Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Microwave (MW)-induced oxidation and ultraviolet (UV)/TiO2 photocatalytic technologies are widely used for organic wastewater treatment. Furthermore, the combination of these technologies (MW/UV/TiO2) result in a new advanced oxidation process. As a green and efficient photocatalytic degradation technology, MW/UV/TiO2 is favored for its advantages of high removal rate, short time use, wide concentration range, low cost, good stability, and no secondary pollution.
- microwave
- UV
- TiO2
- MW/UV/TiO2 photocatalysis
- organic waste water
1. Microwave Radiation Technology
Microwave (MW) is a part of the electromagnetic spectrum that occurs in the frequency range of 300 MHz to 300 GHz and lies between infrared and radio frequencies, corresponding to wavelengths of 1 cm and 1 m (see Figure 1) [1][2]. The scientific consensus is that MW is barely absorbed when passing through non-polar compounds such as glass, plastic, and porcelain. However, MW can be rapidly absorbed by polar compounds such as polar organic solutes, water, and food, and these matters are then heated via polar molecules and ions [1]. Dielectric parameters are linked to the molecular structure that affects the parity of materials. As such, the change in the dielectric parameters of the processed materials significantly affects the efficiency and economy of microwave heating processes [3].
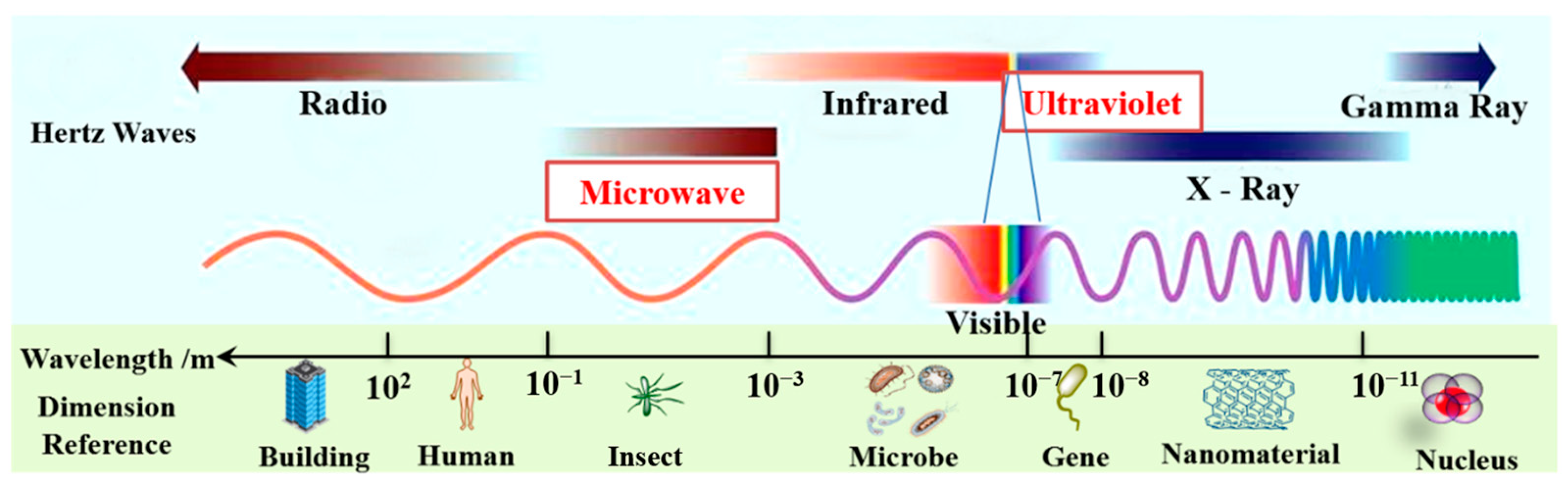
Figure 1. The wavebands of microwave and ultraviolet in the electromagnetic waves.
Compared with traditional heating methods, MW-based technology offers several advantages, including high speed, high efficiency, mild conditions, high selectivity, easy controllability, and no contact with heating materials [4][5]. As a result, MW is widely applied in environmental pollution control, the food industry, plasma processing, organic synthesis, analytical chemistry, polymer treatment, metal sintering, disinfection and sterilization, regeneration and preparation of functional materials, and other physico-chemical and biological research fields [5][6][7][8][9][10][11].
It is scientifically known that MW irradiation can enhance the removal reaction rates and induce selective heating of contaminants through internal molecular vibrations in the treatment of organic wastewater/flue gas/landfill leaching [12][13][14], sludge recycling [15], and solid waste reduction [16][17]. For example, some substances (e.g., Cu/GAC) have minimal degradation efficiencies under conventional conditions and belong to the non-catalysts in the degradation of organic pollutants [18]. However, a hotspot effect radiated by MW can be on the surface of these substances, resulting in the easy degradation of organic pollutants [5][19][20][21]. This process would therefore transform the materials into highly efficient catalysts. Similarly, MW irradiation can be used to improve the catalytic efficiency of conventional catalysts [4].
In addition, MW heating can pyrolyze sewage sludge to produce activated carbon (AC) with high adsorption properties, and AC has been confirmed to effectively remove phenolic organic compounds in an aqueous solution [15]. Moreover, MW irradiation can treat solid waste to promote its solidification, reduce its volume, stabilize heavy metals, and reduce leaching [16][17]. More importantly, landfill leachate can be rapidly discolored, and total organic carbon (TOC) can be reduced by microwave treatment combined with strong oxidizers. Meanwhile, the elimination rates of chemical oxygen demand (COD) and NH3-N are significantly higher than that of the non-microwave treatment [14][22].
2. Ultraviolet Photodegradation Technology
As shown in Figure 1, the wavelength range of ultraviolet (UV) light (10~400 nm) falls between visible light and X-ray electromagnetic radiation [23]. The energy of the UV wave is high and UV can directly destroy the structure of organic matter in wastewater. Currently, UV radiation technology is primarily used to disinfect and sterilize water in conventional water treatment applications due to its low-toxic by-products, chemical-free process, and no overdose risk [24][25]. UV irradiation has a variety of applications in advanced oxidation processes (AOPs) for the degradation of organic wastewater contaminants. [26]. For example, two water pollutants, ranitidine and nizatidine, could be effectively degraded under UV irradiation [27]. In principle, some oxidants (e.g., H2O2, HClO, and S2O82−) radiated by UV could produce the corresponding higher oxidation of free radicals, which would accelerate the degradation of organic pollutants; then, these oxidants could be excited by UV to generate highly reactive radical species such as OH·, Cl·, and SO4−·, respectively [28][29][30]. The OH·, for example, could rapidly and non-selectively destroy most organic pollutants, degrading them to CO2, H2O, and mineral salts in water [31]. The previous literature showed that organic effluents, such as azathioprine [32], sulfamethoxazole [28], ibuprofen [33], thiamphenicol [34], cyclophosphamide, and 5-fluorouracil [35] have been investigated with UV photodegradation technology. Furthermore, ronidazole, metoprolol, ibuprofen, carbamazepine, chloramphenicol, and benzalkonium chloride were effectively decomposed using UV/chlorine as an advanced oxidation process [30][36][37][38][39][40].
3. UV/TiO2 Photocatalysis Technology
Titanium dioxide (TiO2), as a special type of semiconductor, has demonstrated great potential as an ideal photocatalyst material due to its advantages of high reactivity, non-toxicity, water insolubility, resistance to photocorrosion, relatively cheap price, and stability [41][42][43]. Thus, it has been applied to significant applications as the most widely used benchmark photocatalyst in the field of environmental protection [44][45]. Figure 2 depicts the UV/TiO2 photoactivation mechanism. Under the action of UV irradiation, the electron (e−)~hole (h+) pairs are formed on the surface of the TiO2 catalyst in the aqueous solution. Subsequently, O2 and OH− can trap the e−~h+ pairs and adsorb on the surface, and then generate the highly reactive O2−· and OH·, respectively, to degrade organic pollutants [46][47].
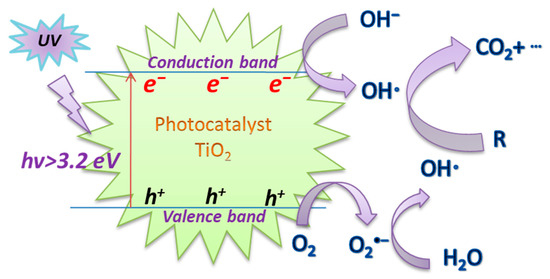
Figure 2. The mechanism of UV/TiO2 photoactivation.
UV/TiO2 photocatalysis has been shown to have strong redox potential, high photocatalytic efficiency, low cost, and no secondary pollution [46][48][49]. Therefore, it is a promising technology for wastewater treatment. For example, the decolorization rate of acid blue 113 and acid red 114 treated with UV/TiO2 reached up to 96% and 99%, respectively, after 240 min [50]. In total, 97% of p-chlorophenol in the solution could be degraded by UV/TiO2 in combination with a Fenton reagent [51]. In addition, UV/TiO2 could be effective in treating dyes (e.g., rhodamine B, methyl orange, methyl blue, amaranth dye), antibiotic compounds, and endocrine disruptors in water [43][52][53].
4. MW/UV/TiO2 Photocatalysis Technology
MW/UV/TiO2 photocatalysis, a novel technology for environmental organic pollutant disposal, was developed by combining microwave irradiation with UV/TiO2 photocatalysis. In essence, it is a new advanced oxidation process for the improvement of photocatalytic technology. In addition, it congregates all the advantages of MW irradiation and UV/TiO2 photocatalysis. As a result, the degradation efficiency of organic pollutants is greatly improved, and the concentration range is wider, especially for refractory organics, with fast and efficient treatment effects through the synergistic actions of MW, UV, and TiO2 [54][55].
4.1. Technical Features
In detail, TiO2 catalysts, microwave electrodeless lamps (MEDLs), and oxidants are added to the reactor vessel containing wastewater. Then, organic pollutants can be degraded by UV emitted from MEDLs under MW irradiation.
The types of organic pollutants removed by MW/UV/TiO2 are phenol, benzene, dyes, pesticides, carboxylic acids, endocrine substances, etc. Among them, dyes and pesticides are the main ones. Overall, all the removal efficiencies of pollutants are very high. Most of the removal efficiencies exceed 80%, with efficiencies approaching 100% in more than half of the studies. The initial concentration of the treated organic pollutants is higher, with a maximum value of 400 mg/L, and the degradation process is very rapid. For example, when methylene blue dye was degraded under the optimized conditions using MW/UV/TiO2, its removal rate could be as high as 96% within 15 min [56]. Notably, herbicide could be completely degraded in 5 min [57].
The majority of the catalysts in the MW/UV/TiO2 are newer and more effective commercial powders of P25 [58][59]. The P25 powders are a mixture of anatase and rutile crystallites, and the reported ratio is typically 70:30 or 80:20 [60]. As the co-presence of anatase and rutile crystallites increases the density defects in the TiO2 lattice, it can enhance the charge separation and improve the utilization efficiency of electron–hole pairs [58][59]. Scientifically, nanoscale TiO2 has a greater ability to trap solution components (e.g., water, oxygen, and organic matter) on its surface than conventional TiO2 [61][62][63]. More importantly, nano-sized TiO2 has excellent UV absorption capability [64]. Therefore, nano-P25 has a better effect on the degradation of organic pollutants and has become the most widely used catalyst in the MW/UV/TiO2 photocatalytic system.
The UV lamp sources for the MW/UV/TiO2 are microwave-powered electrodeless discharge lamps (MEDLs), which have many advantages including the absence of electrodes, low price, low energy cost, and simple reactor configuration [65]. The luminescence mechanism of MEDLs is that the UV light is generated by gas discharge through the excited emission of electrons from metal electrodes. More specifically, the production approach has generally been to fill a mixture of vaporizable metals and rare gases into a sealed body made of quartz, glass, etc., and then UV will be produced by irradiating the mixture with MW. [66][67][68]. Microwave irradiation can heat the metals to form a vapor state and stimulate rare gases to produce a low-pressure plasma, eventually leading to a high luminescence efficiency [69][70][71]. It has been reported that the MEDLs can emit photons in the UV as well as in the visible regions (254, 313, 365, 405, 436, 546, and 577–579 nm) [65]. This degradation method using MEDLs is a promising technology for the treatment of recalcitrant organic compounds based on economy and efficiency [65].
Generically, most of the MW reactors used in studies operated at 2.45 GHz [1]. The MW power in the process was predominantly in the range of 200–900 W, the wavelength of the effective UV was around 254 nm, and the initial volume was 10–1000 mL. In addition, the temperature, air flow, light intensity, pH, and solution circulation velocity were critical technological conditions for MW/UV/TiO2 photocatalysis to remove the organic pollutants.
4.2. Technical Advantages
As shown in Figure 3, MW/UV/TiO2 provided the best photodegradation effect compared to other technologies (i.e., MW/UV, MW/TiO2, MW only, UV only, or TiO2 only). Essentially, MW was recommended to prevent the recombination between the generated positive holes and electrons on the surface of TiO2. Actually, MW could raise the pollutants to a higher electronic excited state. This would favor the formation of more OH· [2]. Even in the absence of the TiO2 catalyst, the effect of organic pollutant removal could therefore be achieved. However, the degradation efficiency of the pollutants was significantly reduced when UV light irradiation was not available, even with the presence of microwave and TiO2. The scientific foundation was that the catalytic system of MW/TiO2, MW, and TiO2 only could not effectively produce hydroxyl radicals if UV was absent [72][73]. The energy of MW only and the adsorption of TiO2 only were insufficient to break the chemical bonds of organic pollutants. Thus, it was clear that the microwave played an auxiliary role in the degradation of organic pollutants using the MW/UV/TiO2 technology. For example, the difference between the effects of MW/UV/TiO2 and heating/UV/TiO2 on the degradation of bisphenol A was insignificant, indicating that microwave radiation might play a role in heating [74]. It should also be noted that the addition of oxidizing agents (e.g., H2O2) would further improve the degradation efficiency of target organic pollutants in the MW/UV/TiO2 process [75].
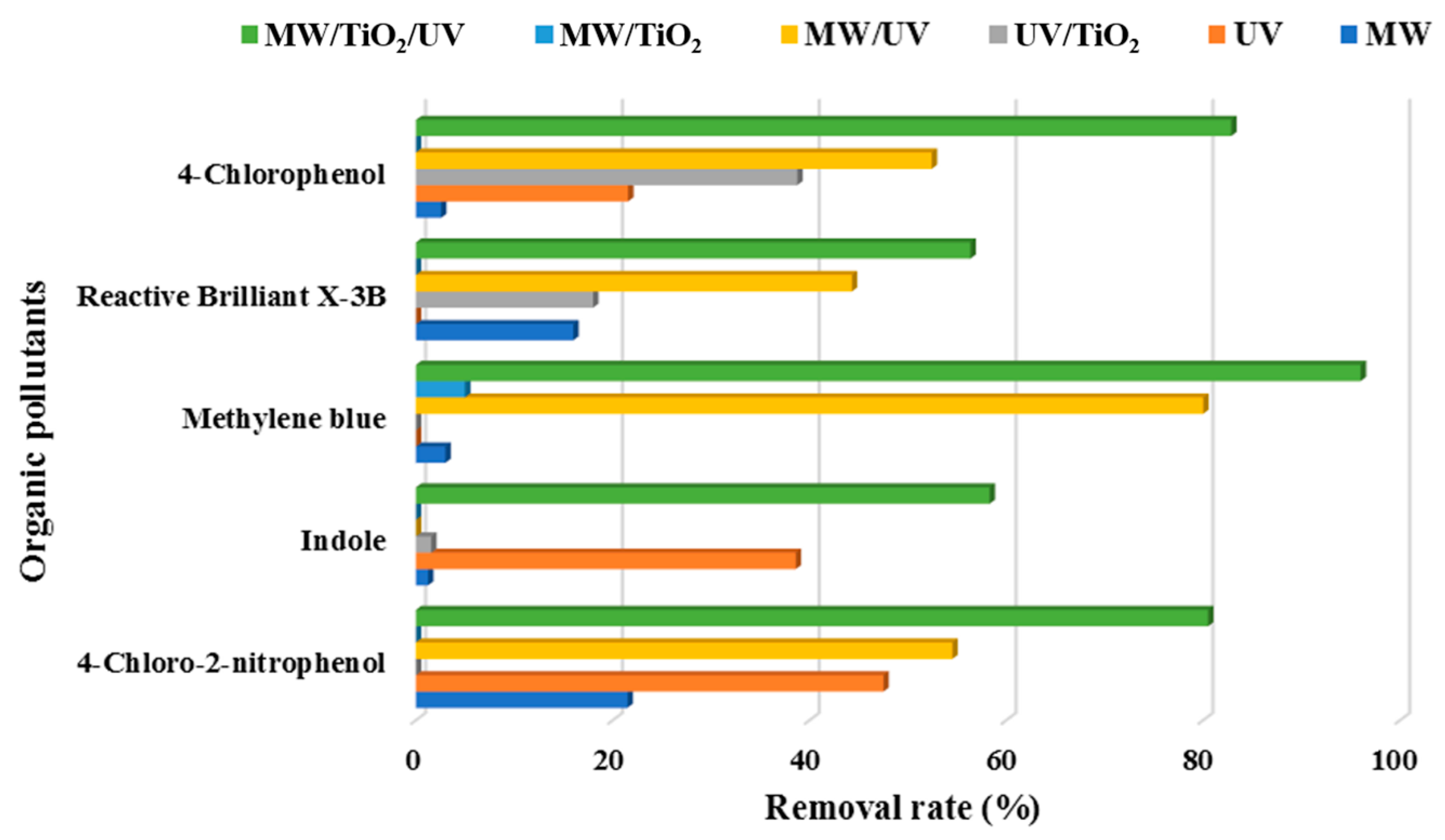
Figure 3. Degradation of organic pollutants efficiency under different process conditions.
4.3. Photocatalytic Degradation Mechanism
The MW/UV/TiO2 photocatalytic degradation technology is an advanced oxidation technology based on using hydroxyl radicals to degrade organic pollutants. The principle of OH· generation is the same as that of UV/TiO2. However, it is true that more OH· can be produced with MW radiation than without MW radiation, as shown in Figure 4. The OH·, with a redox potential of 2.8 V, is the second most reactive after the fluorine atom [76]. It is very highly oxidative and can attack organic contaminants non-selectively [77][78]. As a result, the photodegradation efficiency of MW/UV/TiO2 was better than that of UV/TiO2.
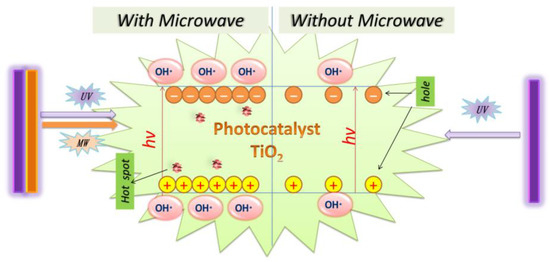
Figure 4. The role of microwave in MW/UV/TiO2 photocatalytic technology.
Scientifically, the degradation process of organic pollutants in the MW/UV/TiO2 system is achieved via OH generated by catalytic TiO2 absorption of UV with microwave assistance according to Equations (1)–(8), where the reaction formula (3) occurs in the alkaline condition, notably [44][45][57][79]. Environmental organic contaminants (EOCs) are eventually mineralized to CO2, H2O, and salts by OH· oxidation [57][80].
TiO2 + hv → e− + h+
h+ + H2O → OH· + H+
h+ + OH− → OH
e− + O2 → O2−·
O2− + H+ → OOH
2OOH· → O2 + H2O2
H2O2 + O2−· → OH· + OH− + O2
EOCs + OH· → CO2 + H2O + salts
It can be explained that the microwave causes the TiO2 surface to produce the additional defect spot, increases the probability of h+ to e− transfer, and simultaneously reduces the reorganization of h+ to e−. Thus, the MW radiation can enhance the surface activity of the TiO2. The surface becomes more hydrophobic under the irradiation of both MW and UV, and it is beneficial to generate a large amount of OH− and O2, which can be easily converted to OH· by Equations (3)–(7) [81]. Horihoshi et al. demonstrated that the amount of OH· produced by microwave-assisted photocatalysis was 20% higher than that of conventional photocatalysis, and therefore MW increased the production rate of OH· [82]. Additionally, it was found that EOCs could be directly broken down by UV light under the MW/UV/TiO2 photocatalytic system [57].
4.4. Treatment Application of Wastewater Selection
It can safely be concluded that in the MW/UV/TiO2 system, TiO2 is the catalyst and UV is the basic requirement to excite the catalyst to produce high-energy hydroxyl radicals. Microwave radiation serves to enhance the degradation efficiency of the pollutants. Essentially, MW/UV/TiO2 technology belongs to an advanced oxidation process based on free-radical degradation reactions. Therefore, this technology has certain requirements for the selection of wastewater treatment. Technically, MW/UV/TiO2 is more suitable for refractory organic wastewater containing high concentrations of substances (e.g., phenol, benzene, dyes, pesticides, etc.). However, it is not capable of treating wastewater involving heavy metals due to the different removal mechanisms.
4.5. Potential Applications
Synthetically, the catalyst of TiO2 is green and economical, and the cost of the UV-producing MEDLs is low. Therefore, the MW/UV/TiO2 is an economically viable and promising technology for the treatment of refractory high-concentration organic wastewater. However, the current research was mostly carried out on a laboratory scale. The lack of integrated large-scale equipment is a basic constraint to its practical application on an industrial scale. There is thus an urgent need for manufacturers to develop specialized equipment tailored to the technical characteristics that meet the requirements for the wastewater treatment process.
This entry is adapted from the peer-reviewed paper 10.3390/catal13040754
References
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Renew. Sust. Energ. Rev. 2017, 77, 12–27.
- Remya, N.; Lin, J.-G. Current status of microwave application in wastewater treatment—A review. Chem. Eng. J. 2011, 166, 797–813.
- Kovacs, P.V.; Lemmer, B.; Keszthelyi-Szabo, G.; Hodur, C.; Beszedes, S. Application of dielectric constant measurement in microwave sludge disintegration and wastewater purification processes. Water Sci. Technol. 2018, 77, 2284–2291.
- Wang, N.; Wang, P. Study and application status of microwave in organic wastewater treatment—A review. Chem. Eng. J. 2016, 283, 193–214.
- Sun, J.; Wang, W.; Yue, Q.; Ma, C.; Zhang, J.; Zhao, X.; Song, Z. Review on microwave–metal discharges and their applications in energy and industrial processes. Appl. Energy 2016, 175, 141–157.
- Jones, D.A.; Lelyveld, T.P.; Mavrofidis, S.D.; Kingman, S.W.; Miles, N.J. Microwave heating applications in environmental engineering—A review. Resour. Conserv. Recycl. 2002, 34, 75–90.
- Martínez, E.J.; Gil, M.V.; Rosas, J.G.; Moreno, R.; Mateos, R.; Morán, A.; Gómez, X. Application of thermal analysis for evaluating the digestion of microwave pre-treated sewage sludge. J. Therm. Anal. Calorim. 2016, 127, 1209–1219.
- Swamy, G.J.; Muthukumarappan, K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017, 220, 108–114.
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172.
- Faraji, S.; Ani, F.N. Microwave-assisted synthesis of metal oxide/hydroxide composite electrodes for high power supercapacitors—A review. J. Power Sources 2014, 263, 338–360.
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sust. Energ. Rev. 2018, 92, 958–979.
- Tian, L.; Lv, G.; Liu, M.; Lei, X.; Rao, W.; Liao, L. Reviews: Microwave-induced oxidation technology and its applications. Prog. Nat. Sci. Mater. 2022, 32, 665–673.
- Lee, D.-J.; Park, Y.-K.; Kim, S.-J.; Lee, H.; Jung, S.-C. Photo-catalytic destruction of ethylene using microwave discharge electrodeless lamp. Korean J. Chem. Eng. 2015, 32, 1188–1193.
- Yeh, C.J.; Lo, S.L.; Kuo, J.; Chou, Y.C. Optimization of landfill leachate treatment by microwave oxidation using the Taguchi method. Int. J. Environ. Sci. Technol. 2017, 15, 2075–2086.
- dos Reis, G.S.; Adebayo, M.A.; Sampaio, C.H.; Lima, E.C.; Thue, P.S.; de Brum, I.A.S.; Dias, S.L.P.; Pavan, F.A. Removal of Phenolic Compounds from Aqueous Solutions Using Sludge-Based Activated Carbons Prepared by Conventional Heating and Microwave-Assisted Pyrolysis. Water Air Soil Pollut. 2016, 228, 33.
- Menéndez, J.A.; Domínguez, A.; Inguanzo, M.; Pis, J.J. Microwave-induced drying, pyrolysis and gasification (MWDPG) of sewage sludge: Vitrification of the solid residue. J. Anal. Appl. Pyrolysis 2005, 74, 406–412.
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486.
- Bo, L.L.; Zhang, Y.B.; Quan, X.; Zhao, B. Microwave assisted catalytic oxidation of p-nitrophenol in aqueous solution using carbon-supported copper catalyst. J. Hazard. Mater. 2008, 153, 1201–1206.
- Xu, D.; Cheng, F.; Zhang, Y.; Song, Z. Degradation of Methyl Orange in Aqueous Solution by Microwave Irradiation in the Presence of Granular-Activated Carbon. Water Air Soil Pollut. 2014, 225, 1983.
- Yuan, D.; Fu, D.Y.; Luo, Z.W. Study on Microwave Combined with Granular Active Carbon for Treatment of P-Nitrophenol Wastewater. Adv. Mater. Res. 2012, 602–604, 2287–2290.
- Zhang, Z.; Shan, Y.; Wang, J.; Ling, H.; Zang, S.; Gao, W.; Zhao, Z.; Zhang, H. Investigation on the rapid degradation of congo red catalyzed by activated carbon powder under microwave irradiation. J. Hazard. Mater. 2007, 147, 325–333.
- Foo, K.Y.; Lee, L.K.; Hameed, B.H. Preparation of activated carbon from sugarcane bagasse by microwave assisted activation for the remediation of semi-aerobic landfill leachate. Bioresour. Technol. 2013, 134, 166–172.
- Verhoeven, G.J.; Schmitt, K.D. An attempt to push back frontiers—Digital near-ultraviolet aerial archaeology. J. Archaeol. Sci. 2010, 37, 833–845.
- Bowker, C.; Sain, A.; Shatalov, M.; Ducoste, J. Microbial UV fluence-response assessment using a novel UV-LED collimated beam system. Water Res. 2011, 45, 2011–2019.
- Kheyrandish, A.; Mohseni, M.; Taghipour, F. Development of a method for the characterization and operation of UV-LED for water treatment. Water Res. 2017, 122, 570–579.
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016, 86, 24–44.
- Dong, H.; Qiang, Z.; Lian, J.; Qu, J. Degradation of nitro-based pharmaceuticals by UV photolysis: Kinetics and simultaneous reduction on halonitromethanes formation potential. Water Res. 2017, 119, 83–90.
- Yang, Y.; Lu, X.; Jiang, J.; Ma, J.; Liu, G.; Cao, Y.; Liu, W.; Li, J.; Pang, S.; Kong, X.; et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate. Water Res. 2017, 118, 196–207.
- Fujioka, T.; Masaki, S.; Kodamatani, H.; Ikehata, K. Degradation of N-Nitrosodimethylamine by UV-Based Advanced Oxidation Processes for Potable Reuse: A Short Review. Curr. Pollut. Rep. 2017, 3, 79–87.
- Dong, H.; Qiang, Z.; Hu, J.; Qu, J. Degradation of chloramphenicol by UV/chlorine treatment: Kinetics, mechanism and enhanced formation of halonitromethanes. Water Res. 2017, 121, 178–185.
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325.
- Zhang, Y.; Zhang, J.; Xiao, Y.; Chang, V.W.C.; Lim, T.-T. Kinetic and mechanistic investigation of azathioprine degradation in water by UV, UV/H2O2 and UV/persulfate. Chem. Eng. J. 2016, 302, 526–534.
- Kwon, M.; Kim, S.; Yoon, Y.; Jung, Y.; Hwang, T.-M.; Lee, J.; Kang, J.-W. Comparative evaluation of ibuprofen removal by UV/H2O2 and UV/S2O82− processes for wastewater treatment. Chem. Eng. J. 2015, 269, 379–390.
- Wang, F.; Wang, W.; Yuan, S.; Wang, W.; Hu, Z.-H. Comparison of UV/H2O2 and UV/PS processes for the degradation of thiamphenicol in aqueous solution. J. Photoch. Photobio. A 2017, 348, 79–88.
- Zhang, Y.; Xiao, Y.; Zhang, J.; Chang, V.W.C.; Lim, T.-T. Degradation of cyclophosphamide and 5-fluorouracil in water using UV and UV/H2O2: Kinetics investigation, pathways and energetic analysis. J. Environ. Chem. Eng. 2017, 5, 1133–1139.
- Huang, N.; Wang, T.; Wang, W.L.; Wu, Q.Y.; Li, A.; Hu, H.Y. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: Synergistic effect, transformation products and toxicity evaluation. Water Res. 2017, 114, 246–253.
- Qin, L.; Lin, Y.L.; Xu, B.; Hu, C.Y.; Tian, F.X.; Zhang, T.Y.; Zhu, W.Q.; Huang, H.; Gao, N.Y. Kinetic models and pathways of ronidazole degradation by chlorination, UV irradiation and UV/chlorine processes. Water Res. 2014, 65, 271–281.
- Nam, S.W.; Yoon, Y.; Choi, D.J.; Zoh, K.D. Degradation characteristics of metoprolol during UV/chlorination reaction and a factorial design optimization. J. Hazard. Mater. 2015, 285, 453–463.
- Wang, W.-L.; Wu, Q.-Y.; Huang, N.; Wang, T.; Hu, H.-Y. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species. Water Res. 2016, 98, 190–198.
- Xiang, Y.; Fang, J.; Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res. 2016, 90, 301–308.
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Silver nanoparticles and defect-induced visible light photocatalytic and photoelectrochemical performance of 2 nanocomposite. Sol. Energy Mater. Sol. Cells 2015, 141, 162–170.
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644.
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571.
- Laxma Reddy, P.V.; Kavitha, B.; Kumar Reddy, P.A.; Kim, K.H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303.
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349.
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241.
- Tong, A.; Braund, R.; Warren, D.; Peake, B. TiO2-assisted photodegradation of pharmaceuticals—A review. Open Chem. 2012, 10, 989–1027.
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B 2004, 49, 1–14.
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986.
- Garg, A.; Sangal, V.K.; Bajpai, P.K. Photocatalytic Treatment of Binary Mixture of Dyes using UV/TiO2 Process: Calibration, Modeling, Optimization and Mineralization Study. Int. J. Chem. React. Eng. 2017, 15, 1–14.
- Yousefi, M.; Ghanbari, F.; Zazouli, M.A.; Ahmadi, M.M.; Akbari, S. Investigation of the Efficiency of Electro-Fenton and UV/TiO2 Processes for Para-Chlorophenol Removal from Aqueous Solutions. J. Health 2016, 7, 600–610.
- Verma, P.; Samanta, S.K. Degradation kinetics of pollutants present in a simulated wastewater matrix using UV/TiO2 photocatalysis and its microbiological toxicity assessment. Res. Chem. Intermed. 2017, 43, 6317–6341.
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds—A review. Chem. Eng. J. 2017, 310, 437–456.
- Chen, H.; Bramanti, E.; Longo, I.; Onor, M.; Ferrari, C. Oxidative decomposition of atrazine in water in the presence of hydrogen peroxide using an innovative microwave photochemical reactor. J. Hazard. Mater. 2011, 186, 1808–1815.
- Církva, V.; Relich, S. Microwave Photochemistry and Photocatalysis. Part 1: Principles and Overview. Curr. Org. Chem. 2011, 15, 248–264.
- Hong, J.; Sun, C.; Yang, S.G.; Liu, Y.Z. Photocatalytic degradation of methylene blue in TiO2 aqueous suspensions using microwave powered electrodeless discharge lamps. J. Hazard. Mater. 2006, 133, 162–166.
- Gao, Z.; Yang, S.; Ta, N.; Sun, C. Microwave assisted rapid and complete degradation of atrazine using TiO2 nanotube photocatalyst suspensions. J. Hazard. Mater. 2007, 145, 424–430.
- Bessergenev, V.G.; Mateus, M.C.; do Rego, A.M.B.; Hantusch, M.; Burkel, E. An improvement of photocatalytic activity of TiO2 Degussa P25 powder. Appl. Cataly. A-Gen. 2015, 500, 40–50.
- Wang, A.N.; Teng, Y.; Hu, X.F.; Wu, L.H.; Huang, Y.J.; Luo, Y.M.; Christie, P. Diphenylarsinic acid contaminated soil remediation by titanium dioxide (P25) photocatalysis: Degradation pathway, optimization of operating parameters and effects of soil properties. Sci. Total Environ. 2016, 541, 348–355.
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photoch. Photobio. A 2010, 216, 179–182.
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549.
- Haynes, V.N.; Ward, J.E.; Russell, B.J.; Agrios, A.G. Photocatalytic effects of titanium dioxide nanoparticles on aquatic organisms-Current knowledge and suggestions for future research. Aquat. Toxicol. 2017, 185, 138–148.
- Pettibone, J.M.; Cwiertny, D.M.; Scherer, M.; Grassian, V.H. Adsorption of Organic Acids on TiO2 Nanoparticles: Effects of pH, Nanoparticle Size, and Nanoparticle Aggregation. Langmuir 2008, 24, 6659–6667.
- Xiu, H.; Qi, X.; Bai, H.; Zhang, Q.; Fu, Q. Simultaneously improving toughness and UV-resistance of polylactide/titanium dioxide anocomposites by adding poly(ether)urethane. Polym. Degrad. Stab. 2017, 143, 136–144.
- Hong, J.; Han, B.; Yuan, N.; Gu, J. The roles of active species in photo-decomposition of organic compounds by microwave powered electrodeless discharge lamps. J. Environ. Sci. 2015, 33, 60–68.
- Zukawa, T.; Sasaki, Y.; Nagao, N. Ultraviolet Light Emitting Device. U.S. Patent 9691600 B2, 27 June 2017.
- Volkova, G.A.; Kirillova, N.N.; Pavlovskaya, E.N.; Yakovleva, A.V. Vacuum-ultraviolet lamps with a barrier discharge in inert gases. J. Appl. Spectrosc. 1984, 41, 1194–1197.
- Horikoshi, S.; Kajitani, M.; Sato, S.; Serpone, N. A novel environmental risk-free microwave discharge electrodeless lamp (MDEL) in advanced oxidation processes. J. Photoch. Photobio. A 2007, 189, 355–363.
- Kuwano, K.; Nezu, A.; Matsuura, H.; Akatsuka, H. Dissociation degree of nitrogen molecule in low-pressure microwave-discharge nitrogen plasma with various rare-gas admixtures. Jpn. J. Appl. Phys. 2016, 55, 086101.
- Ho, G.S.; Faizal, H.M.; Ani, F.N. Microwave induced plasma for solid fuels and waste processing: A review on affecting factors and performance criteria. Waste Manag. 2017, 69, 423–430.
- Literák, J.ı.; Klán, P. The electrodeless discharge lamp: A prospective tool for photochemistry Part 2. Scope and limitation. J. Photoch. Photobio. A 2000, 137, 29–35.
- Xiong, Z.; Xu, A.; Li, H.; Ruan, X.; Xia, D.; Zeng, Q. Highly Efficient Photodegradation of Alizarin Green in TiO2 Suspensions Using a Microwave Powered Electrodeless Discharged Lamp. Ind. Eng. Chem. Res. 2012, 52, 362–369.
- Qu, D.; Qiang, Z.; Xiao, S.; Liu, Q.; Lei, Y.; Zhou, T. Degradation of Reactive Black 5 in a submerged photocatalytic membrane distillation reactor with microwave electrodeless lamps as light source. Sep. Purif. Technol. 2014, 122, 54–59.
- Horikoshi, S.; Tokunaga, A.; Hidaka, H.; Serpone, N. Environmental remediation by an integrated microwave/UV illumination method. J. Photochem. Photobiol. A Chem. 2004, 162, 33–40.
- Ki, S.J.; Jeon, K.-J.; Park, Y.-K.; Jeong, S.; Lee, H.; Jung, S.-C. Improving removal of 4-chlorophenol using a TiO2 photocatalytic system with microwave and ultraviolet radiation. Catal. Today 2017, 293–294, 15–22.
- Masten, S.J.; Davies, S.H. The use of ozonation to degrade organic contaminants in wastewaters. Environ. Sci. Technol. 1994, 28, 180A–185A.
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598.
- Zhang, Y.; Han, C.; Zhang, G.; Dionysiou, D.D.; Nadagouda, M.N. PEG-assisted synthesis of crystal TiO2 nanowires with high specific surface area for enhanced photocatalytic degradation of atrazine. Chem. Eng. J. 2015, 268, 170–179.
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119.
- Zhang, X.; Sun, D.D.; Li, G.; Wang, Y. Investigation of the roles of active oxygen species in photodegradation of azo dye AO7 in TiO2 photocatalysis illuminated by microwave electrodeless lamp. J. Photoch. Photobio. A 2008, 199, 311–315.
- Kataoka, S.; Tompkins, D.T.; Zeltner, W.A.; Anderson, M.A. Photocatalytic oxidation in the presence of microwave irradiation:observations with ethylene and water. J. Photochem. Photobiol. A Chem. 2002, 148, 323–330.
- Horikoshi, S.; Saitou, A.; Hidaka, H. Environmental remediation by all integrated microwave/UV-illumination method. 1. Microwave assisted degradation of Rhodamine-B dye in aqueous TiO2 dispersions. Environ. Sci. Technol. 2003, 37, 5813–5822.
This entry is offline, you can click here to edit this entry!
