Slowly progressive spastic paraparesis with bladder dysfunction, the main clinical feature of human T-cell leukemia virus-1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP), is induced by chronic inflammation in the spinal cord, mainly the lower thoracic cord. A long-standing bystander mechanism, such as the destruction of surrounding tissues by inflammatory cytokines, etc., induced under the interaction between infiltrated HTLV-1-infected CD4+ T cells and HTLV-1-specific CD8+ cytotoxic T cells, has been considered implicated for the induction of chronic inflammation. As this bystander mechanism is triggered conceivably by the transmigration of HTLV-1-infected CD4+ T cells to the spinal cord, heightened transmigrating activity of HTLV-1-infected CD4+ T cells to the spinal cord might play a crucial role as the first responder in the development of HAM/TSP.
1. Introduction
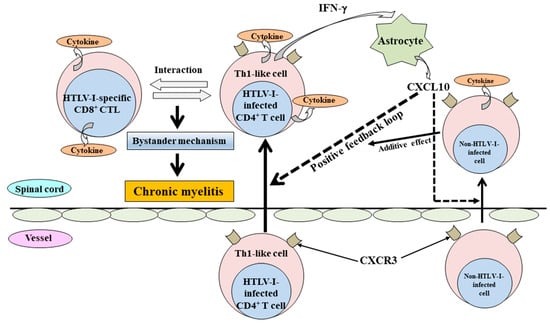
Human T-cell leukemia virus-1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a chronic progressive myelopathy characterized by bilateral pyramidal tract involvement with sphincteric disturbances [
1,
2]. Although the exact reasons why HTLV-1 induces HAM/TSP in a very small population, such as 0.3–3.8% of HTLV-1-infected individuals [
3,
4], are still unsolved, the involvement of numerous immunological dysregulations based on high HTLV-1 proviral load in the peripheral blood mononuclear cells (PBMC) are strongly suggested in the development of HAM/TSP [
5,
6,
7]. Although the abundance of interferon-γ (IFN-γ) and tumor necrosis factor-α(TNF-α) producing cells in the HTLV-1 tax-expressing cell population in PBMC of HAM/TSP patients was observed in a comparative study of intracellular cytokine expression levels in HAM/TSP patients and HTLV-1 asymptomatic carriers with a high HTLV-1 proviral load equivalent to those of HAM/TSP patients [
8], this finding suggested that HTLV-1-infected cells having the characteristic of Th1 increased in HAM/TSP patients. Araya et al. clearly demonstrated that IFN-γ-producing CD4
+CCR4
+ T cells expressing Th1 marker CXCR3 based on the activation of the Th1 master regulator T box transcription factor (T-bet) induced by HTLV-1 tax in cooperation with specificity protein 1 increased in cerebrospinal fluid (CSF) and spinal cord lesions of HAM/TSP patients [
9]. In addition, the frequency of this cell population in PBMC was correlated with disease activity of HAM/TSP [
10]. Thus, a Th1-like status based on a high HTLV-I proviral load in the peripheral blood may be very critical in the immunopathogenesis of HAM/TSP. The primary neuropathological feature of HAM/TSP is chronic myelitis, such as chronic inflammation in the spinal cord, mainly the lower thoracic cord, with perivascular cuffing and parenchymal infiltration of mononuclear cells [
11]. The exact mechanism of how HTLV-1 infection causes chronic inflammation of the spinal cord is still unclear. However, a long-standing bystander mechanism, such as the destruction of surrounding tissues by inflammatory cytokines, etc., induced under the interaction between HTLV-1-infected Th1-like CD4
+ T cells and HTLV-1-specific CD8
+ cytotoxic T cells in the spinal cord, is probably a critical cause of the induction of chronic myelitis [
2,
12,
13] (
Figure 1). Although there is no significant difference in a functional CD8
+ cell assay for the anti-viral efficacy of HTLV-1-specific CD8
+ cytotoxic T cells between HAM/TSP patients and HTLV-1 carriers [
14], the finding that HTLV-1-specific CD8
+ cytotoxic T cells of HAM/TSP patients as the response to HTLV-1 tax produces high levels of proinflammatory cytokines, such as IFN-γ and TNF-α, etc., [
15] strongly suggests that HTLV-1-specific CD8
+ cytotoxic T cells can function for the induction of bystander mechanism. In addition to this situation, a positive feedback loop, which is formed by chemokine CXCL10 induced from astrocytes via stimulation by IFN-γ produced by infiltrated HTLV-1-infected Th1-like CD4
+ T cells, might be involved in the maintenance and promotion of chronic myelitis [
16]. Furthermore, non-HTLV-1-infected CXCR3
+ cells, which are attracted by CXCL10, provide the additive effect for a positive feedback loop (
Figure 1).
Figure 1. The proposed mechanism for the induction of chronic myelitis in HAM/TSP. In the induction of chronic myelitis, a long-standing bystander mechanism, such as the destruction of surrounding tissues by inflammatory cytokines, etc., induced under the interaction between infiltrated HTLV-1-infected Th1-like CD4+ T cells and HTLV-1-specific CD8+ cytotoxic T cells in the spinal cord, is probably critical. For the bystander mechanism, a positive feedback loop formed through chemokine CXCL10 (a ligand of CXCR3) from astrocytes via stimulation by IFN-γ produced from infiltrated HTLV-1-infected Th1-like CD4+ T cells might be involved in the maintenance and promotion of chronic myelitis. In addition, non-HTLV-1-infected CXCR3+ cells, which are attracted by CXCL10, provide the additive effect for a positive feedback loop.
As this bystander mechanism is triggered conceivably by the transmigration of HTLV-1-infected CD4
+ T cells to the spinal cord, heightened transmigrating activity of HTLV-1-infected CD4
+ T cells to the spinal cord might play a crucial role in the first stage of the development of HAM/TSP. The fact that HTLV-1 provirus accumulated in the CSF in HAM/TSP patients compared to HTLV-1 asymptomatic carriers [
17,
18] and that the main lesion of the spinal cord in HAM/TSP is in the lower thoracic cord (the watershed zone providing stagnant blood flow in hemodynamic condition) [
19,
20] might indicate that the outcome is induced by heightened transmigrating activity of HTLV-1-infected CD4
+ T cells of HAM/TSP patients. Several inflammatory diseases, including uveitis, pulmonary disorder, arthritis, myositis, and Sjögren syndrome, occasionally occur in conjunction with HAM/TSP [
21]. Even in this situation, heightened transmigrating activity of HTLV-1-infected CD4
+ T cells to the tissues might be involved in the triggering of these inflammatory diseases.
2. The Change of Cell Adhesion-Related Molecules Expression on HTLV-1-Infected CD4+ T Cells Leading to the Heightened Transmigrating Activity through Vascular Endothelial Cells in HAM/TSP Patients
Studies have demonstrated that the expression of several kinds of adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), is upregulated in HTLV-1-infected cells [
22].
In the process leading to the transmigration of T cells into the tissues, the rolling process of T cells on vascular endothelial cells (ECs) functions as the first step [
23]. Selectin and its ligands, which are expressed on ECs and T cells, respectively, play an important role in this step [
24,
25]. Of these, sialyl Lewis
x antigen (sLe
x) is a ligand for both E- and P-selectin [
26]. Therefore, T cells expressing sLe
x (sLe
x+ cell) might have the potential to transmigrate into tissues. The scholars previously compared the frequency of sLe
x+ cells together with IFN-γ production in peripheral blood CD4
+ T cells between 8 HAM/TSP patients and 14 controls, including four anti-HTLV-1-seropositive carriers [
27]. As shown in the results, the frequency of sLe
x+ cells in peripheral blood CD4
+ T cells of HAM/TSP patients was significantly higher than in controls. In addition, the activity of IFN-γ production in the sLe
x+ cell population in the peripheral blood CD4
+ T cells of HAM/TSP patients had significantly increased compared to controls.
The upregulation of cell adhesion molecule expression on HTLV-1-infected cells of HAM/TSP patients might have enough potential to facilitate the transmigration of HTLV-1-infected cells through ECs. However, although the expressions or the activities of LFA-1, LFA-3, ICAM-1, VCAM-1, and ALCAM, etc., are associated with HTLV-1 tax or HBZ activity [
22,
35,
41], the effects of selective adhesion molecules on HTLV-1-infected T cells of HAM/TSP have not been clarified yet. Therefore, when considering the potential therapeutic target for HAM/TSP patients, the identification of a specific adhesion molecule might have an important significance in the future.
3. The Role of Small GTPase Activation Leading to the Heightened Transmigration Activity of HTLV-1-Infected CD4+ T Cells into Tissues in HAM/TSP Patients
Small GTPases function as the propulsion for the transmigration of the cells through ECs. That is, small GTPase activation can induce the upregulation of transmigration activity of the cells into tissues through the rearrangement of the cytoskeleton involved in the adhesion and migration of cells [
42]. The finding that HTLV-1 tax regulates cell adhesion and migration by the interaction with small GTPases, such as Cdc42, Rac, and Rho, was previously demonstrated [
43]. A report indicated that Gem, which is one of the small GTP-binding proteins belonging to the Ras superfamily, is involved in the increase of the cellular migration of HTLV-1-infected cells through cytoskeleton remodeling [
44].
It previously reported the significantly increased adherence to ECs and subsequent transmigration through ECs of activated CD4
+ T cells with heightened LFA-1 expression in the peripheral blood T cells of HAM/TSP patients as mentioned above [
30,
31], suggesting the upregulation of signaling based on integrin/ligand interaction in the peripheral blood CD4
+ T cells of HAM/TSP patients. Subsequently, the upregulation of integrin/ligand signaling induces the activation of small GTPases, which are the downstream targets, followed by the rearrangement of cytoskeletal components [
45,
46]. Therefore, small GTPases might be activated in the HTLV-1-infected cells of HAM/TSP patients. To confirm this, scholars analyzed the activity of small GTPases, such as Cdc42, Rac, and Rho, in HTLV-1-infected T cell lines derived from HAM/TSP patients in comparison with those in HTLV-1-infected T cell lines derived from other origins [
47]. As a result, it was revealed that all small GTPases were strongly activated in all cell lines derived from HAM/TSP patients. Of these small GTPases, the difference in the degree of activation between both kinds of cell lines was the most obvious in Cdc42. Cdc42 plays an important role in the polarization of the cytoskeleton following integrin-mediated activation [
48] with the involvement in cell migration [
49,
50]. Therefore, the activation of Cdc42 in the HTLV-1-infected cells of HAM/TSP patients suggests that these cells have upregulated transmigrating activity into the tissues. Thus, activation of the outside-in signaling from integrin signaling in HTLV-1-infected cells of HAM/TSP patients suggests that this activity functions as one of the first triggers in the development of HAM/TSP.
4. The Mediators Involved in the Heightened Tissue Transmigration of HTLV-1-Infected CD4+ T Cells in HAM/TSP Patients
When considering the transmigration of T cells into the tissues after passing through the endothelium barrier, the extracellular matrix, including the vascular basement membrane, functions as the next barrier. Although collagens, gelatine, fibronectin, and laminin are the main components of the vascular basement membrane, matrix metalloproteinases (MMPs), such as MMP-2 and MMP-9, can cleave these components followed by the disruption of the basement membrane of the endothelium [
55]. Indeed, the immunopathological analysis of spinal cord lesions in HAM/TSP patients revealed that MMP-2 and MMP-9 are expressed in infiltrating mononuclear cells with disruption of the vascular endothelium in chronic active lesions with the findings that higher levels of MMP-2 and/or MMP-9 were detected in the CSF of HAM/TSP patients [
56,
57]. In addition, the importance of MMPs in the tissue transmigration of T cells by the degradation of the extracellular matrix is also supported by the finding that the transmigration of CD4
+ T cells of HAM/TSP patients was significantly inhibited by selective MMP inhibitor [
58].
Recent proteomic analysis of CSF revealed an increased level of soluble VCAM-1 (sVCAM-1) in HAM/TSP patients [
60,
61]. It is reported that the production of MMPs under the inflammatory status induces the shedding of sVCAM-1 from the surface of ECs [
62,
63]. Therefore, the up-regulated expression of MMPs in CSF, as mentioned above, might account for the increase of sVCAM-1 in CSF of HAM/TSP patients.
5. The Polysulfate Treatment for HAM/TSP Patients Focusing on the Inhibition of the Transmigration of HTLV-1-Infected Cells into the Spinal Cord
The ideal treatment for HAM/TSP patients is the complete elimination of HTLV-1-infected cells. However, the therapeutic strategy against HAM/TSP is yet to be established. Therefore, a regimen with an inhibitory activity against the transmigration of HTLV-1-infected cells as the first responder into the spinal cord might be recommended as one of the therapeutic strategies. The main regions in which pathological changes occur in HAM/TSP are in the lower thoracic spinal cord [
11]. These regions are anatomical watershed zones [
19,
20], where lymphocytes stagnate because of the decreased blood flow, can easily transmigrate to the tissues and evoke immune reactions. Therefore, manipulation of the microcirculation and interaction between lymphocyte and vascular ECs might be one of the therapeutic strategies against HAM/TSP.
Pentosan polysulfate sodium (PPS), which was developed as a heparin-like agent and has been used in Europe for thrombosis prophylaxis and treatment of phlebitis, is a semisynthetic drug manufactured from European beech-wood hemicellulose by sulfate esterification [
79]. Therefore, PPS is safe and has also been approved by the US Food and Drug Administration as an oral medication for treating interstitial cystitis. In addition to the activity of improving microcirculation, polysulfates, such as heparin and PPS, have the potential to inhibit the intercellular spread of HTLV-1 by blocking the binding of the virus to heparan sulfate proteoglycans [
80,
81]. Indeed, the multiple activities of PPS experimentally verified included (i) the inhibition of the adhesion to and transmigration of HTLV-1-infected cells through ECs; (ii) inhibition of HTLV-1 cell to cell transmission; (iii) suppression of HTLV-1 production; and (iv) blockage of interaction of HTLV-1 infection and ECs with the inhibition of subsequent induction of inflammatory cytokines [
82].
6. Conclusions and Perspectives
Considering the mechanism of chronic inflammation induced in the spinal cord of HAM/TSP patients, HTLV-1-infected CD4+ T cells might play a crucial role as the first responder. Therefore, the activities of HTLV-1-infected CD4+ T cells in HAM/TSP patients were evaluated based on the transmigration activity into the tissues. Consequently, it was demonstrated that HTLV-1-infected CD4+ T cells in HAM/TSP were supposed to have enough heightened transmigrating activity into the tissues based on the activation of integrin signaling followed by small GTPase activation with the up-regulated expression of MMPs. This activity appears sufficient to allow HTLV-1-infected CD4+ T cells to function as the first responders in the development of HAM/TSP. In addition, the HTLV-1-infected CD4+ T cells in HAM/TSP patients might also have the potential to induce the trigger for the development of another systemic inflammatory status, including Sjögren’s syndrome, myositis, and uveitis, etc., which occasionally occur in conjunction with HAM/TSP, as the first responder.
Although the exact reasons why HTLV-1 induces HAM/TSP in a very small population of HTLV-1-infected individuals are still unsolved, a very important task in future HAM/TSP research is to clarify the molecular mechanisms leading to the establishment of HTLV-1-infected CD4+ T cells as the first responder in HAM/TSP patients. At this point, the acquisition of heightened transmigrating activity of HTLV-1-infected CD4+ T cells into the tissues among HTLV-1-infected individuals seems to be the key event leading to the development of HAM/TSP.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12030492