Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Horticulture
Adventitious root formation is defined as the formation of new roots on above-ground plant parts and is considered crucial for the survival of a plant under harsh environmental conditions (i.e., flooding, salt stress, and other abiotic stresses) as well as in the nursery industry. Clonal propagation is based on the ability of a plant part to grow and generate a completely new plant, genetically identical to the mother plant, where the plant part came from.
- auxins
- cuttings
- reactive oxygen species
- reactive nitrogen species
1. Introduction
A plant root system is composed of the primary roots, mainly formed at the embryogenic and later seedling stage, the lateral and the adventitious roots (ARs) [1,2,3]. The latter are mainly formed on above-ground plant parts [4] when the conditions are favoring their formation and development and are considered post-embryogenic roots [5]. Organs, where ARs can be formed, are shoots, leaves, stems, nodes, and hypocotyls, while underground stems and old root parts can also be the source of ARs [3,5,6]. In contrast, lateral roots (LRs) are formed on primary roots [7]. Another major difference between ARs and LRs is their origin, as LRs come from the pericycle cells, while the ARs originate from regions around the cambial zone or other meristematic tissues (Figure 1) [4,6,7]. Both ARs and LRs present similar properties, as both serve for nutrient and water uptake, while they are also sources of endogenous cytokinin production. On the other hand, the formation of ARs is mostly observed under harsh stress conditions, such as flooding or hypoxia, after mechanical wounding or in the case of vegetative plant propagation where the main objective is the formation of ARs on plant parts and organs (propagules) in order to produce a new line of plants [8,9,10,11]. In clonal propagation nowadays, the most commonly used propagation material is the cutting. In general, every plant part, which can be severed from the mother plant and, under favorable conditions, can regenerate the plant it originates from, can be a potential cutting. Many organs can be used as cuttings, from a simple leaf, cut into pieces, to a stem with or without leaves, a single bud, a root cutting, an explant in in vitro propagation, and other types of propagules [12].
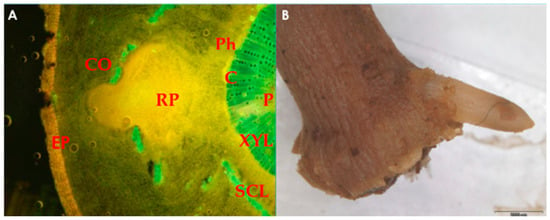
Figure 1. (A) Microscopic image of root primordium (RP) development in olive leafy cutting, originating from the area around the cambium (C), which disrupts the continuum of the sclerenchymatous ring (SCL) and passes through the cortex (CO); (B) root emergence at the base of olive sub-apical shoot cutting. EP, epidermis; XYL, xylem; P, pith; Ph, phloem. The photo is courtesy of Prof. Nikoleta-Kleio Denaxa.
Adventitious root formation (ARF) in cuttings is a prerequisite step in the clonal propagation of economically important plant species, taking place in nurseries multiplying horticultural, ornamental, forestry, or medicinal plants (Figure 2) [4,9,10,13]. The aim of ARF is the production of a perfect, complete, and autonomous plant deriving from a cutting. For the nursery to be successful, the losses during, as well as after, the ARF should be minimized. Such losses are usually due to either insufficient rooting performance or poor quality of the rooting system produced, which presents a barrier to the successful acclimatization of the rooted plantlets [9,14,15].

Figure 2. Rooted olive softwood cuttings (A), details of the root system emergence (B), and (C) rooted hardwood cuttings of Rootpac-90 peach rootstock.
Stem cuttings are the most popular among the organs, which can be used in the vegetative propagation of plants (i.e., leaves, roots, buds, and other plant parts able to regenerate the mother plant) [12]. There are two ways ARF can occur in stem cuttings: via preformed root initials and through wound-induced rooting (Figure 3) [16]. The pre-formed or latent root initials already exist in the cut stem and lay dormant by the beginning of stem development [16]. They are activated through the severance of cutting from the mother plant, and under favorable environmental conditions, they can develop into functional roots [16]. On the other hand, wound-induced ARF is the major type of rhizogenesis taking place in stem cuttings [16]. For the ARF to be efficient, two major factors are crucial, i.e., the presence of the necessary auxin (either the endogenous and/or the applied one), and a tissue predisposed to form roots, as it is known that there is a great difference between juvenile and mature tissues in their ability to form roots, deriving even from the same plant source [16,17]. Besides these two, many other factors are essential, to achieve high rooting percentages, either endogenous or exogenous (hormonal balance, water relationships, oxygen, relative humidity, light, pathogens, and others) [12]. High rooting percentages during asexual plant propagation are the aim of all nurseries, along with short rooting period, high root number, dense rooting system, medium root length (not too short but also not too long roots, which are quite fragile during transplanting), which are all considered as good markers for a successful rooting.

Figure 3. Pre-formed root initials in cherry rootstock COLT (A) with a close look at their emergence (B) and wound plus auxin-induced roots in peach-almond rootstock GF677 softwood cuttings (C).
Nonetheless, insufficient rooting still occurs, and economic losses are faced despite the optimized environmental conditions, the state-of-the-art equipment, and the careful organization and management of the production line in the modern propagation industry. A deeper and more holistic understanding of the physiological, biochemical, and molecular mechanisms of ARF is therefore essential for improving the existing propagation protocols.
2. Insights into Adventitious Root Formation and Factors Affecting It
ARF is usually divided into various phases, based on physiological, biochemical, and molecular markers. De Klerk et al. [14] have described these phases starting from the dedifferentiation of already differentiated cells at the base of the cuttings and their reprogramming, which are usually found in the vicinity of the vascular cambium, secondary phloem, and surrounding tissues. In poplar, the adventitious roots seem to originate from the area between the phloem and cambium [18], while in apple, the interfascicular cambium cells are responsible for the origin of adventitious roots [18]. In raspberry, on the other hand, the ray cells are those to be dedifferentiated and become the progenitor cells for the root primordium formation [18]. These cells should then become competent to respond to the rooting stimulus, which in most cases is being played by auxin, thus promoting the formation of root meristemoids (for some researchers, this is considered the early induction phase) [6,16]. During the next phase (the induction or late induction phase), these cells become committed to forming the first root initials [9,16]. Right after, a series of cell divisions occur, where the organized mass of cells is visible, forming the first root meristem, which will gradually develop into root primordium (root initiation) [4,9,19,20,21]. The latter will continue to grow in order to connect to the vascular system of the cutting [7] while at the same time, if the conditions are favorable, it will emerge from the stem as a functional, developing root (expression phase or root emergence) [4,18].
Although this description of the phases of ARF seems like a simplistic approach and a simple procedure, it is far from that, as it is a complex phenomenon regulated by both endogenous and exogenous factors.
Once the cutting is separated from the donor plant, the soil–plant–atmosphere continuum is interrupted [9]. The breakdown of the vascular continuum then induces an accumulation of auxin near the wounding zone, within the first hours after excision [6,9,22,23]. Wound-responsive genes activate all the necessary mechanisms of wound sealing and pathogen attack prevention [9]. At the same time, the basipetal polar auxin transport (PAT) is triggered (from the site(s) of synthesis, i.e., the shoot apex and young leaves, to the stem base), and auxin transporters take control and induce the canalization of auxin to the cutting’s base, which results in the prementioned accumulation of auxin around the basal zone [6,10]. This auxin accumulation, along with the potent exogenous supply, may trigger the series of phases described above. Along with this triggering, auxin also induces an accumulation of soluble sugars (mainly in the form of sucrose) [24], which will be primarily used as carbon skeleton donors and energy sources for the sealing of the wound and the initial stages of adventitious roots [9,25,26]. Thus, an auxin-carbohydrate cross-talk takes place during the initial stages, establishing a new carbohydrates sink at the base of the cutting [26]. During that time, the application of auxin seems to accelerate the initial biochemical changes observed at the base of the cuttings, as for some easy-to-root species, this auxin application may not be necessary.
It should be noted, however, that while a transient increase in the levels of auxin is needed during the induction phase, this is followed by a gradient decrease during the initiation and expression phases (even though increases in auxin levels have been detected during the expression phase) [26,27,28]. Overall, auxin concentration within the cutting is controlled through synthesis and degradation, transportation, and conjugation or de-conjugation, as regulatory mechanisms to adjust its desired level [29].
During the induction phase, apart from auxin and carbohydrate accumulation, several other biochemical changes take place, some of which include the local increase in jasmonate as well as of some phenolic compounds, reactive oxygen species (ROS), changes in the levels of other plant growth regulators (especially an increase in ethylene production due to wounding) and changes in the activity of enzymes such as peroxidases (PODs), phenoloxidases, and others [10,13,22,23]. Peroxidases are heme-containing enzymes with multifunctional roles and various organic substrates [30], including the endogenous auxin indole-3-acetic acid (IAA), and this is the reason for which they are considered classical rooting markers [10,11,31,32]. Polyamines have also been implicated in the rooting process since they have been found to promote ARF in some species and improve their response to the external application of auxin [11,16,33,34,35,36]. All classes of phytohormones (cytokinins, ethylene, abscisic acid, and gibberellins) have also been implicated (positively, negatively, or neutral) in the capacity of a cutting to form roots [6,13,22,37,38].
Apart from the prementioned organic molecules, nutrients also play an important role in cutting behavior during ARF, as structural elements, enzyme co-factors, and signaling molecules [1,10,39,40,41]. All the above-mentioned organic and inorganic constituents of a cutting may act either as the co-factors or inhibitors of ARF, which largely depends on the genetic material and their concentration changes during ARF as well as their ratios. It has been shown that the ease of ARF is a quantitative heritable trait, which is under the influence of many factors [42].
The actual list of environmental and endogenous factors that affect ARF includes nearly every factor that can affect plant growth and development. Among these, the following have been recognized as potent ARF regulators:
-
Traditional plant growth regulators (auxins, cytokinins, gibberellins, abscisic acid, and ethylene);
-
Light intensity, quality, and photoperiod;
-
Oxygen and carbon dioxide levels;
-
Free radicals;
-
Relative air and soil humidity;
-
The pH and physical properties of the substrate;
-
Antioxidants;
-
Polyamines;
-
Nutrients;
-
Specific growth regulators such as strigolactones, jasmonates, brassinosteroids, melatonin, and generally indoleamines and catecholamines;
-
Hydrogen;
-
Hydrogen sulfide;
-
Methane;
-
Calmodulin;
-
Salicylic acid;
-
Amino acids;
-
Mitogen-activated protein kinase (MAPK);
-
Ca2+-dependent protein kinase (CDPK);
It becomes obvious then that ARF is anything but simple, with an array of factors interacting with each other, regulating its outcome. However, in recent years, and by exploiting the potential of instrumental analysis (both at the biochemical and molecular level), several novel signal molecules have been identified as potential regulators of ARF. Among those, ROS, as well as the reactive nitrogen species (RNS), have been recognized as important players in both lateral root formation as well as ARF [3,45,46]. Among these, hydrogen peroxide (H2O2) and nitric oxide (NO) have been implicated as modulators of induction and initiation phases.
3. Oxidative Species (OS) and Their Role
Reactive oxygen species are products of aerobic metabolism. They are produced in all living organisms, and under normal growth and developmental conditions, their production and scavenging are efficiently balanced. The incomplete reduction of molecular oxygen is the generative force for their production. The most important ROS are hydrogen peroxide (H2O2), superoxide anion (O2•−), hydroxyl radicals (•OH), singlet oxygen (1O2), and others [47,48]. Mitochondria, peroxisomes, and chloroplasts are all cellular organelles where the production of ROS occurs [48], while an apoplastic oxidative burst also confers to the accumulation of ROS in the extracellular space [23]. On the other hand, alternative oxidase (AOX) may also have a role in the production of both ROS and RNS in the mitochondria [10,34,49], as it is a terminal oxidase of the mitochondrial electron transport chain, which can detoxify the OS and restore, to some extent, an equilibrium of their production [10,49].
Under unfavorable conditions, their generation may exceed the capacity of the plant to effectively control their concentration within the cell, so an accumulation of ROS and or RNS is inevitable. This elevated concentration causes oxidative damage, which is encountered at both cellular and molecular levels. To counteract oxidative stress, plants have evolved an array of defenses, including both enzymatic and non-enzymatic factors. Among the enzymatic ones, the major part is being played by superoxide dismutase (SOD), which dismutases O2•– into H2O2, catalase (CAT), which detoxifies H2O2, peroxidase (POD), and enzymes of the ascorbate–glutathione cycle (such as ascorbate peroxidase (APX) and glutathione reductase (GR)) as well as other enzymes [1,3,45,50]. Among the non-enzymatic defense molecules against oxidative stress, phenolic compounds and ascorbic acid have a major role, along with sulfur-containing antioxidants such as glutathione, cysteine, tocopherols, and other antioxidant molecules [3,45,50,51,52].
At the physiological level, their role is quite important, since they serve as signal molecules and are involved in many developmental processes and plant morphogenesis. Among others, ROS take part in cell cycle and elongation, root hair formation, stomatal function, gravitropism, embryogenesis, lateral and adventitious root formation, root elongation, and other physiological processes [51,53,54].
It has been found that ROS may act downstream of several signaling pathways, which originate from the action of plant growth regulators [23,53]. Part of that action is the signal production for the formation of adventitious roots, especially considering that OS are actively produced after physical damage or wound, which is the starting point for the production of cuttings [23]. Nonetheless, there is not much known on the action of ROS or RNS in lateral as well as adventitious root formation; however, quite recently, enough research has been carried out to elucidate their role in this important physiological and economic process.
This entry is adapted from the peer-reviewed paper 10.3390/antiox12040862
This entry is offline, you can click here to edit this entry!
