Essential oils (EOs) are mixtures of volatile molecules endowed with health-promoting biological activities that go beyond their role as aromas and natural preservatives and can be exploited to develop functional foods and diet supplements. Some of the potential health benefit of human diet supplementation with EOs are in the area of: (1) irritable bowel syndrome; (2) inflammatory bowel disease; (3) regulation of microbiota; (4) gastroprotection; (5) hepatoprotection; (6) protection of the urinary tract and diuresis; (7) management of metabolic disorders including hyperglycemia and hyperlipidemia; (8) anti-inflammatory and pain control; (9) immunomodulation and protection from influenza; and (10) neuroprotection and modulation of mood and cognitive performance.
- essential oils
- health
- diet supplements
- nutraceutical
- functional food
1. Introduction
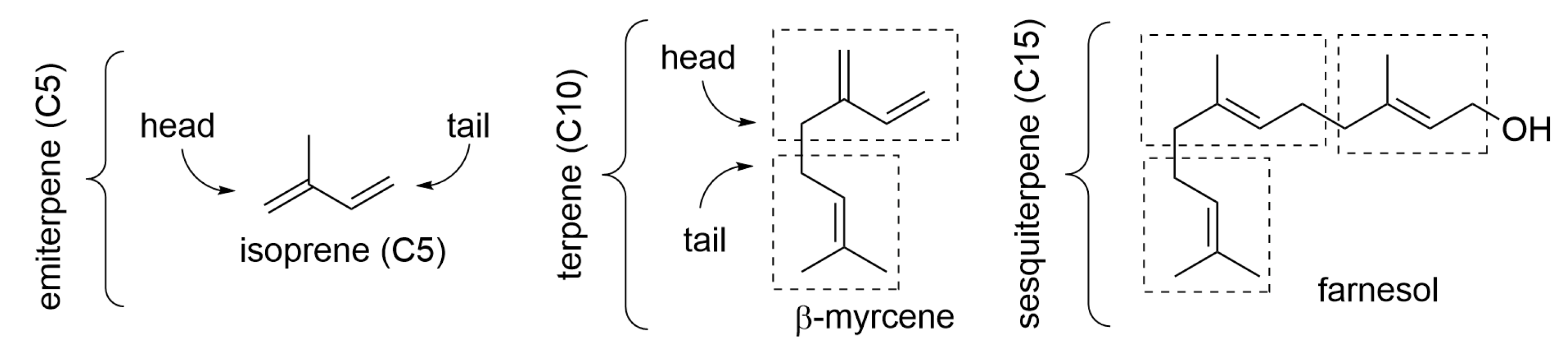
2. Composition of Essential Oils

3. Essential Oils Helpful in the Irritable Bowel Syndrome (IBS)
4. Essential Oils Helpful in Inflammatory Bowel Disease (IBD) and in the Prevention of Colorectal Cancer (CRC)
5. Essential Oils in Probiotic Food and Supplements for Regulation of Gut Microflora
6. Essential Oils for Gastric Protection and to Alleviate Peptic Ulcer
7. Essential Oils Useful in the Management of Metabolic Disorders
Inhibition of carbohydrate hydrolyzing enzymes α-amilase and α-glucosidase is a key target for oral hypoglycemic treatments for type 2 diabetes mellitus (T2DM). While pancreatic α-amylase hydrolyzes starch into oligosaccharides, these are further hydrolyzed by intestinal α-glucosidase into glucose, which is finally absorbed into systemic circulation, raising the plasma glucose level. In T2DM subjects with reduced glucose tolerance, postprandial plasma glucose rise can be smoothed by enzyme inhibitors, which slow down carbohydrate digestion, ameliorating their condition. In a comparative study on several terpenoid EO components, citral was the only one that showed mild inhibition of α-amilase compared to the reference drug acarbose. Instead, several monoterpenes (10 mM) showed inhibition of α-glucosidase with the following order of potency: (R)-(+)-limonene = (S)-(−)-perillyl alcohol > α-terpineol, while (R)-(+)-β-citronellol, terpinolene, citral, (R)-(−)-linalool, nerol, geraniol, and (S)-(−)-β-citronellol exerted weaker inhibition, and (L)-menthol and γ-terpinene did not show any significant α-glucosidase-inhibitory activity [31]. The same terpenes were tested for stimulation of glucose uptake in 3T3-L1 adipocytes, which represents another strategy to reduce glucose plasma levels. Geraniol, citral, limonene, and (R)-(+)-β-citronellol (1 μM) had the highest activity, while nerol, (S)-(−)-perillyl alcohol, γ-terpinene, and α-terpineol were weaker stimulants; and (S)-(−)-b-citronellol, terpinolene, and linalool did not affect the glucose uptake [32]. Thus, to a different degree, several terpenes of widespread presence in EOs, such as limonene, citral, and citronellol, have hypoglycemic activity, which helps rationalize the efficacy recorded for the whole oils.
High levels of circulating total cholesterol (TC), of low-density lipoprotein-cholesterol (LDL-C), and of plasma triglycerides along with low levels of high-density lipoprotein-cholesterol represent well-established risk factors for cardiovascular disease and might be associated with diabetes or dysregulated glycemic parameters in metabolic syndrome. Treating these unbalances with suitable dietary approaches is not less important than addressing hyperglycemia alone. Some EOs, most notably green and black cumin, show parallel reduction of lipidemic factors along with glycemic.
8. Essential Oils to Protect Liver Function and Stimulate Digestion
9. Essential Oils for Diuresis and Protection of the Urinary Tract
10. Essential Oils to Reduce Inflammation and Pain
11. Essential Oils with Immunomodulatory and Anti-Influenza Activity
A recent literature survey concerning the immunostimulant activity of EOs pointed out some differences among studies depending on their type, distinguishing in vitro, pre-clinical, clinical, or those based on diet supplementation of animals. It suggested that further studies are needed to clarity the matter although a few EOs were found to stand out in all families of studies: eucalypt and ginger [42]. However, other EOs have accumulated evidence for their efficacy in many studies, including human; these are thyme, lavender, clove, tea tree, and citruses (lemon, orange, and bergamot) [36]. The mechanism of immune stimulation is often complex to rationalize, as the same EO typically also expresses anti-inflammatory activity through the down-regulation of pro-inflammatory cytokines, and these, in principle, should stimulate immune response. Although the modulation varies with the actual EO and, even within the same botanical species, with the actual composition of the EO used in the study (i.e., with the chemotype), often, immune stimulation is linked to boosting innate immune response, and it is associated with interfering with the NFkB, p38, or ERK/MAPK signaling pathways [36]. Eucalyptus EO, with prevalent 1,8-cyneole content, was found to increase phagocytosis by inducing monocyte-derived macrophages with increased phagocytic activity. The activity of tea tree oil rich in tepinen-4-ol was attributed to activation of NF-kB factor, which increases phagocytic activity. Both tea tree oil and terpinen-4-ol induce the differentiation of immature myelocytes into active phagocytizing monocytes and increase the expression of CD11b, a receptor that is partially responsible for the phagocytosis of opsonized bacteria and fungi by leukocytes [36].
12. Essential Oils for Neuroprotection and Modulation of Mood and Cognitive Function
With the global increase of life expectancy, the prevalence of age-related diseases is also increasing, particularly including neurodegenerative conditions such as Alzheimer’s disease (AD). Neurodegenerative diseases and other chronic inflammatory conditions characterized by cognitive impairment are related to lifestyle and to the diet; hence, a dietary intervention can help their course and patients’ quality of life. In AD, the two main microscopic hallmarks of disease, the abnormal accumulation of extracellular protein material in the amyloid–beta plaques and the formation of intracellular neurofibrillary tangles, are associated with abnormal expression of acetylcholinesterase (AChE), which leads to decreased levels of neurotransmitter acetylcholine (ACh), synaptic alteration, and impaired memory and learning [43]. Increased AChE expression appears to be related both to the formation of plaques and to the impaired cognitive function; hence, inhibiting AChE is currently the main pharmacological strategy to treat AD [43][44]. On the other hand, research efforts are clarifying the association between AD, inflammation, and oxidative stress, outlining that antioxidants are key to effective AD treatment strategies [44][45][46]. One additional point is the association between AD and impaired glycemic control, with increased incidence of T2DM in patients with dementia [43]. The above constitutes a solid rational basis to understand the potential of dietary EO supplementation in protecting from neurodegenerative conditions such as AD because (1) many EOs have excellent anti-inflammatory activity with low incidence of side effects; (2) many EOs have good antioxidant activity; (3) many EOs are effective in improving glycemic control and T2DM parameters; and (4) EOs are small, lipophilic molecules with high diffusivity, and hence, they have excellent ability to cross the blood–brain barrier and reach the central nervous system. A fifth reason is that some EOs [47][48] and EO components [47][48][49] have been demonstrated to possess good inhibition activity toward AChE, thereby representing a promising aid to tread AD and other cognitive-impairment conditions [49]. Therefore, several essential oils have the potential to ameliorate the cognitive function and protect from AD; these include thyme, sage, eucalypt, lemon balm, peppermint, oregano, rosemary, lavender, basil, and citrus EOs (rich in limonene and citral) owing to the combination of glucose-lowering, anti-inflammatory, antioxidant, and anti-AChE activities [43][47][48][49].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28020901
References
- Marongiu, B.; Porceddu, S.; Piras, A.; Falconieri, D. Traditional and modern methods for the preparation of essential oils. In Essential Oils and Natural Food Additives: Composition, Applications, Antioxidant and Antimicrobial Properties; Valgimigli, L., Ed.; Nova Science Publishing: New York, NY, USA, 2012; pp. 25–46. ISBN 978-1-62100-241-3.
- Zhang, J.; Zhang, M.; Ju, R.; Chen, K.; Bhandari, B.; Wang, H. Advances in efficient extraction of essential oils from spices and its application in food industry: A critical review. Crit. Rev. Food Sci. Nutr. 2022, 2092834.
- Mancianti, F.; Ebani, V.V. Biological Activity of Essential Oils. Molecules 2020, 25, 678.
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25.
- Valgimigli, L. Essential oils: An overview on origins, chemistry, properties and uses. In Essential Oils and Natural Food Additives: Composition, Applications, Antioxidant and Antimicrobial Properties; Valgimigli, L., Ed.; Nova Science Publishing: New York, NY, USA, 2012; pp. 1–24. ISBN 978-1-62100-241-3.
- Valnet, J. The Practice of Aromatherapy. A Classic Compendium of Plant Medicines and Their Healing Properties; Tisserand, R.B., Ed.; Healing Arts Press: Rochester, NY, USA, 1982; ISBN 0-89281-398-9.
- Price, S.; Price, L. Aromatherapy for Health Professionals, 4th ed.; Elsevier: London, UK, 2012; ISBN 978-0-7020-3564-7.
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504.
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611.
- Rahimi, R. Herbal medicines for the management of irritable bowel syndrome: A comprehensive review. World J. Gastroenterol. 2012, 18, 589–600.
- Balakrishnan, A. Therapeutic Uses of Peppermint—A Review. J. Pharm. Sci. Res. 2015, 7, 474.
- Hawthorn, M.; Ferrante, J.; Luchowski, E.; Rutledge, A.; Wei, X.Y.; Triggle, D.J. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment. Pharmacol. Ther. 1988, 2, 101–118.
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847.
- Gabbai-Armelin, P.R.; Sales, L.S.; Ferrisse, T.M.; De Oliveira, A.B.; De Oliveira, J.R.; Giro, E.M.A.; Brighenti, F.L. A systematic review and meta-analysis of the effect of thymol as an anti-inflammatory and wound healing agent. Phytother. Res. 2022, 36, 3415–3443.
- Subramaniyam, S.; Yang, S.; Diallo, B.N.; Fanshu, X.; Lei, L.; Li, C.; Bishop, O.T.; Bhattacharyya, S. Oral Phyto-thymol ameliorates the stress induced IBS symptoms. Sci. Rep. 2020, 10, 13900.
- Toschi, A.; Tugnoli, B.; Rossi, B.; Piva, A.; Grilli, E. Thymol modulates the endocannabinoid system and gut chemosensing of weaning pigs. BMC Vet. Res. 2020, 16, 289.
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587.
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Sarti, M.D.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152.
- Nutrition Division, FAO/WHO. Probiotics in food. Health and nutritional properties and guidelines for evaluation. FAO Food Nutr. Pap. (FAO) 2006, 85, 1–56. Available online: https://www.fao.org/publications/card/en/c/7c102d95-2fd5-5b22-8faf-f0b2e68dfbb6/ (accessed on 1 December 2022).
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21.
- Probiotics. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/ (accessed on 1 December 2022).
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502.
- Deep, S.; Karmakar, S.; Khare, R.S.; Ojha, S.; Kundu, K.; Kundu, S. Development of Probiotic Candidate in Combination with Essential Oils from Medicinal Plant and Their Effect on Enteric Pathogens: A Review. Gastroenterol. Res. Pract. 2012, 2012, 457150.
- Unusan, N. Essential oils and microbiota: Implications for diet and weight control. Trends Food Sci. Technol. 2020, 104, 60–71.
- Jaiswal, S.; Kundu, K.; Karmakar, S.; Kundu, S. Bacterial strains from local curd, ice-cream and natural milk cultures as po-tential probiotic candidate: Isolation, characterization and in vitro analysis. Int. J. Probiotics Prebiotics 2009, 4, 187–194.
- Oliveira, F.D.A.; Andrade, L.N.; De Sousa, B.V.; de Sousa, D. Anti-Ulcer Activity of Essential Oil Constituents. Molecules 2014, 19, 5717–5747.
- Alomair, M.K.; Alabduladheem, L.S.; Almajed, M.A.; Alobaid, A.A.; Alkhalifah, E.A.R.; Younis, N.S.; Mohamed, M.E. Achillea millefolium Essential Oil Mitigates Peptic Ulcer in Rats through Nrf2/HO-1 Pathway. Molecules 2022, 27, 7908.
- Guesmi, F.; Ali, M.B.; Barkaoui, T.; Tahri, W.; Mejri, M.; Ben-Attia, M.; Bellamine, H.; Landoulsi, A. Effects of Thymus hirtus sp. algeriensis Boiss. Et Reut. (Lamiaceae) essential oil on healing gastric ulcers according to sex. Lipids Health Dis. 2014, 13, 138. Available online: http://www.lipidworld.com/content/13/1/138 (accessed on 3 January 2023).
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254.
- Khosropour, P.; Sajjadi, S.-E.; Talebi, A.; Minaiyan, M. Anti-inflammatory effect of Myrtus communis hydroalcoholic extract and essential oil on acetic acid-induced colitis in rats. J. Rep. Pharm. Sci. 2019, 8, 204.
- De Blasio, A.; D’Anneo, A.; Lauricella, M.; Emanuele, S.; Giuliano, M.; Pratelli, G.; Calvaruso, G.; Carlisi, D. The Beneficial Effects of Essential Oils in Anti-Obesity Treatment. Int. J. Mol. Sci. 2021, 22, 11832.
- Pramanik, K.C.; Biswas, R.; Bandyopadhyay, D.; Mishra, M.; Ghosh, C.; Chatterjee, T.K. Evaluation of anti-ulcer properties of the leaf extract of Juniperus communis L. in animals. J. Nat. Remedies 2007, 7, 207–213.
- Valussi, M. Functional foods with digestion-enhancing properties. Int. J. Food Sci. Nutr. 2012, 63, 82–89.
- Saddiqi, H.A.; Iqbal, Z. Usage and Significance of Fennel (Foeniculum vulgare Mill.) Seeds in Eastern Medicine. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Elsevier: London, UK, 2011; pp. 461–467.
- Darwish, R.S.; Hammoda, H.M.; Ghareeb, D.A.; Abdelhamid, A.S.; El Naggar, E.M.B.; Harraz, F.M.; Shawky, E. Efficacy-directed discrimination of the essential oils of three Juniperus species based on their in-vitro antimicrobial and anti-inflammatory activities. J. Ethnopharmacol. 2020, 259, 112971.
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory Activities of Selected Essential Oils. Biomolecules 2020, 10, 1139.
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A Systematic Review of the Anti-Inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid. Based Complement. Altern. Med. 2020, 2020, 2020–8878927.
- Guimarães, A.G.; Quintans, J.S.S.; Quintans-Júnior, L.J. Monoterpenes with Analgesic Activity—A Systematic Review. Phytother. Res. 2013, 27, 1–15.
- de Cássia Da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. Sesquiterpenes from Essential Oils and Anti-Inflammatory Activity. Nat. Prod. Commun. 2015, 10, 1767–1774.
- Sá, R.D.C.D.S.E.; Andrade, L.N.; Oliveira, R.D.R.B.D.; De Sousa, D.P. A Review on Anti-Inflammatory Activity of Phenylpropanoids Found in Essential Oils. Molecules 2014, 19, 1459–1480.
- Matos, M.S.; Anastácio, J.D.; Nunes dos Santos, C. Sesquiterpene Lactones: Promising Natural Compounds to Fight Inflammation. Pharmaceutics 2021, 13, 991.
- Peterfalvi, A.; Miko, E.; Nagy, T.; Reger, B.; Simon, D.; Miseta, A.; Czéh, B.; Szereday, L. Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System. Molecules 2019, 24, 4530.
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Essential oils and functional herbs for healthy aging. Neural Regen. Res. 2019, 14, 441–445.
- De Simone, A.; Bartolini, M.; Baschieri, A.; Apperley, K.Y.; Chen, H.H.; Guardigni, M.; Montanari, S.; Kobrlova, T.; Soukup, O.; Valgimigli, L.; et al. Hydroxy-substituted trans-cinnamoyl derivatives as multifunctional tools in the context of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 378–389.
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J. Alzheimer’s Dis. Rep. 2020, 4, 175–183.
- Tarozzi, A.; Bartolini, M.; Piazzi, L.; Valgimigli, L.; Amorati, R.; Bolondi, C.; Djemil, A.; Mancini, F.; Andrisano, V.; Rampa, A. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharmacol. Res. Perspect. 2014, 2, e00023.
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Für Nat. C 2016, 71, 393–402.
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and Antiacetylcholinesterase Activities of Some Commercial Essential Oils and Their Major Compounds. Molecules 2011, 16, 7672–7690.
- Min, S.L.S.; Liew, S.Y.; Chear, N.J.Y.; Goh, B.H.; Tan, W.-N.; Khaw, K.Y. Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy. Biology 2022, 11, 307.
- Zhang, N.; Yao, L. Anxiolytic Effect of Essential Oils and Their Constituents: A Review. J. Agric. Food Chem. 2019, 67, 13790–13808.
- Veiskaramian, A.; Gholami, M.; Yarahmadi, S.; Baharvand, P.A.; Birjandi, M. Effect of aromatherapy with Melissa essential oil on stress and hemodynamic parameters in acute coronary syndrome patients: A clinical trial in the emergency department. Complement. Ther. Clin. Pract. 2021, 44, 101436.
- Lari, Z.N.; Hajimonfarednejad, M.; Riasatian, M.; Abolhassanzadeh, Z.; Iraji, A.; Vojoud, M.; Heydari, M.; Shams, M. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J. Ethnopharmacol. 2020, 251, 112560.
- Zamanifar, S.; Bagheri-Saveh, M.I.; Nezakati, A.; Mohammadi, R.; Seidi, J. The Effect of Music Therapy and Aromatherapy with Chamomile-Lavender Essential Oil on the Anxiety of Clinical Nurses: A Randomized and Double-Blind Clinical Trial. J. Med. Life 2020, 13, 87–93.
- Cho, M.-Y.; Min, E.S.; Hur, M.-H.; Lee, M.S. Effects of Aromatherapy on the Anxiety, Vital Signs, and Sleep Quality of Percutaneous Coronary Intervention Patients in Intensive Care Units. Evid. Based Complement. Altern. Med. 2013, 2013, 381381.
