The intake of fruit has a notable effect on the prevention of signs of aging, cardiovascular diseases, cataracts, and strokes, presenting anti-inflammatory, anticancer, antidiabetic, and neuroprotective properties. In addition, fruit juices are considered alternative food products, being developed as probiotic substrates in recent years as an alternative to dairy products. Because they are well accepted by consumers and have a high nutritional value with positive health effects, fruit juices are ideal vehicles for probiotics.
- fruit
- processing
- fruit juice
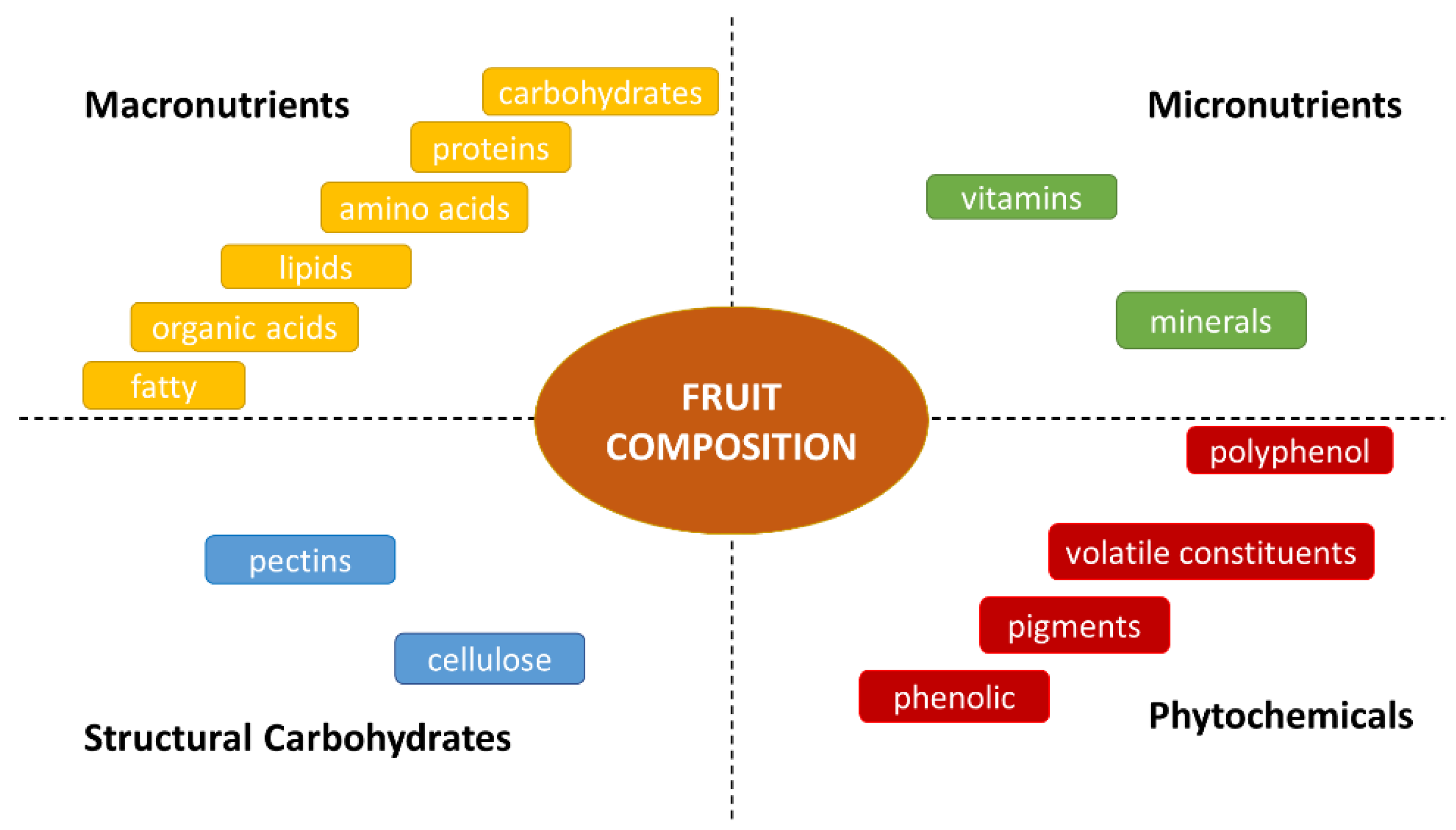
1. The Chemical Composition of Fruit
|
Fruits |
Minerals (mg/100 g of Fresh Weight) |
Vitamins |
References |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ca |
P |
K |
Mg |
Na |
Fe |
Se |
Cu |
Mn |
Zn |
C |
A |
B6 |
B2 |
||
|
Apple |
6 |
11 |
107 |
5 |
1 |
120 a |
0 |
30 a |
40 a |
40 a |
4.6 |
3 a |
- |
- |
[9] |
|
Apricot |
13 |
23 |
259 |
10 |
1 |
390 a |
0.1 a |
80 a |
80 a |
200 a |
10 |
96 a |
- |
- |
[9] |
|
Banana |
5 |
22 |
358 |
27 |
1 |
260 a |
1 a |
80 a |
270 a |
150 a |
8.7 |
3 a |
- |
- |
[9] |
|
Blackberry |
6–29 |
2–29 d |
77–349 |
6–44.8 |
2–4 d |
0.28–1.28 |
- |
0.02–0.04 d |
1.2–2.6 d |
0.07–0.44 |
34–52 |
- |
- |
- |
|
|
Blackcurrant |
35–45 d |
35–40 d |
300–320 d |
15–18 d |
1.7–2.5 d |
1.3–2.5 d |
- |
0.15–0.20 d |
0.35–0.52 d |
0.25–0.31 d |
122.4–193.2 |
- |
- |
- |
|
|
Blueberry |
15–35 d |
8.6 |
56–80 d |
4.9 |
0.11–0.22 d |
1.24 |
- |
0.02–0.04 d |
- |
0.13 |
10–100 |
- |
1999 c |
216 c |
|
|
Cherry |
13 |
12.2–15 |
90.9–173 |
11–12.2 |
0 |
1.16 |
0 |
60 a |
- |
0.69 |
10–62.4 |
3 a |
790 c |
247 c |
|
|
Clementine |
30 |
21 |
177 |
10 |
1 |
140 a |
10 a |
40 a |
20 a |
60 a |
48.8 |
- |
- |
- |
[9] |
|
Cranberry |
15–30 d |
1–4 d |
24–30 d |
3–7 d |
4–6 d |
0.16–0.4 d |
0.13–0.2 d |
0.3–0.10 d |
0.02–0.04 d |
10 b |
- |
606 c |
69 c |
||
|
Fig |
35 |
14 |
14 |
17 |
1 |
370 a |
0.2 a |
70 a |
130 a |
150 a |
2 |
7 a |
- |
- |
[9] |
|
Grapes |
10 |
20 |
191 |
7 |
2 |
360 a |
0.1 a |
130 a |
70 a |
70 a |
3.2 |
3 a |
- |
- |
[9] |
|
Litchis |
5 |
31 |
171 |
10 |
1 |
310 a |
0.6 a |
150 a |
60 a |
70 |
71.5 |
0 |
- |
- |
[9] |
|
Mango |
11 |
14 |
168 |
10 |
1 |
160 a |
0.6 a |
110 a |
60 a |
90 a |
36.4 |
54 a |
0.1–0.16 |
0.02–0.1 |
[9] |
|
Melon |
9 |
15 |
267 |
12 |
16 |
210 a |
0.4 a |
40 a |
- |
180 a |
36.7 |
169 a |
- |
- |
[9] |
|
Orange |
41 |
14 |
181 |
10 |
0 |
100 a |
0.5 a |
40 a |
30 a |
70 a |
53.2 |
11 a |
- |
- |
[9] |
|
Papaya |
20 |
10 |
182 |
21 |
8 |
250 a |
0.6 a |
40 a |
40 a |
80 a |
60.9 |
47 a |
- |
- |
[9] |
|
Peach |
6 |
20 |
190 |
9 |
0 |
250 a |
0.1 a |
70 a |
60 a |
170 a |
6.6 |
16 a |
- |
- |
[9] |
|
Pear |
9 |
12 |
116 |
7 |
1 |
180 a |
0.1 a |
80 a |
50 a |
100 a |
4.3 |
1 a |
- |
- |
[9] |
|
Pineaplle |
13 |
8 |
109 |
12 |
1 |
290 a |
0.1 a |
110 a |
930 a |
120 a |
47.8 |
3 a |
- |
- |
[9] |
|
Plum |
6 |
16 |
157 |
7 |
0 |
170 a |
0 |
60 a |
50 a |
100 a |
9.5 |
17 a |
- |
- |
[9] |
|
Raspberry |
1.14 |
5.7 |
71.8 |
15.9 |
0.5–1 d |
1.06 |
- |
- |
1.5–2.0 d |
.37 |
5–92.2 |
- |
- |
- |
|
|
Strawberry |
2.2–16 |
6.6–24 |
51.2–153 |
13–15.9 |
1 |
410 |
0.4 |
50 |
390 |
140 |
5–90 |
1 |
1744 c |
93 c |
|
|
Watermelon |
7 |
11 |
112 |
10 |
1 |
240 |
0.4 |
40 |
40 |
100 |
8.1 |
28 |
- |
- |
[9] |
2. Juice Composition vs. Processing Technologies
|
Juice |
Conditions |
Effect |
References |
|---|---|---|---|
|
Pasteurization-Conventional heating |
|||
|
Orange juice |
95 ℃, 1 min |
Reduction of pectin methylesterase activities (88.3%) |
[61] |
|
90 ℃, 50 s |
Sensory quality was the limiting factor for the shelf life of conventionally pasteurized juice, at 50 days |
[62] |
|
|
90 ℃, 30 s |
Significant loss of the content of total carotenoid pigment |
[63] |
|
|
Pulsed electric field (PEF) |
|||
|
Orange juice |
35 kV/cm, 4 μs, 40 °C |
8% loss of vitamin A, 1% loss of citric acid no change in Brix, pH, vitamin C, and viscosity |
[64] |
|
40 kV/cm, 97 ms, 45 °C |
PEF-processed juice retained more ascorbic acid, flavor, and color than thermally processed juice (90 °C/90 s) PEF-processed juice sensory evaluation of texture, flavor, and overall acceptability was ranked highest than thermally processed juice |
[65] |
|
|
20 kV/cm, 25 µs |
PEF treatments preserved the characteristic compounds associated with a fresh flavor (e.g., dl-limonene, β-myrcene, α-pinene, and valencene) more effectively than an intensive thermal treatment (121 °C/20 min) |
[66] |
|
|
Apple Juice |
35 kV/cm, 94 µs |
No change in natural color and Vitamin C |
[67] |
|
35 kV/cm, 4 µs |
pH, total acidity, phenolic and volatile compounds were less affected by PEF than by HTST treatment (90 °C /30 s) |
[56] |
|
|
High-pressure processing (HHP) |
|||
|
Orange juice |
600 MPa, 4 min at 40 °C |
High-pressure treatment led to lower degradation of ascorbic acid compared with pasteurization (80 °C/60 s) |
[68] |
|
500 MPa, 5 min at 25 °C |
2% loss of vitamin C, no change in Brix, pH, and color |
[64] |
|
|
400 MPa, 1 min at 40 °C |
5%-8% loss of vitamin C, no change in Brix, pH, and color |
[69] |
|
|
600 MPa, 15 min |
93.4% retention rate of anthocyanin (cyanidin-3-glucoside); 85% retention rate of ascorbic acid |
[70] |
|
|
Lemon juice |
450 MPa, 2, 5, or 10 min |
Slight effects of HPP on the compounds and physicochemical properties |
[71] |
|
Apple juice |
400 MPa, 10 min |
High-pressure treated apple juice sensory quality was higher compared to pasteurization (80 °C, 20 min) |
[72] |
|
Strawberry juice |
200–500 MPa, 20 min, 20 °C |
No major changes in strawberry juice aromatic volatile profile composition after HP treatment. Changes appeared in the composition of aromatic compounds after sterilization (120 °C, 20 min) |
[73] |
|
Ultra-sonication (US) |
|||
|
Orange juice |
20 kHz, 1500 W, 10 min, 32–38 °C |
No changes in pH, °Brix, and titratable acidity |
[74] |
|
20 kHz, 1500 W, 8 min, 10 °C |
Changes in color and ascorbic acid concentration during storage |
[75] |
|
|
Grapefruit juice |
28 kHz, 30, 60, and 90 min, 20 °C |
Improvement in the ascorbic acid, total phenolics, flavonoids, and flavonols. No changes in the pH, acidity, and °Brix value. Differences in the color values with overall quality improved |
[76] |
|
Cold plasma |
|||
|
Pomegranate juice |
5 min; 4 cm3; 0.75 dm3/min |
Pasteurization and plasma treatment resulted in total phenolic content increasing by 29.55% and 33.03%, respectively |
[77] |
|
3 min; 5 cm3; 0.75 dm3/min |
Anthocyanin content increased after cold plasma treatment by between 21% and 35% Higher anthocyanin stability |
[78] |
|
|
Ultraviolet-C radiation (UV-C) |
|||
|
Orange juice |
>230 J/L |
No changes in aroma and color 11% loss of vitamin C |
[64] |
|
12–48 kJ/L |
Ascorbic acid losses increased with the UV-C application No changes in total phenols and antioxidant capacity No changes in pH, total soluble solids, and titration acidity |
[79] |
|
|
Pomegranate juice |
12–62 J/mL |
No changes in total phenol content No changes in pH, total soluble solids, and titration acidity |
[80] |
|
Ohmic heating (OH) |
|||
|
Watermelon juice |
90 °C/15–60 s |
No changes in lycopene High color stability Decrease in total phenolic compounds |
[81] |
|
95 °C/1, 3 and 5 min/voltage gradients of 10, 13.33, 16.66, 20 and 23.33 V/cm at 50 Hz |
Voltage gradient and treatment time was statistically significant with change in pH and total color difference |
[82] |
|
This entry is adapted from the peer-reviewed paper 10.3390/beverages8020033
References
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red Fruits Composition and Their Health Benefits—A Review. Foods 2022, 11, 644.
- Charlton, K.; Kowal, P.; Soriano, M.M.; Williams, S.; Banks, E.; Vo, K.; Byles, J. Fruit and Vegetable Intake and Body Mass Index in a Large Sample of Middle-Aged Australian Men and Women. Nutrients 2014, 6, 2305–2319.
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073.
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; MoustaidMoussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623.
- De Souza, V.R.; Pereira, P.A.; Da Silva, T.L.; Lima, L.C.O.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368.
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144.
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and Vegetables, as a Source of Nutritional Compounds and Phytochemicals: Changes in Bioactive Compounds during Lactic Fermentation. Food Res. Int. 2018, 104, 86–99.
- Sinha, P.S.; Rosen, H.N. Clinical Pharmacology of Bisphosphonates. In Encyclopedia of Bone Biology; Zaidi, M., Ed.; Academic Press: London, UK, 2020; pp. 579–589. ISBN 9780128140826.
- USDA-ARS (US Department of Agriculture, Agricultural Research Service). USDA Nutrient Database for Standard Reference, Release 25, Software 1.2.2, from the Nutrient Data Laboratory. Available online: http://www.nal.usda.gov/fnic/foodcomp (accessed on 20 December 2021).
- Hakala, M.; Lapvetelainen, A.; Houpalahti Kallio, H.; Tahvonen, R. Effects of varieties and cultivation conditions on the composition of strawberries. J. Food Compos. Anal. 2003, 16, 67–80.
- Djordjević, B.; Šavikin, K.; Zdunić, G.; Janković, T.; Vulić, T.; Pljevljakušić, D.; Oparnica, C. Biochemical properties of the fresh and frozen black currants and juices. J. Med. Food. 2013, 16, 73–81.
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1-57881-072-8.
- Fayet-Moore, F.; Cassettari, T.; Tuck, K.; McConnell, A.; Petocz, P. Dietary fibre intake in Australia. Paper II: Comparative examination of food sources of fibre among high and low fibre consumers. Nutrients 2018, 10, 1223.
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727.
- Terry, P.; Giovannucci, E.; Michels, K.B.; Bergkvist, L.; Hansen, H.; Holmberg, L.; Wolk, A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J. Natl. Cancer Inst. 2001, 93, 525–533.
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706.
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, 10.
- Brat, P.; Georgé, S.; Bellamy, A.; Du Chaffaut, L.; Scalbert, A.; Mennen, L.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373.
- Omoregie, E.S.; Osagie, A.U. Antioxidant properties of methanolic extracts of some Nigerian plants on nutritionally-stressed rats. Niger. J. Basic Appl. Sci. 2012, 20, 7–20.
- Mokhtar, M.; Bouamar, S.; Di Lorenzo, A.; Temporini, C.; Daglia, M.; Riazi, A. The Influence of Ripeness on the Phenolic Content, Antioxidant and Antimicrobial Activities of Pumpkins (Cucurbita moschata Duchesne). Molecules 2021, 26, 3623.
- Carocho, M.; Ferreira, I. The role of phenolic compounds in the fight against cancer—A review. Anti-Cancer Agents Med. Chem. 2013, 13, 1236–1258.
- Panickar, K.S.; Anderson, R.A. Effect of polyphenols on oxidative stress and mitochondrial dysfunction in neuronal death and brain edema in cerebral ischemia. Int. J. Mol. Sci. 2011, 12, 8181–8207.
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977.
- Jakobek, L.; Seruga, M.; Novak, I.; Medvidović-Kosanović, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensm. Rundsch. 2007, 103, 369–378.
- Može, Š.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Ulrih, N.P.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004.
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic acids in berries, fruits and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199.
- Pilat, B.; Zadernowski, R.; Czaplicki, S.; Jez, M. Cold storage, freezing and lyophilisation and its effect on transformations of ˙phenolic compounds in lingonberry (Vaccinium vitis-idaea L.). Pol. J. Nat. Sci. 2018, 33, 101–113.
- D'Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361.
- Hara, Y. Tea catechins and their applications as supplements and pharmaceutics. Pharmacol. Res. 2011, 64, 100–104.
- Gu, L.; Kelm, M.A. Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617.
- Clifford, M.N. Anthocyanins-nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Costa, E.; Cosme, F.; Jordão, A.M.; Mendes-Faia, A. Anthocyanin profile and antioxidant activity from 24 grape varieties cultivated in two Portuguese wine regions. OENO ONE 2014, 48, 51–62.
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917.
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M'hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070.
- Fernández-Mar, M.I.; Mateos, R.; Garcıa-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797.
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042.
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673.
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126.
- Jaros, D.; Thamke, I.; Raddatz, H.; Rohm, H. Single-cultivar cloudy juice made from table apples: An attempt to identify the driving force for sensory preference. Eur. Food Res. Technol. 2009, 229, 51–61.
- Medina, S.; Perestrelo, R.; Santos, R.; Pereira, R.; Câmara, J.S. Differential volatile organic compounds signatures of apple juices from Madeira Island according to variety and geographical origin. Microchem. J. 2019, 150, 104094.
- Estrada-Beltran, A.; Salas-Salazar, N.A.; Parra-Quezada, R.A.; Gonzalez-Franco, A.C.; Soto-Caballero, M.C.; Rodriguez-Roque, M.J.; Flores-Cordova, M.A.; Chavez-Martinez, A. Effect of conventional and organic fertilizers on volatile compounds of raspberry fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 862–870.
- Pinto, T.; Vilela, A.; Pinto, A.; Nunes, M.F.; Cosme, F.; Anjos, R. Influence of cultivar and conventional and organic agricultural practices on phenolic and sensory profile of blackberries (Rubus fruticosus). J. Sci. Food Agric. 2018, 98, 4616–4624.
- Anjos, R.; Cosme, F.; Gonçalves, A.; Nunes, F.M.; Vilela, A.; Pinto, T. Effect of agricultural practices, conventional vs organic, on the phytochemical composition of 'Kweli' and 'Tulameen' raspberries (Rubus idaeus L.). Food Chem. 2020, 328, 126833.
- Perestrelo, R.; Silva, C.; Silva, P.; Medina, S.; Câmara, J.S. Differentiation of fresh and processed fruit juices using volatile composition. Molecules 2019, 24, 974.
- Kebede, B.; Ting, V.; Eyres, G.; Oey, I. Volatile changes during storage of shelf stable apple juice: Integrating GC-MS fingerprinting and chemometrics. Foods 2020, 9, 165.
- Sobhana, A.; Mathew, J.; Ambili Appukutan, A.; Mredhula Raghavan, C. Blending of cashew apple juice with fruit juices and spices for improving nutritional quality and palatability. Acta Hortic. 2015, 1080, 369–375.
- Curi, P.N.; Almeida, A.B.D.; Tavares, B.D.S.; Nunes, C.A.; Pio, R.; Pasqual, M.; Souza, V.R.D. Optimization of tropical fruit juice based on sensory and nutritional characteristics. Food Sci. Technol. 2017, 37, 308–314.
- Buzrul, S.; Hami, A.; Largeteau, A.; Demazeau, G. Inactivation of Escherichia coli and Listeria innocua in kiwifruit and pineapple juices by high hydrostatic pressure. Int. J. Food Microbiol. 2008, 124, 275–278.
- Aadil, R.M.; Zeng, X.-A.; Sun, D.-W.; Wang, M.-S.; Liu, Z.-W.; Zhang, Z.-H. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT-Food Sci. Technol. 2015, 62, 890–893.
- Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Wurlitzer, N.J.; Fernandes, F.A.; Rabelo, M.C.; Rodrigues, S. Evaluation of thermal and non-thermal processing effect on non-prebiotic and prebiotic acerola juices using 1H qNMR and GC–MS coupled to chemometrics. Food Chem. 2018, 265, 23–31.
- Moyer, J.C.; Aitken, H.C. Apple juice. In Fruit and Vegetable Juice Processing Technology; Nelson, P.E., Tressler, D.K., Eds.; AVI: Westport, CT, USA, 1980; pp. 212–267.
- Braddock, R.J. Single-strength orange juice and concentrates. In Handbook of Citrus By-Products and Processing Technology; Braddock, R.J., Ed.; Wiley: New York, NY, USA, 1999; pp. 53–83.
- Plaza, L.; Sanchez-Moreno, C.; Elez-Martinez, P.; Ancos, B.; Martin-Belloso, O.; Cano, M.P. Effect of refrigerated storage on vitamin C and antioxidant activity of orange juice processed by high-pressure or pulsed electric fields with regard to low pasteurization. Eur. Food Res. Technol. 2006, 223, 487–493.
- Vegara, S.; Mena, P.; Martí, N.; Saura, D.; Valero, M. Approaches to understanding the contribution of anthocyanins to the antioxidant capacity of pasteurized pomegranate juices. Food Chem. 2013, 141, 1630–1636.
- Aguilar-Rosas, S.F.; Ballinas-Casarrubias, M.L.; Nevarez-Moorillon, G.V.; Martin-Belloso, O.; Ortega-Rivas, E. Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. J. Food Eng. 2007, 83, 41–46.
- Mena, P.; Vegara, S.; Martí, N.; García-Viguera, C.; Saura, D. Changes on indigenous microbiota, colour, bioactive compounds and antioxidant activity of pasteurised pomegranate juice. Food Chem. 2013, 141, 2122–2129.
- de Jesus, A.L.T.; Cristianini, M.; dos Santos, N.M.; Maróstica Júnior, M.R. Effects of high hydrostatic pressure on the microbial inactivation and extraction of bioactive compounds from açai (Euterpe oleracea Martius) pulp. Food Res. Int. 2020, 130, 108856.
- de Jesus, A.L.T.; Leite, T.S.; Cristianini, M. High isostatic pressure and thermal processing of açai fruit (Euterpe oleracea Martius): Effect on pulp color and inactivation of peroxidase and polyphenol oxidase. Food Res. Int. 2018, 105, 853–862.
- de Castro, D.R.G.; Mar, J.M.; da Silva, L.S.; da Silva, K.A.; Sanches, E.A.; de Araújo Bezerra, J.; Fernandes, F.A.N.; Campelo, P.H. Dielectric barrier atmospheric cold plasma applied on camu-camu juice processing: Effect of the excitation frequency. Food Res. Int. 2020, 131, 109044.
- Demirdöven, A.; Baysal, T. Optimization of Ohmic Heating Applications for Pectin Methylesterase Inactivation in Orange Juice. J. Food Sci. Technol. 2014, 51, 1817–1826.
- Leizerson, S.; Shimoni, E. Effect of Ultrahigh-temperature Continuous Ohmic Heating Treatment on Fresh Orange Juice. J. Agric. Food Chem. 2005, 53, 3519–3524.
- Lee, H.S.; Coates, G.A. Effect of Thermal Pasteurization on Valencia Orange Juice Color and Pigments. LWT Food Sci. Technol. 2003, 36, 153–156.
- Rupasinghe, H.V.; Yu, L.J. Emerging Preservation Methods for Fruit Juices and Beverages. In Food Additive; InTech.: Rijeka, Croatia, 2012.
- Min, S.; Jin, Z.T.; Min, S.K.; Yeom, H.; Zhang, Q.H. Commercial-scale pulsed electric field processing of orange juice. J. Food Sci. 2003, 68, 1265–1271.
- Lee, H.; Choi, S.; Kim, E.; Kim, Y.-N.; Lee, J.; Lee, D.-U. Effects of Pulsed Electric Field and Thermal Treatments on Microbial Reduction, Volatile Composition, and Sensory Properties of Orange Juice, and Their Characterization by a Principal Component Analysis. Appl. Sci. 2021, 11, 186.
- Evrendilek, G.A.; Jin, Z.T.; Ruhlman, K.T.; Qiu, X.; Zhang, Q.H.; Richter, E.R. Microbial safety and shelf-life of apple juice and cider processed by bench and pilot scale PEF systems. Innov. Food Sci. Emerg. Technol. 2000, 1, 77–86.
- Polydera, A.C.; Stoforos, N.G.; Taoukis, P.S. Effect of high hydrostatic pressure treatment on post processing antioxidant activity of fresh Navel orange juice. Food Chem. 2005, 91, 495–503.
- Deliza, R.; Rosenthal, A.; Abadio, F.B.D.; Silva, C.H.; Castillo, C. Application of High Pressure Technology in the Fruit Juice Processing: Benefits Perceived by Consumers. J. Food Eng. 2005, 67, 241–246.
- Torres, B.; Tiwari, B.K.; Patras, A.; Cullen, P.J.; Brunton, N.; ODonnell, C.P. Stability of anthocyanins and ascorbic acid of high pressure processed blood orange juice during storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 93–97.
- Donsi, G.; Ferrari, G.; Di Matteo, M. High-pressure stabilization of orange juice: Evaluation of the effects of process conditions. Ital. J. Food Sci. 1996, 8, 99–106.
- Novotna, P.; Valentova, H.; Strohalm, J.; Kyhos, K.; Landfeld, A.; Houska, M. Sensory evaluation of high pressure treated apple juice during its storage. Czech J. Food Sci. 1999, 17, 196–198.
- Lambert, Y.; Demazeau, G.; Largeteau, A.; Bouvier, J.-M. Changes in aromatic volatile composition of strawberry after high pressure treatment. Food Chem. 1999, 67, 7–16.
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Colour degradation and quality parameters of sonicated orange juice using response surface methodology. LWT-Food Sci. Technol. 2008, 41, 1876–1883.
- Gómez-López, V.M.; Orsolani, L.; Martínez-Yépez, A.; Tapia, M.S. Microbiological and sensory quality of sonicated calcium-added orange juice. LWT-Food Sci. Technol. 2010, 43, 808–813.
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sun, D.-W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206.
- Herceg, Z.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672.
- Bursać Kovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Režek Jambrak, A.; Herceg, Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323.
- Pala, C.U.; Toklucu, A.K. Microbial, physicochemical and sensory properties of UV-C processed orange juice and its microbial stability during refrigerated storage. LWT Food Sci. Technol. 2013, 50, 426–431.
- Pala, C.U.; Toklucu, A.K. Effect of UV-C on anthocyanin content and other quality parameters of pomegranate juice. J. Food Compos. Anal. 2011, 24, 790–795.
- Makroo, H.A.; Saxena, J.; Rastogi, N.K.; Srivastava, B. Ohmic heating assisted polyphenol oxidase inactivation of watermelon juice: Effects of the treatment on pH, lycopene, total phenolic content, and color of the juice. J. Food Processing Preserv. 2017, 41, e13271.
- Ishita, C.; Athmaselvi, K.A. Changes in pH and colour of watermelon juice during ohmic heating. Int. Food Res. J. 2017, 24, 741–746.
- Linhares, M.F.D.; Alves Filho, E.G.; Silva, L.M.A.; Fonteles, T.V.; Wurlitzer, N.J.; Brito, E.S.; Fernandes, F.A.N.; Rodrigues, S. Thermal and non-thermal processing effect on açai juice composition. Food Res. Int. 2020, 136, 109506.
- Basak, S.; Ramaswamy, H.S.; Simpson, B.K. High pressure inactivation of pectin methyl esterase in orange juice using combination treatments. J. Food Biochem. 2001, 25, 509–552.
- Fernández-García, A.; Butz, P.; Bognar, A.; Tauscher, B. Antioxidative capacity, nutrient content and sensory quality of orange juice and an orange–lemon–carrot juice product after high pressure treatment and storage in different packaging. Eur. Food Res. Technol. 2001, 213, 290–296.
- Ferrari, G.; Maresca, P.; Ciccarone, R. The application of high hydrostatic pressure for the stabilization of functional foods: Pomegranate juice. J. Food Eng. 2010, 100, 245–253.
- Varela-Santos, E.; Ochoa-Martinez, A.; Tabilo-Munizaga, G.; Reyes, J.E.; Pérez-Won, M.; Briones-Labarca, V.; Morales-Castro, J. Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 13–22.
- Sánchez-Moreno, C.; Plaza, L.; Elez-Martínez, P.; De Ancos, B.; Martín-Belloso, O. Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. J. Agric. Food Chem. 2005, 53, 4403–4409.
- Oms-Oliu, G.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Elez-Martinez, P.; Martin-Belloso, O. Stability of health related compounds in plant foods through the application of non thermal processes. Trends Food Sci. Technol. 2012, 23, 111–123.
- Jin, Z.T.; Zhang, Q.H. Pulsed electric field inactivation of microorganisms and preservation of quality of cranberry juice. J. Food Process. Preserv. 1999, 23, 481–497.
- Agcam, E.; Akyıldız, A.; Akdemir Evrendilek, G. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurization. Food Chem. 2014, 143, 354–361.
- Shi, X.M.; Zhang, G.J.; Wu, X.L.; Li, Y.X.; Ma, Y.; Shao, X.J. Effect of low-temperature plasma on microorganism inactivation and quality of freshly squeezed orange juice. IEEE Trans. Plasma Sci. 2011, 39, 1591–1597.
- Surowsky, B.; Frohling, A.; Gottschalk, N.; Schluter, O.; Knor, D. Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms. Int. J. Food Microbiol. 2014, 174, 63–71.
- Dasan, B.G.; Boyaci, I.H. Effect of cold atmospheric plasma on inactivation of Escherichia coli and physicochemical properties of apple, orange, tomato juices, and sour cherry nectar. Food Bioprocess Technol. 2018, 11, 334–343.
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135.
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86.
- Paixão, L.M.N.; Fonteles, T.V.; Oliveira, V.S.; Fernandes, F.A.N.; Rodrigues, S. Cold plasma effects on functional compounds of siriguela juice. Food Bioprocess Technol. 2019, 12, 110–121.
- Park, I.-K.; Ha, J.-W.; Kang, D.-H. Investigation of optimum ohmic heating conditions for inactivation of Escherichia coli O157: H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes in apple juice. BMC Microbiol. 2017, 17, 117.
- Kim, N.; Ryang, J.; Lee, B.; Kim, C.; Rhee, M. Continuous ohmic heating of commercially processed apple juice using five sequential electric fields results in rapid inactivation of Alicyclobacillus acidoterrestris spores. Int. J. Food Microbiol. 2017, 246, 80–84.
- Kim, S.-S.; Kang, D.-H. Comparison of pH effects on ohmic heating and conventional heating for inactivation of Escherichia coli O157: H7, Salmonella enterica Serovar Typhimurium and Listeria monocytogene s in orange juice. LWT-Food Sci. Technol. 2015, 64, 860–866.
- Funcia, E.S.; Gut, J.A.W.; Sastry, S.K. Effect of Electric Field on Pectinesterase Inactivation during Orange Juice Pasteurization by Ohmic Heating. Food Bioprocess Technol. 2020, 13, 1206–1214.
- Elzubier, A.S.; Thomas, C.S.Y.; Sergie, S.Y.; Chin, N.L.; Ibrahim, O.M. The effect of buoyancy force in computational fluid dynamics simulation of a two-dimensional continuous ohmic heating process. Am. J. Appl. Sci. 2009, 6, 1902–1908.
- Hashemi, S.M.B.; Roohi, R. Ohmic heating of blended citrus juice: Numerical modeling of process and bacterial inactivation kinetics. Innov. Food Sci. Emerg. Technol. 2019, 52, 313–324.
- Darvishi, H.; Salami, P.; Fadavi, A.; Saba, M.K. Processing kinetics, quality and thermodynamic evaluation of mulberry juice concentration process using Ohmic heating. Food Bioprod. Processing 2020, 123, 102–110.
