Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Slipped capital femoral epiphysis (SCFE) is the most common adolescent hip disorder in children 9–15 years old with an incidence that ranges from 0.33:100,000 to 24.58:100,000. Idiopathic SCFE is strongly associated with obesity, while atypical SCFE is associated with endocrinopathies, metabolic and renal disease, radiation therapy, and chemotherapy.
- slipped capital femoral epiphysis
- obesity
- children
1. Introduction
There are several etiological factors that can lead to slipped capital femoral epiphysis (SCFE), and which can act both separately or collectively. These include mechanical and anatomic factors [1][2], hormonal and endocrine factors [3][4], obesity [5][6], and immunological abnormalities [7].
Most cases of SCFE are idiopathic, presenting without any other underlying condition. However, a strong relation with childhood obesity has been demonstrated [8]. Obesity is recognized as a global health issue, particularly in Western industrialized nations [9][10][11], and is a well-established risk factor for SCFE. Three key elements in the pathophysiology of SCFE include an increase in body mass, the maintenance of the perichondrial ring, and high levels of activity [2]. In the presence of preexisting physeal pathology, the additional forces induced by obesity could result in a slip [6].
Obesity has been linked to leptin resistance, primarily by providing adequate satiety feedback and secondarily resulting in greater signaling at other sites of action [5]. This is achieved when the serum leptin levels exceed the normal ones in non-obese individuals. Leptin levels are elevated in children with obesity. As observed in SCFE, leptin has the potential to enhance the proliferative zone’s width. Leptin stimulates chondrocyte hypertrophy and proliferation; variations in leptin levels result in an extended physis, disrupted columnar structure, cellular apoptosis, and diminished expression and organization of the type-II and type-X collagen, normally observed throughout the hypertrophic zone [12]. Regarding the histologic similarities between SCFE-affected physes and hyperleptinemic physes, in addition to the connection between increased leptin and body mass index (BMI), researchers can suspect that leptin may be the cause of physeal pathology necessary for the emergence of SCFE [12][13]. In tissues obtained from biopsies in SCFE, alterations in the longitudinal orientation of the physeal cartilage cells, which are typically parallel to the axis of the bone, are observed. The columnar organization and the extracellular matrix composition of the physis in SCFE differ significantly histologically from those of a healthy physis (Figure 1). Although all the above changes are performed in sequence and in response, their role is still unknown. It remains a question as to whether they are of causal or adaptive reason. Additionally, some of these modifications can be present in endocrine or metabolic disorders [3].

Figure 1. The effect of leptin on the pathophysiology of slipped capital femoral epiphysis.
Obesity causes accelerated growth earlier in the prepubescent stage, advancing puberty in females and delaying it in males [14][15]. The positive effect of insulin and insulin like growth factor 1 (IGF-1) in the proliferation and hypertrophy of differentiated chondrocytes of the physis has been well demonstrated [16][17][18]. Wu et al. [19], using a mice model, demonstrated that mice fed with a high fat diet developed greater body and tibial growth and higher serum insulin levels. Montanez-Alvarez et al. [4], in a case-control study of 14 children with SCFE compared to 23 age and BMI matched obese children without the disease conducted in Mexico, found elevated serum insulin, triglycerides, and very low density lipoprotein levels in the first group. Hyperinsulinemia impairs normal apoptosis of chondrocytes, which is necessary in the transition from physis to bone, therefore contributing in the broadening and weakening of the physis [17][20]. In a histologic study of the proximal femoral growth plate of eight core biopsies of the proximal femoral growth plate during in situ epiphysiodesis in patients with SCFE in the pre-slipping stage in two cases and at the mild slipping stage (Southwick angle < 30°) in six cases, there were significant structural changes. Chondrocytes were smaller, elongated, and arranged in clusters rather than columns, with thinner collagen fibrils and impaired physeal architecture [21], events suggesting that there are probably hormonal and metabolic factors rather than mechanical that cause the physis to deteriorate before the slip occurs.
Patients with SCFE frequently have delayed sexual maturation, which could indicate a delay in physeal closure. This produces an extended period of weakness, resulting in a physis prone to the impacts of augmented load, mainly in the pre-existence of obesity. This interruption is implicated in the slip. Children with obesity are most commonly diagnosed with the above-mentioned condition [3].
It is fundamental to consider that atypical SCFE manifests with distinct clinical and radiological features, as well as different complications from idiopathic SCFE. Atypical SCFE is linked to endocrine abnormalities, neoplastic or metabolic disorders, including renal osteodystrophy, chemotherapy, or radiation therapy on the pelvic region [22].
Both biomechanical and biochemical alterations developing during puberty are generally established etiologies [23].
2. Endocrine Factors
Hypogonadal diseases, hypothyroidism, as well as growth hormone (GH) suppletion that constitute systemic or endocrine abnormalities deteriorate the physis. SCFE is prevalent among pediatric patients diagnosed with apparent endocrinopathies, which include hypopituitarism, hyperparathyroidism, and hypogonadal states but also hypo- and hyperthyroidism. Thyroid hormone via signaling of the Indian hedgehog/parathyroid hormone-related protein (IHH–PTHrH) pathway affects the physis both directly and indirectly [3].
Thyroid hormone facilitates growth, development, and maturation of the skeleton by stimulating chondrocyte proliferation, inducing differentiation of bone progenitor cells, mineralization, and angiogenesis. Moreover, it facilitates pituitary GH secretion and GH-dependent IGF-1 production in the bone, which both have regulatory effects on the activity of GH [24]. The epiphyseal plate becomes hypoplastic and endochondral and intramembranous ossification are delayed in children with thyroid hormone insufficiency. The growth hormone/insulin-like growth factor axis is rendered inactive by thyroid hormone deprivation. Recent animal studies have shown that hypothyroid swine displays much lower aggrecan and type X collagen gene expression, demonstrating the effect of hypothyroidism on the development plate [25]. Such modifications probably reduce the overall strength and resilience of the epiphysis, which may provide insight on the diseases of the human orthopedic growth plate. In patients with SCFE with atypical presentation, such as children younger than the age of 10 or older than 16 years old, those who have bilateral SCFE, or those whose height is less than 10% of the normal range for their age and sex, suggested thyroid function screening is suggested [26][27].
A rare pediatric condition, known as pseudohypoparathyroidism (PHP) type 1b, is marked by renal resistance to the parathyroid hormone (PTH), which results in biochemical hypoparathyroidism with skeletal sensitivity to PTH. Despite otherwise normal renal function, PHP is characterized by renal resistance to PTH, which leads to hypocalcemia and hyperphosphatemia and increased PTH concentrations. Since there is osseous sensitivity to PTH, bone resorption occurs [28].
In pediatric patients, long-lasting ailments provoke growth deficiency through altered mechanisms, which manifest on the GH/IGF-1 axis [29].
Physeal disorders mimic those developed in SCFE, mostly in young children with chronic renal failure that have higher risk of developing renal osteodystrophy. High and long-lasting concentrations of glucocorticoids decrease growth, directly and indirectly. Nevertheless, there is no clear evidence indicating the relation between glucocorticoids and SCFE [3].
In patients with malignancies, the survival rates have significantly improved, leading to the hypothesis of atypical SCFE as a late complication of radiotherapy and chemotherapy due to the systemic effects on the physis. Although physeal chondrocyte radiosensitivity, radiation’s indirect endocrine effects, and chemotherapeutic radiosensitization have all been proposed as potential reasons, the exact mechanisms are still unknown. The fact that the majority of studies to date have not particularly addressed the clinical and radiologic characteristics of atypical SCFE is likely related to the relatively small number of subjects. Chemotherapy as a systemic treatment for pediatric malignancies may be the cause of atypical SCFE associated with a secondary endocrine dysfunction. Up to date, there is lack of evidence about an immediate association between chemotherapy and SCFE [22].
3. Immunilogical/Biochemical Factors
In their study, Eisenstein and Rothschild [30] investigated the biochemical abnormalities associated with SCFE and chondrolysis. They found that patients with SCFE had elevated levels of serum immunoglobulins, particularly IgA, as well as an elevated C3 component of complement. Interestingly, IgA had the highest values despite other laboratory tests being normal. Additionally, urinary glycosaminoglycan levels were elevated and increased with the severity of the illness. The authors speculated that these findings could suggest either that slipping of the epiphysis generates an antigen that triggers an autoimmune response, or that slipping is a localized manifestation of a generalized process replicating a connective tissue disorder or an inflammatory state. They also noted that while no other biochemical abnormalities were detected in patients with chondrolysis, the IgM fraction was significantly increased. These preliminary findings suggest that SCFE may be associated with underlying immunological and connective tissue disorders. Furthermore, it is possible that a subset of patients with SCFE may be genetically predisposed to chondrolysis. However, further research is needed to confirm these hypotheses and to fully understand the underlying mechanisms of SCFE and its associated complications. Overall, the study by Eisenstein and Rothschild sheds light on the complex pathophysiology of SCFE and provides important insights into potential underlying mechanisms. Further studies are needed to confirm and expand upon these findings, which may ultimately lead to more effective prevention and treatment strategies for this condition.
4. Mechanical Factors
Mechanical factors are categorized as contributing factors in the pathogenesis of SCFE. Direct or indirect injuries are fairly uncommon, in most cases, mild and rarely of decisive importance [2]. The proximal femoral physis’ mechanical insufficiency is assumed to be part of the pathogenesis of SCFE. The slip can be caused either due to an abnormally high load across a normal physis or due to a physiological load across an abnormally weak physis, or a combination of both. Obesity, femoral and acetabular retroversion, the persistence of the perichondrial ring, epiphyseal tubercle height and location, and physeal cupping [31][32] are part of the mechanical factors contributing to the disease [2][33]. The accepted etiology postulates that as biomechanical forces increase, they eventually exceed the physis’ structural capacity, causing the epiphysis to slip or translate from its starting position, usually posteroinferiorly [12]. Many children during their growth expose their hip joints to high mechanical loads during both normal as well as strenuous sports activities [3]. Results indicate that during physical activity, the growth plate may undergo an abnormal escalation in tension when subjected to an impact loading. Even under physiological conditions, this could cause the plate to deteriorate and subsequently fracture [34]. Despite being obese and rapidly growing, the majority of adolescents with SCFE do not have hormonal metabolic or chronic disorders. However, it seems unlikely that mechanical overload of the proximal femur epiphysis caused, for instance, by excessive body weight alone, can result in a slip. The aforementioned theory is further supported by the results of Winston et al. [1]. In their research, they measured using computerized tomography, epiphyseal tubercule height, width, and volume, physeal diameter, cupping height, acetabular inclination and rotation, and metaphyseal bone density in 31 obese children and 31 age- and gender-matched children with normal weight. Their results indicate that obesity does not cause morphologic changes in the proximal femoral physis, but there is high metaphyseal metabolic activity, as demonstrated by lower bone mineral density in the metaphysis of obese children [1]. In a finite element 3-dimentional model of the adolescent hip, the combined effect of physeal thickness, physeal-diaphysis angle, body weight, physical activity, and the presence of the perichondrial ring were studied for their combined effect. Their results indicate that the most influential factors that contribute to the stresses along the physis are body mass, the presence of the perichondrial ring, and physical activity. In fact, the presence of the perichondrial ring decreased the stress along the physis by 2.53 MPa and high impact activity, such as jumping, had a double mean stress value than toe-off. Slopping angles between 49.9–51.9 had mean stress value 1.36 times greater than angles 41.9–47.9 [2], therefore indicating that increased obliquity is a stress riser of the physis. A detailed diagram of the factors contributing to the pathophysiology of SCFE is shown in Figure 2.
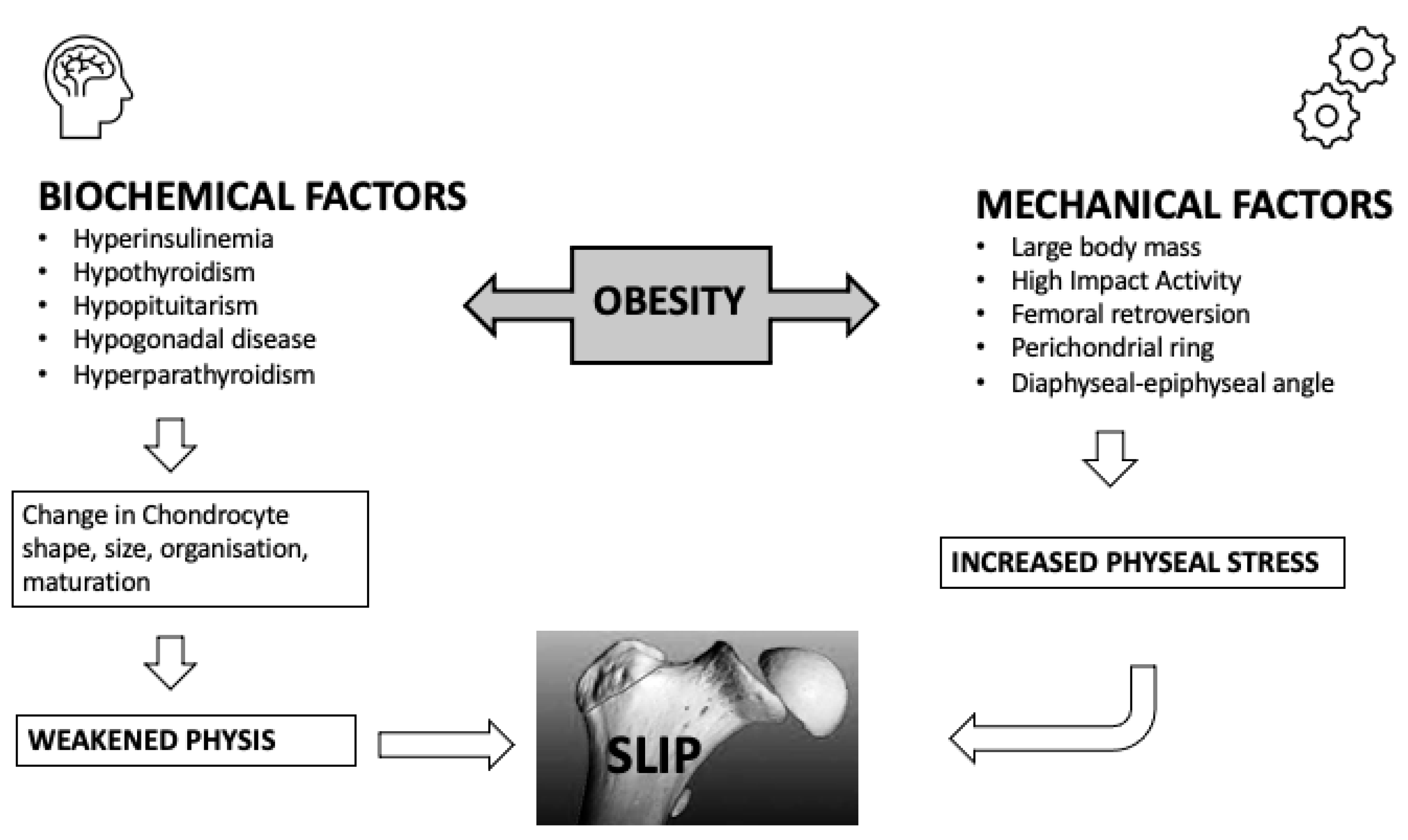
Figure 2. Pathogenesis of slipped capital femoral epiphysis.
This entry is adapted from the peer-reviewed paper 10.3390/surgeries4020017
References
- Winston, T.W.; Landau, A.J.; Hosseinzadeh, P. Proximal Femoral Changes Related to Obesity: An Analysis of Slipped Capital Femoral Epiphysis Pathoanatomy. J. Pediatr. Orthop. B 2022, 31, 216–223.
- Castro-Abril, H.A.; Galván, F.; Garzón-Alvarado, D.A. Geometrical and Mechanical Factors That Influence Slipped Capital Femoral Epiphysis: A Finite Element Study. J. Pediatr. Orthop. B 2015, 24, 418–424.
- Witbreuk, M.; van Kemenade, F.J.; van der Sluijs, J.A.; Jansma, E.P.; Rotteveel, J.; van Royen, B.J. Slipped Capital Femoral Epiphysis and Its Association with Endocrine, Metabolic and Chronic Diseases: A Systematic Review of the Literature. J. Child. Orthop. 2013, 7, 213–223.
- Montañez-Alvarez, M.; Flores-Navarro, H.H.; Cuevas-De Alba, C.; Arana-Hernández, E.I.; Ramírez-Ruiz, M. The Role of Hyperinsulinemia in Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2020, 40, 413–417.
- Galbraith, R.T.; Gelberman, R.H.; Hajek, P.C.; Baker, L.A.; Sartoris, D.J.; Rab, G.T.; Cohen, M.S.; Griffin, P.P. Obesity and Decreased Femoral Anteversion in Adolescence. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1987, 5, 523–528.
- Wabitsch, M.; Horn, M.; Esch, U.; Mayer, H.; Moss, A.; Günther, K.-P.; Nelitz, M. Silent Slipped Capital Femoral Epiphysis in Overweight and Obese Children and Adolescents. Eur. J. Pediatr. 2012, 171, 1461–1465.
- Obana, K.K.; Siddiqui, A.A.; Broom, A.M.; Barrett, K.; Andras, L.M.; Millis, M.B.; Goldstein, R.Y. Slipped Capital Femoral Epiphysis in Children without Obesity. J. Pediatr. 2020, 218, 192–197.e1.
- Murray, A.W.; Wilson, N.I.L. Changing Incidence of Slipped Capital Femoral Epiphysis: A Relationship with Obesity? J. Bone Jt. Surg. Br. 2008, 90, 92–94.
- Perry, D.C.; Metcalfe, D.; Lane, S.; Turner, S. Childhood Obesity and Slipped Capital Femoral Epiphysis. Pediatrics 2018, 142, e20181067.
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; di Luca, N.M.; Grappasonni, I. Fighting Obesity in Children from European World Health Organization Member States. Epidemiological Data, Medical-Social Aspects, and Prevention Programs. Clin. Ter. 2019, 170, e223–e230.
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 January 2023).
- Halverson, S.J.; Warhoover, T.; Mencio, G.A.; Lovejoy, S.A.; Martus, J.E.; Schoenecker, J.G. Leptin Elevation as a Risk Factor for Slipped Capital Femoral Epiphysis Independent of Obesity Status. J. Bone Jt. Surg. 2017, 99, 865–872.
- Bhatia, N.N.; Pirpiris, M.; Otsuka, N.Y. Body Mass Index in Patients with Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2006, 26, 197–199.
- Dunger, D.B.; Ahmed, M.L.; Ong, K.K. Effects of Obesity on Growth and Puberty. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 375–390.
- Burt Solorzano, C.M.; McCartney, C.R. Obesity and the Pubertal Transition in Girls and Boys. Reproduction 2010, 140, 399–410.
- Green, H.; Morikawa, M.; Nixon, T. A Dual Effector Theory of Growth-Hormone Action. Differentiation 1985, 29, 195–198.
- Wu, S.; Zhang, Y.; De Luca, F. The Effect of a High-Calorie Diet on Bone Growth Is Mediated by the Insulin Receptor. Bone 2019, 122, 166–175.
- Zhang, F.; He, Q.; Tsang, W.P.; Garvey, W.T.; Chan, W.Y.; Wan, C. Insulin Exerts Direct, IGF-1 Independent Actions in Growth Plate Chondrocytes. Bone Res. 2014, 2, 14012.
- Wu, S.; Aguilar, A.L.; Ostrow, V.; De Luca, F. Insulin Resistance Secondary to a High-Fat Diet Stimulates Longitudinal Bone Growth and Growth Plate Chondrogenesis in Mice. Endocrinology 2011, 152, 468–475.
- Torres, E.S.; Andrade, C.V.; Fonseca, E.C.; Mello, M.A.; Duarte, M.E.L. Insulin Impairs the Maturation of Chondrocytes In Vitro. Braz. J. Med. Biol. Res. 2003, 36, 1185–1192.
- Tresoldi, I.; Modesti, A.; Dragoni, M.; Potenza, V.; Ippolito, E. Histological, Histochemical and Ultrastructural Study of Slipped Capital Femoral Epiphysis. J. Child. Orthop. 2017, 11, 87–92.
- Chung, C.H.; Ko, K.R.; Kim, J.H.; Shim, J.S. Clinical and Radiographic Characteristics of Atypical Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2019, 39, e742–e749.
- Zelaya, R.; Zarka, A.; Byerly, D. Slipped Capital Femoral Epiphysis as a Presentation of Underlying Metabolic Disorders: Pseudohypoparathyroidism and Juvenile Hypothyroidism. Cureus 2021, 13, e13775.
- Gutch, M.; Philip, R.; Philip, R.; Toms, A.; Saran, S.; Gupta, K.K. Skeletal Manifestations of Juvenile Hypothyroidism and the Impact of Treatment on Skeletal System. Indian J. Endocrinol. Metab. 2013, 17 (Suppl. 1), S181–S183.
- Tank, J.C.; Weiner, D.S.; Jacquet, R.; Childs, D.; Ritzman, T.F.; Horne, W.I.; Steiner, R.; Morscher, M.A.; Landis, W.J. The Effects of Hypothyroidism on the Proximal Femoral Physis in Miniature Swine. J. Orthop. Res. 2013, 31, 1986–1991.
- Moyer, J.; Jacks, L.; Hunter, J.D.; Chan, G. Slipped Capital Femoral Epiphysis and Associated Hypothyroidism. A Review of the Literature with Two Classic Case Examples. J. Pediatr. Endocrinol. Metab. 2016, 29, 427–434.
- Kadowaki, S.; Hori, T.; Matsumoto, H.; Kanda, K.; Ozeki, M.; Shirakami, Y.; Kawamoto, N.; Ohnishi, H.; Fukao, T. Prepubertal Onset of Slipped Capital Femoral Epiphysis Associated with Hypothyroidism: A Case Report and Literature Review. BMC Endocr. Disord. 2017, 17, 59.
- Agarwal, C.; Seigle, R.; Agarwal, S.; Bilezikian, J.P.; Hyman, J.E.; Oberfield, S.E. Pseudohypoparathyroidism: A Rare Cause of Bilateral Slipped Capital Femoral Epiphysis. J. Pediatr. 2006, 149, 406–408.
- Jingushi, S.; Suenaga, E. Slipped Capital Femoral Epiphysis: Etiology and Treatment. J. Orthop. Sci. 2004, 9, 214–219.
- Eisenstein, A.; Rothschild, S. Biochemical Abnormalities in Patients with Slipped Capital Femoral Epiphysis and Chondrolysis. J. Bone Jt. Surg. Am. 1976, 58, 459–467.
- Liu, R.W.; Armstrong, D.G.; Levine, A.D.; Gilmore, A.; Thompson, G.H.; Cooperman, D.R. An Anatomic Study of the Epiphyseal Tubercle and Its Importance in the Pathogenesis of Slipped Capital Femoral Epiphysis. J. Bone Jt. Surg. Am. 2013, 95, e341–e348.
- Kiapour, A.M.; Kiapour, A.; Maranho, D.A.; Kim, Y.-J.; Novais, E.N. Relative Contribution of Epiphyseal Tubercle and Peripheral Cupping to Capital Femoral Epiphysis Stability during Daily Activities. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 1571–1579.
- Chung, S.M.; Batterman, S.C.; Brighton, C.T. Shear Strength of the Human Femoral Capital Epiphyseal Plate. J. Bone Jt. Surg. Am. 1976, 58, 94–103.
- Loder, R.T.; Gunderson, Z.J.; Sun, S.; Liu, R.W.; Novais, E.V. Slipped Capital Femoral Epiphysis Associated with Athletic Activity. Sport. Health 2022, 19417381221093044.
This entry is offline, you can click here to edit this entry!
