prognosis of ovarian yolk sac tumors; an analysis of the National Cancer Data Base. Gynecol. Oncol. 2017, 147, 296–301.
- Kiely, D.; Lewis, C.; Gray, J.; Hall, N. Prevalence of metachronous contralateral mature ovarian teratoma: A systematic review. Pediatr. Blood Cancer 2021, 68, e29237.
- Sauer, M.A.; Coy, S.; Quade, B.J.; Nucci, M.R. Well-developed Cerebellum in an Ovarian Mature Teratoma From a Pregnant Female. Int. J. Gynecol. Pathol. 2022, 41, 615–621.
