Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Photopolymerization offers a unique opportunity to convert liquid monomers to polymers using light as the activation source. Major efforts have been devoted to developing visible light photo-initiating systems, and the search for new dyes that can be incorporated into photocurable resins and polymerize a resin within a few seconds is still ongoing.
- quinoxaline
- photopolymerization
- UV light
- visible light
1. Introduction
During the past decades, photopolymerization has been an active research field, mainly supported by the development of 3D printing but also by the necessity to replace in the near future the historical UV photo-initiating systems with visible light photo-initiating systems in the industry [1][2][3][4][5][6][7][8][9][10][11]. Indeed, UV photopolymerization is more and more the focus of safety concerns, originating from the numerous drawbacks of UV light. Notably, UV light can cause eye damage and skin cancers [12][13][14][15]. Parallel to this, molecular oxygen can be converted to ozone during the polymerization process, constituting an additional drawback of this approach [15]. In addition, photopolymerization constitutes an appealing polymerization technique, which exhibits numerous specificities and advantages compared to traditional thermal polymerization. In order to illustrate this, the possibility of polymerizing without solvents to obtain efficient spatial and temporal control during the polymerization process can be cited as relevant examples [16][17][18][19][20][21][22][23][24][25][26][27][28][29]. Development of photo-initiating systems is not new since the first report mentioning a photoinduced electron transfer between triethanolamine and electron-accepting dyes (xanthenes, acridines, thiazines) was reported as soon as 1954 by Oster and coworkers [30]. Since 1954, photopolymerization has greatly evolved, enabling now polymerization in safer and energy-saving conditions. As a breakthrough, visible light photopolymerization has emerged as a promising alternative to the historical UV photopolymerization. As the main advantage of visible light photopolymerization, a higher light penetration within the photocurable resins can be obtained than in the UV range (See Figure 1). Indeed, if the light penetration is limited to a few hundred micrometers in the UV range, this value can increase up to 4 mm at 450 nm and even reach 5 cm at 800 nm [31]. In these conditions, photopolymerization becomes capable of polymerizing thick samples and is not limited anymore to the polymerization of thin samples, as in the past when UV light was used [32]. However, light penetration within the photocurable resins is an important issue of photopolymerization, and some clarifications should be given. For instance, the light penetration is strongly related to the molar extinction coefficient of the photoinitiator at the wavelength used for irradiation and on the photoinitiator concentration in the system. Only in the case of photo-bleachable initiators can the light penetrate deeply. This concerns the light of any wavelengths, with the exception that short-wavelength UV may also be absorbed by monomers. Figure 1 depicts the light penetration only in the light-scattering system, which cannot be generalized to all photocurable systems because most photocurable resins are transparent and not light-scattering. Consequently, the statement that the main advantage of visible light photopolymerization is the higher light penetration within the photocurable resins than in the case of UV light is not applicable to all resins. In complement to this first point, one of the main drawbacks of visible light photopolymerization remains the difficulty of obtaining colorless coatings [33]. Indeed, UV photoinitiators are colorless compounds, enabling the ability to obtain easily colorless polymers. Conversely, visible light photopolymerization makes use of dyes absorbing in the visible range, and these dyes are often responsible for the final color of the polymers. In order to address this issue, major efforts have been devoted to developing photo-bleachable photo-initiating systems, with more or less success [33].
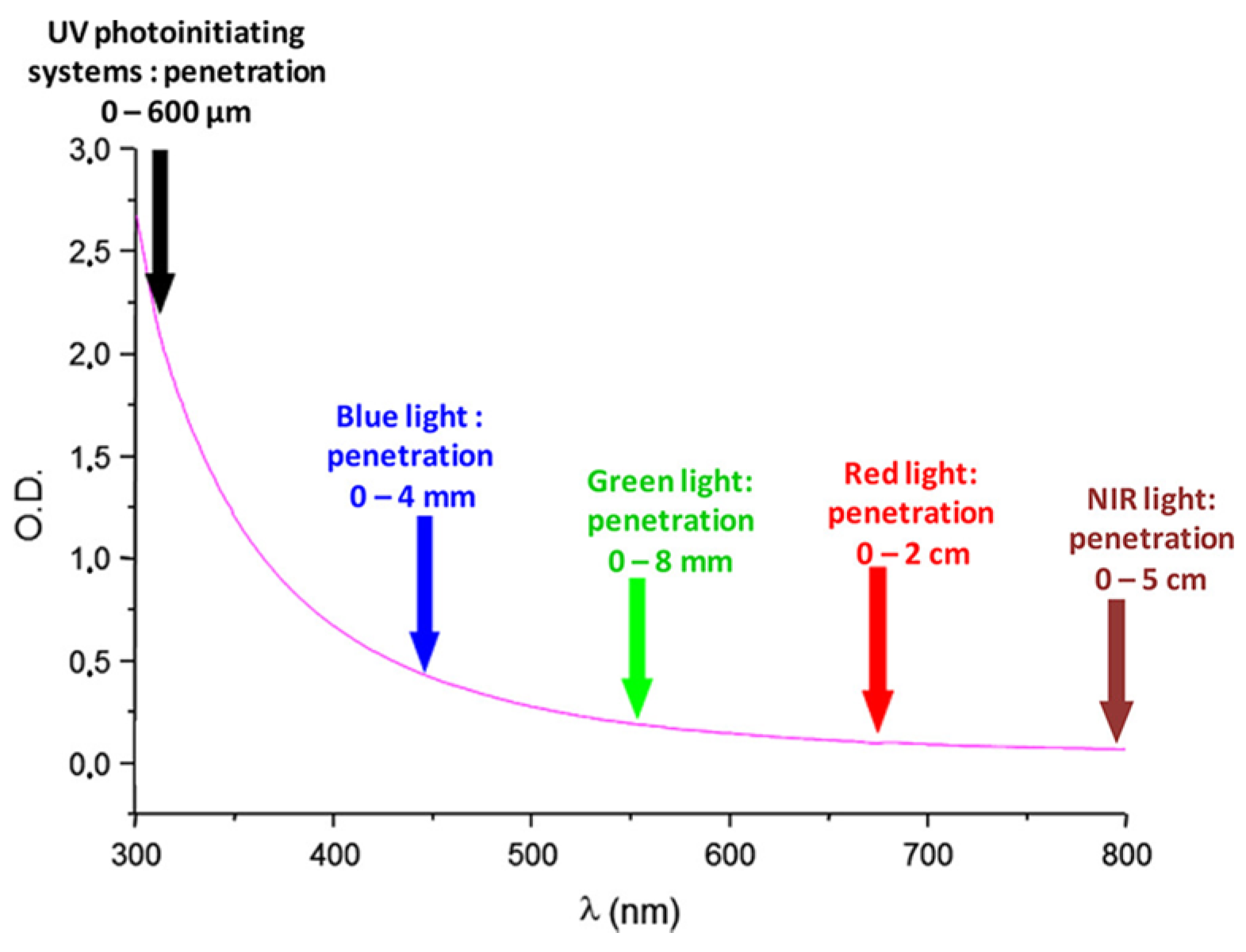
Figure 1. Light penetration in a polystyrene latex with an average particle diameter of 112 nm.
This effort is also supported by the wide range of applications using photopolymerization. Notably, 3D and 4D printing, microelectronics, dentistry, coatings, solvent-free paints, adhesives and varnishes can be cited as the main applications of photopolymerization [1][2][3][4][5][6][7][8][9][10]. Another drawback of visible light photopolymerization is that visible light photons are less energetic than UV photons, so more reactive photo-initiating systems have to be developed in order to overcome this lower energy. In the search for highly reactive photo-initiating systems, numerous structures have been examined as potential candidates capable of addressing the reactivity issue and carbazoles [34][35][36][37][38][39][40][41][42][43][44][45][46][47], dihydroanthraquinones [48], camphorquinone [49][50], chalcones [9][51][52][53][54][55][56][57][58][59][60][61][62][63], naphthalimides [64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82], benzophenones [83][84][85][86][87][88][89][90], silyl glyoximides [91], phenothiazines [92][93][94][95][96][97][98][99][100][101][102][103], thioxanthones [28][104][105][106][107][108][109][110][111][112][113][114][115][116], curcumin [117][118][119][120], pyrenes [121][122][123][124][125][126][127][128][129], iodonium salts [64][130][131][132][133][134][135][136], push-pull dyes [137][138][139], copper complexes [140][141][142][143], iron complexes [144][145] zinc complexes [146] iridium complexes [147][148] and N-heterocyclic carbene boranes [29] can be cited among the most extensively studied structures of the past decade. Beyond the simple selection of the chromophore, the way how to generate initiating species is important. Notably, photoinitiators can be divided into two main categories, namely, Type I and Type II photoinitiators. In the case of Type I photoinitiators, these structures can generate reactive species by homolytic cleavage of a specific bond (See Scheme 1) [149][150][151][152][153][154][155][156][157][158].
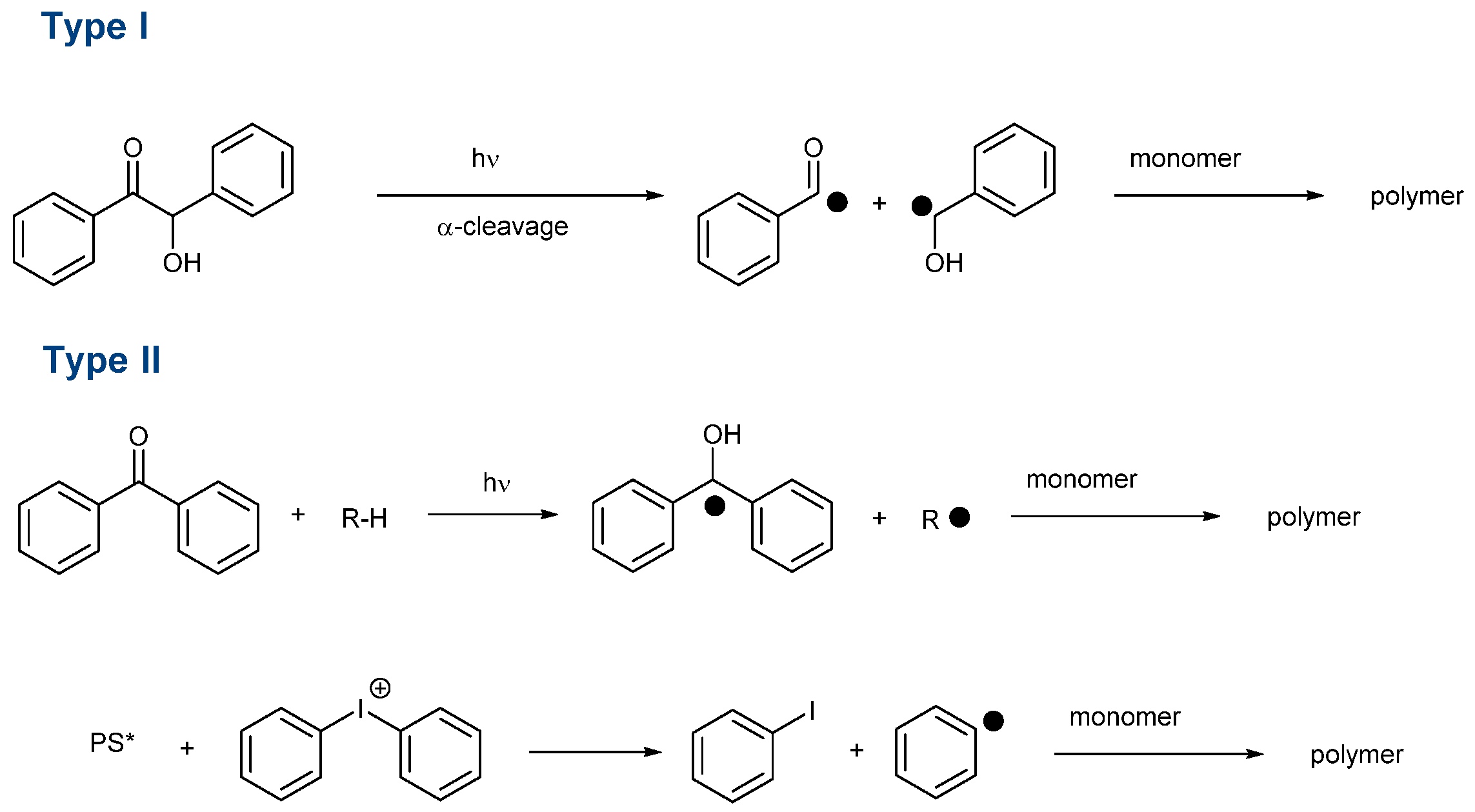
Scheme 1. Formation of initiating species with Type I and Type II photoinitiators. (PS* means that PS is in the excited state).
In this first family, O-acyl-α-oximino ketones, acetophenones, acylgermanes, benzoin ether derivatives, α-aminoketones benzyl ketals, acylphosphine oxides, aminoalkyl phenones, α-hydroxyalkyl ketones, hydroxylalkylphenones and oxime esters can cleave homolytically, generating initiating radicals [103][151][153][158][159][160][161]. As the main interest of these structures, Type I photoinitiators can be used as mono-component systems. As a drawback, the photodecomposition is irreversible, so the concentration continuously decreases during the polymerization process. Conversely, Type II photoinitiators are unable to generate initiating species without additives. However, a few exceptions exist. For instance, free radical polymerization of ether acrylates (such as poly(ethylene glycol) diacrylates) is possible with benzophenone, thioxanthone and different chalcones, the monomer itself acting as a co-initiator because -CH2O- groups are good hydrogen donors [162]. Type II photoinitiators are typically used for the sensitization of onium salts [163][164][165][166][167][168]. A photoinduced electron transfer from the photosensitizer towards the onium salts is the key step to generating initiating radicals. However, Type II photoinitiators are also combined with hydrogen donors, leading to the formation of a ketyl radical with hydrogen abstraction and an additional radical issued from the hydrogen donor (See Scheme 1) [105][110][169][170][171][172][173][174]. Considering that Type II photoinitiators are bimolecular photoinitiators, the introduction of a sacrificial amine can contribute to regenerating the photosensitizer in its initial redox state during the polymerization process so that this latter can be introduced in a catalytic amount. This point is important, considering that the photosensitizer is responsible for the final color of the polymer. By decreasing its content, less colored coatings can be obtained. The search for new structures is also motivated by the recent interest in developing photo-initiating systems activable with sunlight [175][176][177][178][179][180][181][182][183][184] or capable of initiating a polymerization process in water [22][60][116][185][186][187][188][189][190][191][192][193][194][195][196][197][198][199][200]. With the aim of polymerizing in energy-saving conditions, the use of light-emitting diodes (LEDs) is now generalized in photopolymerization due to their low costs, long operating lifetimes, compactness and their precise emission wavelengths. In the search for photo-initiating systems that can be activated under low light intensity, i.e., LEDs, quinoxalines have been identified as potential candidates for visible light photopolymerization. Quinoxalines are heterocyclic compounds in which two nitrogen atoms replace two carbons in the ring of naphthalene. Quinoxalines have been extensively studied for their biological properties. Indeed, quinoxalines are biologically active against bacteria, viruses, cancer, leishmania, tuberculosis, malaria, depression and fungi [201]. Nevertheless, quinoxalines were also used for the design of light-emitting materials for OLEDs [202][203], semiconductors and charge transport materials for solar cells [204][205][206][207], the design of building blocks for covalent organic frameworks [208]. Recently, different quinoxaline derivatives were also proposed as fluorescent probes for near-infrared II (NIR-II, 1000–1700 nm) fluorescent imaging [209].
The first report mentioning the use of quinoxalines as photoinitiators of polymerization was reported in 1999 by Pączkowski and coworkers. By using 3-benzoyl-7-diethylamino-5-methyl-1-phenyl-1H-quinoxalin-2-one (ChAD) as an electron acceptor and N-phenylglycine derivatives (NPG) as electron donors, the free radical polymerization of trimethylolpropane triacrylate (TMPTA) was carried out, using an argon ion laser (emission between 351 and 361 nm, 25.5 mW/cm2) or a He/Ne laser as the light sources (See Figure 2) [210][211]. The best monomer conversion was obtained using (4-methoxyphenyl)glycine as the electron donor. Noticeably, efficiencies of the different photo-initiating systems based on quinoxalines were lower than that of a Rose Bengal derivative, namely RBAX, previously reported in the literature.
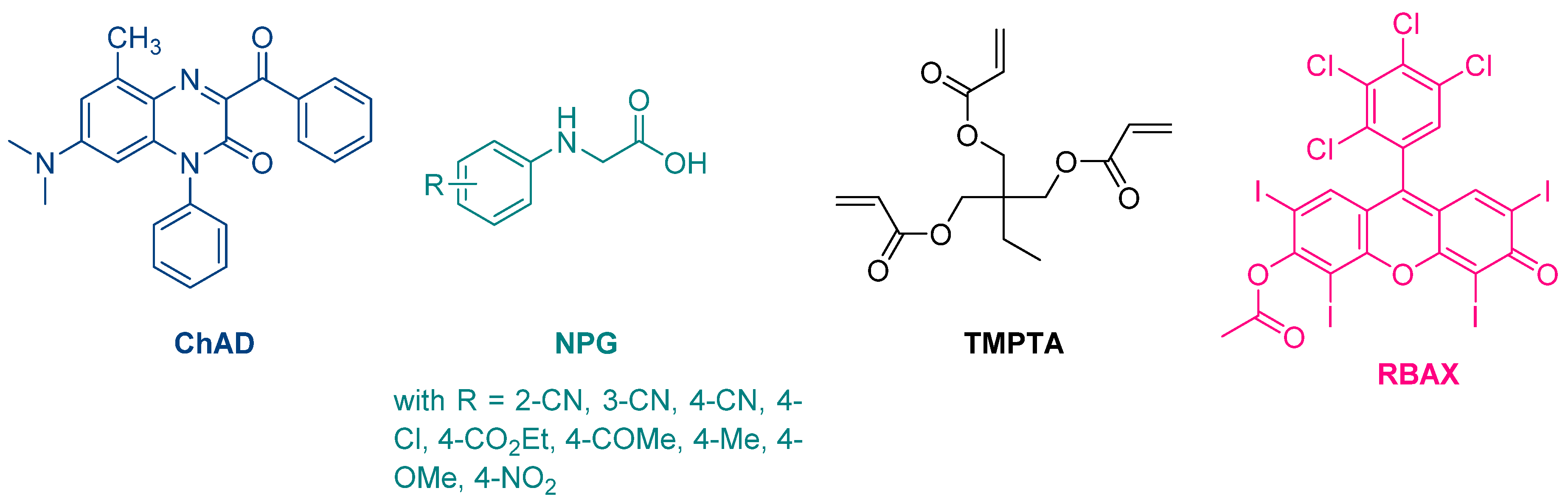
Figure 2. Chemical structures of the chromophore ChAD, the electron donors NPG, the monomer TMPTA and the reference compound RBAX.
The same year, Aydin and coworkers proposed a series of quinoxalines (QNX-1-QNX-5) that proved to be excellent photoinitiators in combination with electron donors such as N-methyldiethanolamine (MDEA) and 2-(N-methyl-N-phenylamino)-1-phenylethanol (MPAPE). By using these two co-initiators, the polymerization of methyl methacrylate (MMA) was carried out upon irradiation with a UV light (λ = 350 nm) (See Figure 3) [212]. A few years later, this strategy was extended to an unusual co-initiator in photopolymerization, namely benzaldehyde [213].
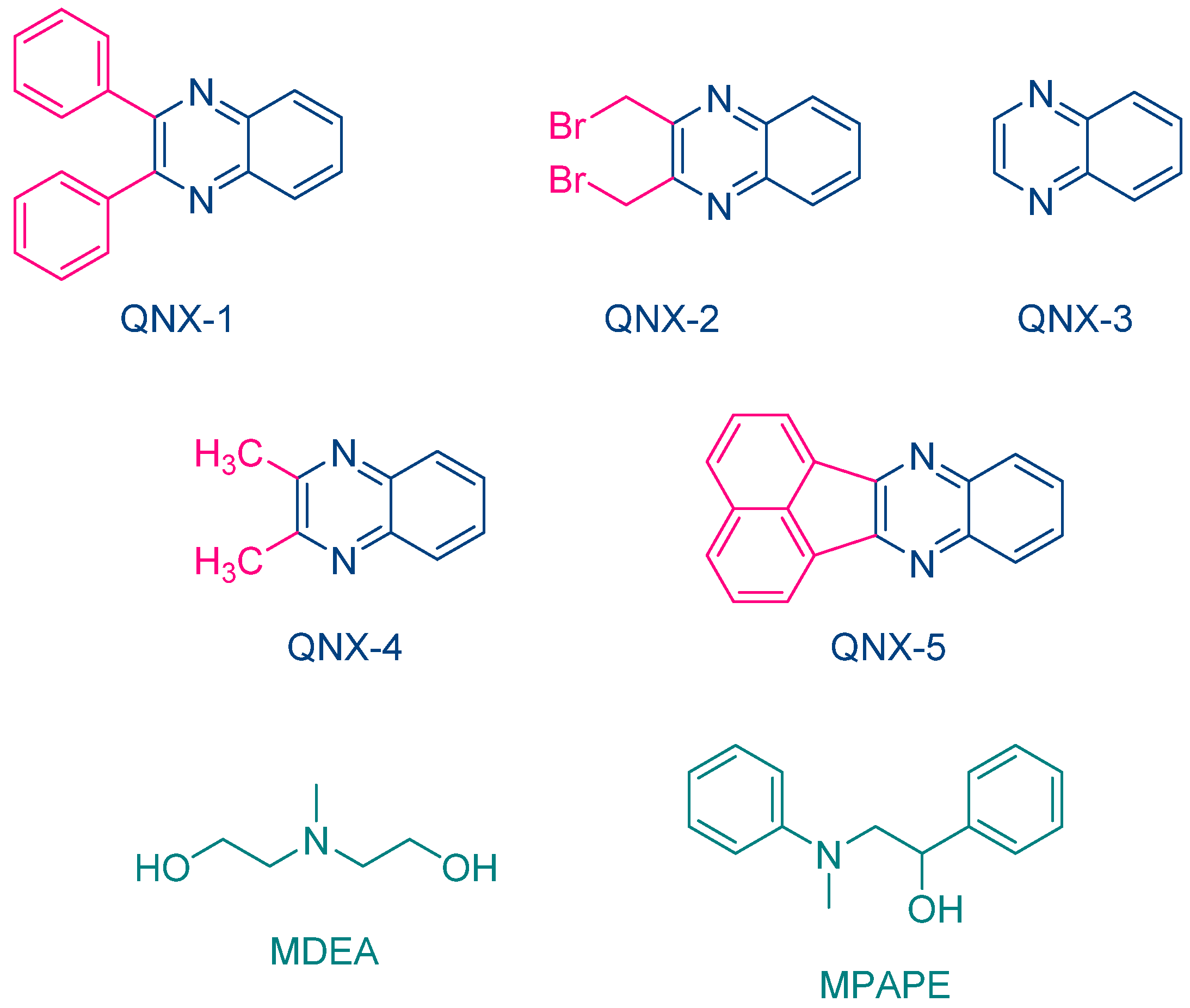
Figure 3. Chemical structures of different quinoxaline derivatives investigated by Aydin and coworkers as photoinitiators of polymerization in combination with MDEA and MPAPE.
Since these pioneering works, numerous quinoxaline derivatives have been proposed, enabling the initiation of free radical polymerizations and cationic polymerizations in the UV and visible range. It has to be noticed that quinoxalines were also investigated as chromophores for two-photon polymerization [214]. Triphenylamine-modified quinoxalines were notably reported as exhibiting a two-photon value higher than 160 GM in the 780–820 nm range, greatly higher than the values reported for most of the benzil derivatives investigated as photoinitiators for two-photon initiated polymerization.
2. Quinoxalines as Photoinitiators of Polymerization
In 2000, Pączkowski and coworkers examined two dyes containing pyrazoloquinoxaline moieties, i.e., ZPG and ZPD, and investigated the photochemical mechanism involved during the free radical polymerization (FRP) of TMPTA (See Figure 4) [215]. Noticeably, similar absorption maxima were found for ZH (λmax = 409 nm in ethyl acetate) and ZPG/ZPD (λmax = 415 nm in ethyl acetate). The photo-initiating abilities of these two dyes were compared with that of the quinoxaline derivative ZH. While the FRP of TMPTA was carried out upon irradiation with an argon ion laser, ZPG and ZPD greatly outperformed the monomer conversion obtained with ZH. To support this, the authors evidenced that the intersystem crossing between the singlet excited state and the triplet excited state was more efficient for ZPG and ZPD than for ZH, enabling these dyes to interact more efficiently with the electron donor N-phenylglycine (NPG) and facilitating the generation of radicals. Application of the Rehm–Weller equation also revealed the free energy change to be more positive for ZH than for ZPD and ZPG so that the rate constant of electron transfer between ZH and NPG was expected to be slower than with ZPD and ZPG. However, the authors also suggested a competitive back electron transfer between ZH and NPG, adversely affecting the photo-initiating ability of this dye.
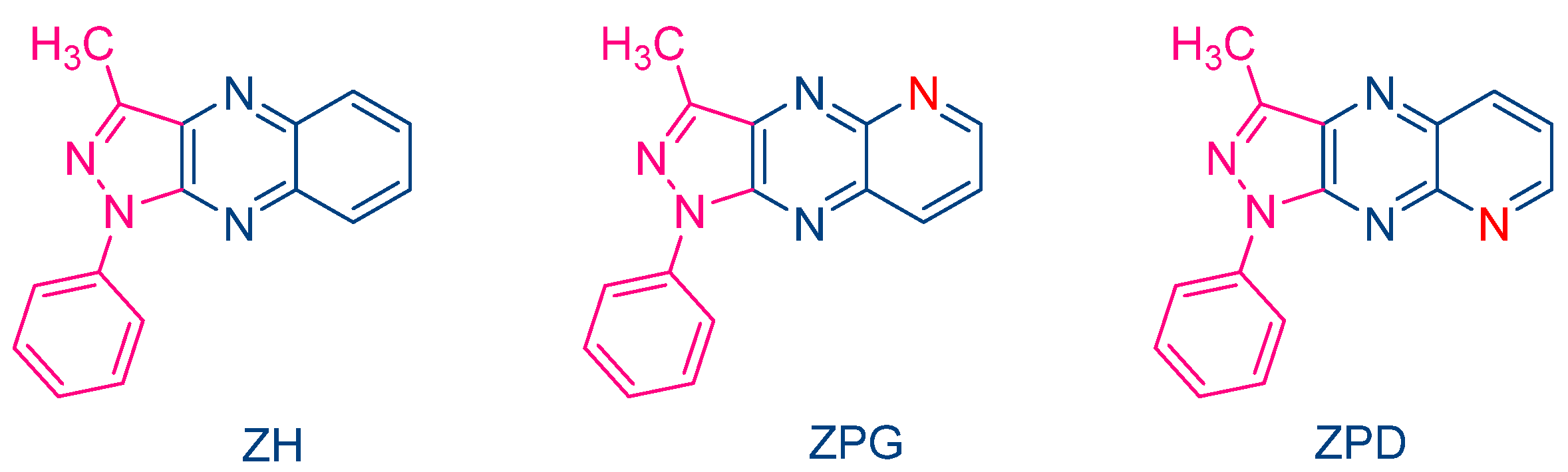
Figure 4. Chemical structures of ZH, ZPG and ZPD.
The occurrence of a back electron transfer was confirmed with a series of ten pyrazoloquinoxalines, including the previously studied quinazoline dye ZH (See Figure 5) [216]. Examination of their UV-visible absorption spectra in ethyl acetate revealed that these structures were relatively insensitive to the substitution pattern. Thus, absorption maxima ranging between 404 nm for ZCD and 435 nm for ZND were determined. It has to be noticed that the positions of the absorption maxima were affected by the substitution pattern. Thus, for the nitro-substituted quinoxaline, positions of the absorption maxima varied from 417 nm for ZNG and up to 435 nm for ZND, in which the nitro group was in a conjugated position with the rest of the molecule. In this series of dyes, the authors could establish a linear relationship between the monomer conversion and the efficiency of singlet oxygen formation, evidencing that the electron transfer between NPG and the different dyes was occurring via the triplet state. By introducing heavy atom in ZCl2, CI and CICl2, quantum yields of the triplet state formation were greatly improved, enhancing the photo-initiating ability.
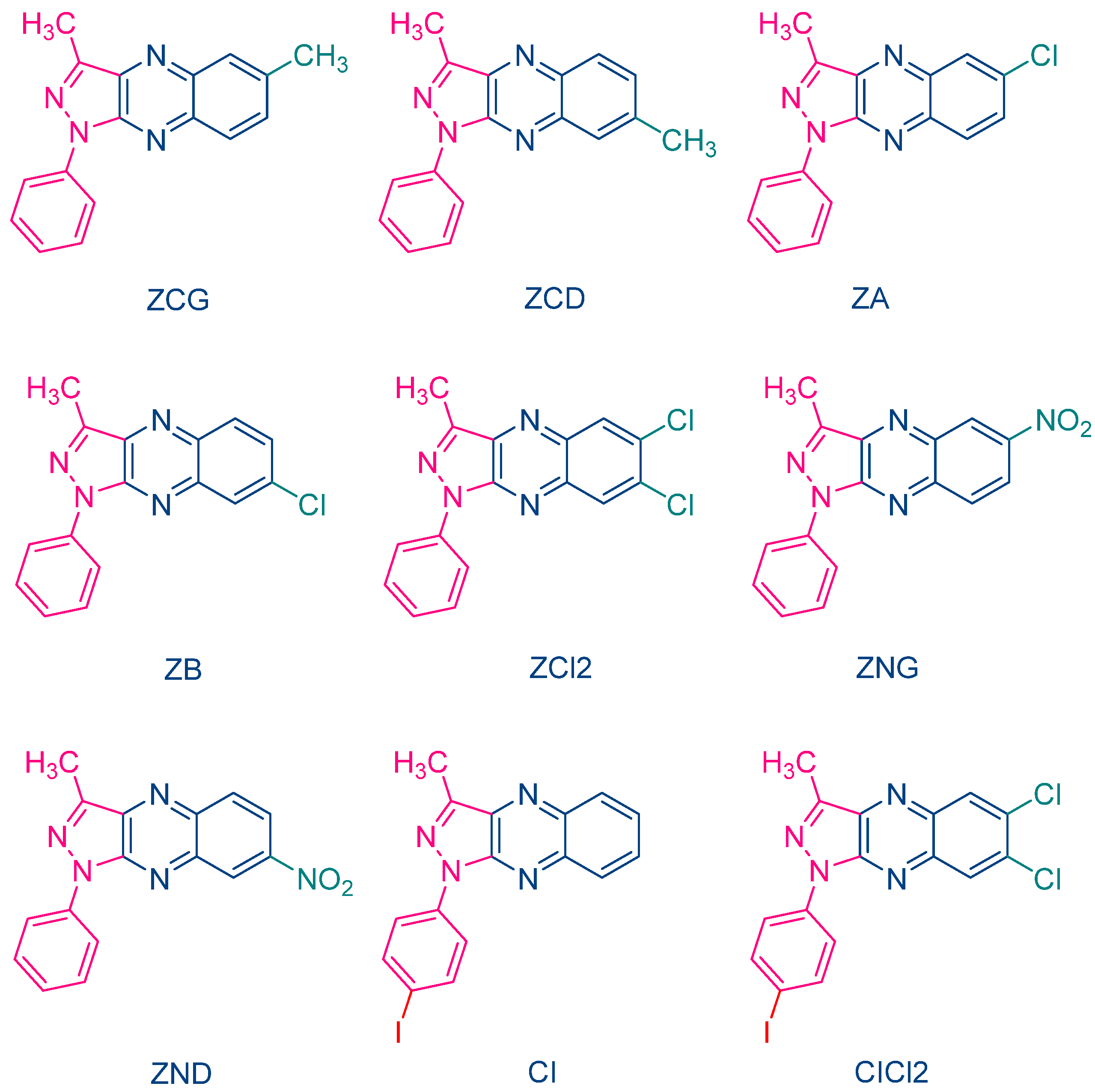
Figure 5. Chemical structures of various pyrazoloquinoxaline dyes.
In benefiting from these elongated excited state lifetimes, interactions between the dyes and NPG in the excited state were favored, improving the polymerization efficiency. As shown in Figure 6, halogenated quinoxalines polymerized TMPTA within 50 s, contrarily to the non-halogenated dyes for which a three-fold elongation of the polymerization time was necessary. To monitor the polymerization process, photo-DSC was used. In this case, access to the monomer conversion was not directly possible. Using photo-DSC, only the heat flow can be determined. Heat flow is proportional to the polymerization rate, which is the derivative of the conversion versus time function. In the present case, the highest heat flows were obtained for CICl2 and CI, the two compounds bearing halogens.
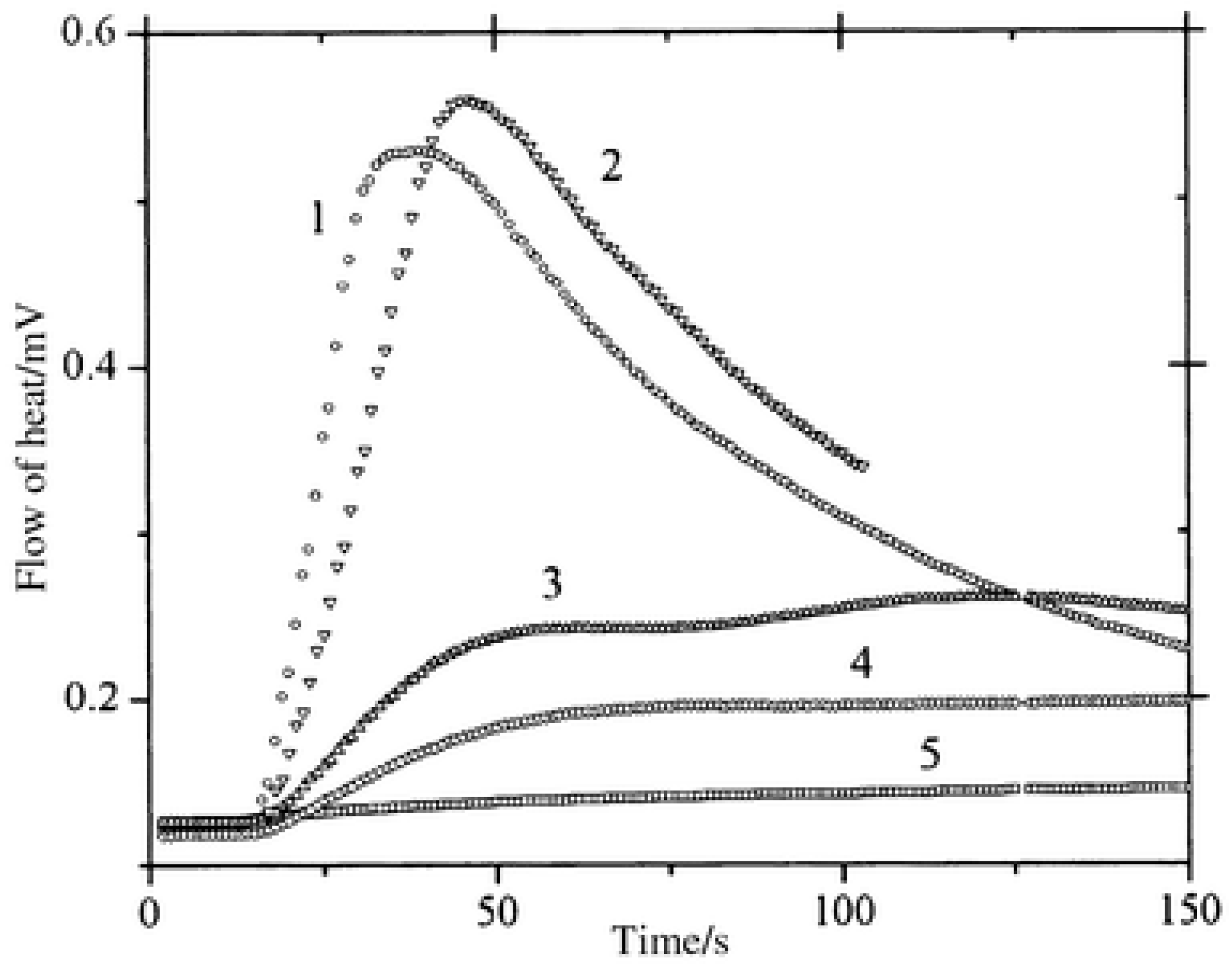
Figure 6. Polymerization profiles obtained by photo-DSC using (1) CICl2, (2) CI, (3) ZB, (4) ZH, (5) CNH2 in combination with NPG.
In 2004, quinoxaline derivatives were tested for the first time in lower light intensity (no use of lasers as the light sources as in the previous works) since dental lamps were used for the polymerization experiments [217]. A series of seven dyes were examined, differing by the substitution pattern and the alkylation or not of the nitrogen groups (See Figure 7). All dyes exhibited an absorption centered in the near UV-visible range, the absorption maxima peaking between 386 nm for IQH and up to 409 nm for IQNO2Cl2. Noticeably, in this series of dyes, the lowest monomer conversions were obtained for the nitro derivatives, namely IQNO2 and IQNO2Cl2. These counter-performances were assigned to the photoreduction of the nitro groups to nitroso groups, constituting efficient free radical scavengers and good inhibitors of free radical polymerization [218]. Polymerization efficiency was also strongly related to the electron donors used. Among the five electron donors tested, namely N-phenylglycine (NPG), N-(4-cyanophenyl)glycine (CN-NPG), (phenylthio)acetic acid (TPAA), ethyl 4-dimethylaminobenzoate (EDAB) and phenoxyacetic acid (PAA), the best monomer conversions were obtained with NPG and CN-NPG while using IQCH3 as the dye (See Figure 8). Using the best electron donors (NPG and CN-NPG), the highest monomer conversions were obtained with IQBr and IQCH3Cl2 bearing halogens. Here again, the beneficial effect of heavy atoms on quinoxalines was demonstrated. Noticeably, no direct correlations could be established between the rates of the electron transfer and the polymerization rates determined for the different dyes. This unexpected result was assigned to differences in molar extinction coefficients between dyes and the diffusion effects of radicals within the resins affecting the polymerization efficiency.
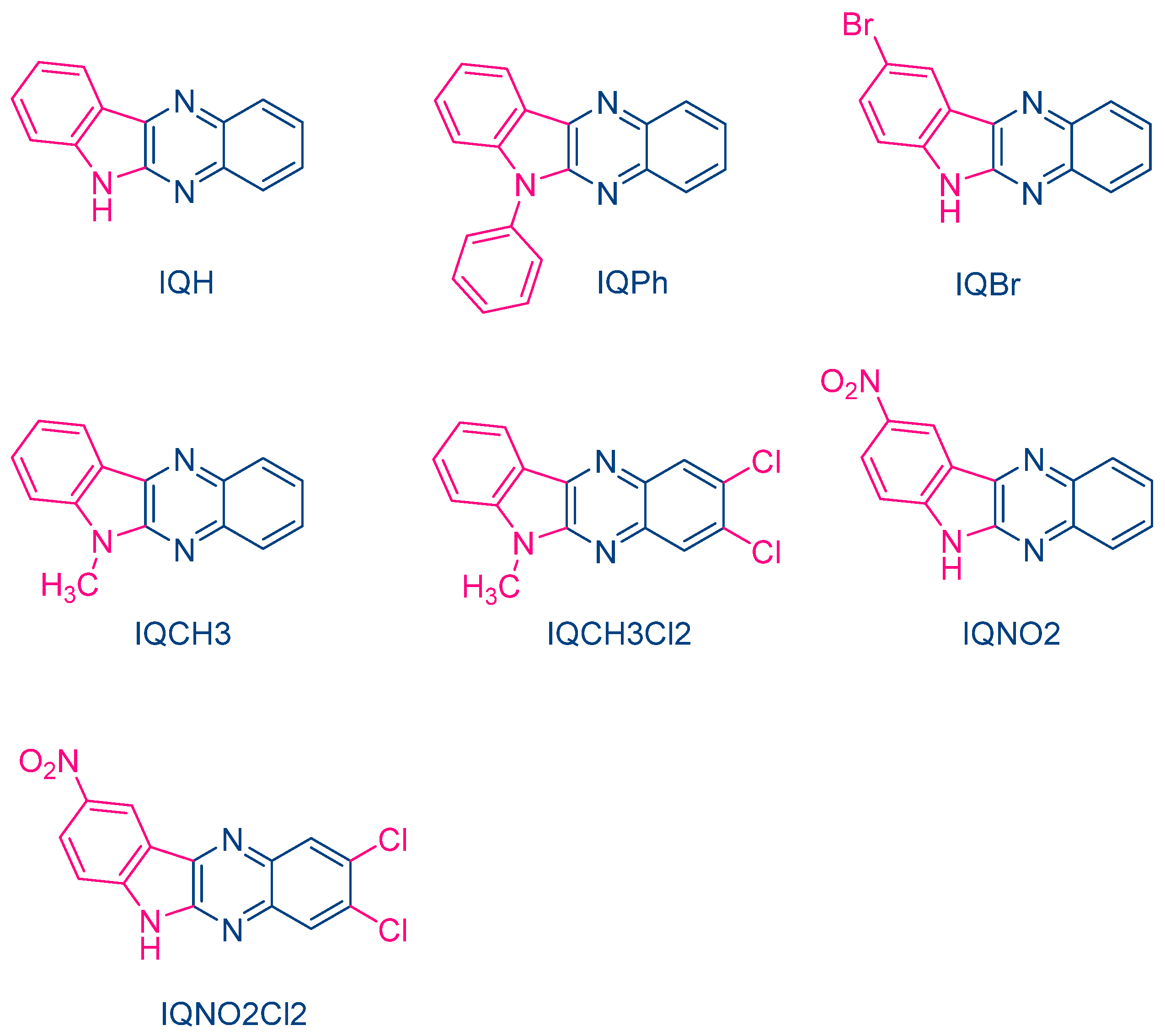
Figure 7. Chemical structures of different 6H-indolo[2,3-b]quinoxalines.
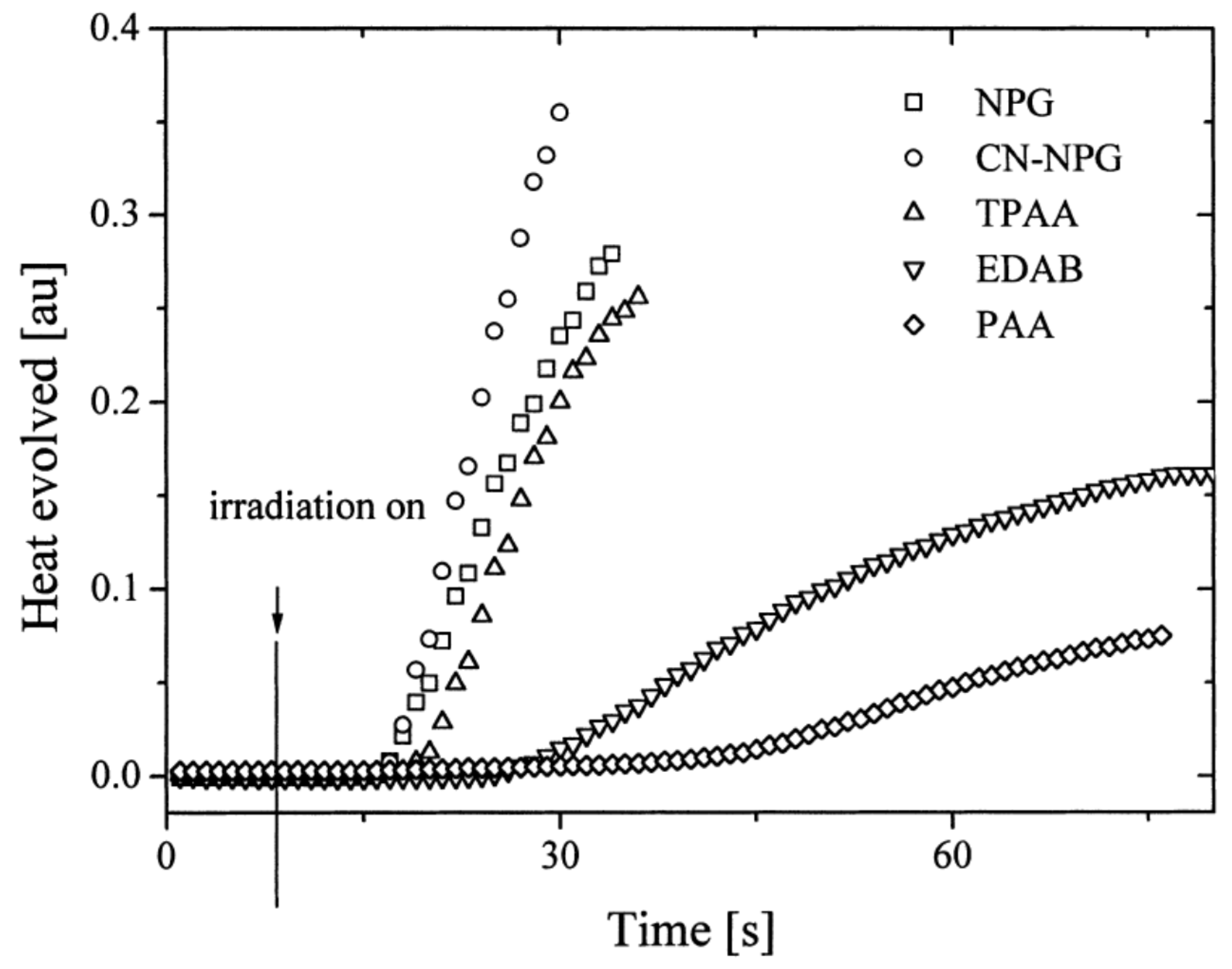
Figure 8. Polymerization profiles obtained by photo-DSC using IQCH3 as the dye and different electron donors (NPG, CN-NPG, TPAA, EDAB and PAA).
Finally, a comparison of the photo-initiating ability of IQBr with that of camphorquinone (CQ) and 2,2-dimethoxy-2-phenylacetophenone (DMPA, Irgacure 651) revealed IQBr furnished similar polymerization rates to these benchmark photoinitiators (See Figure 9).
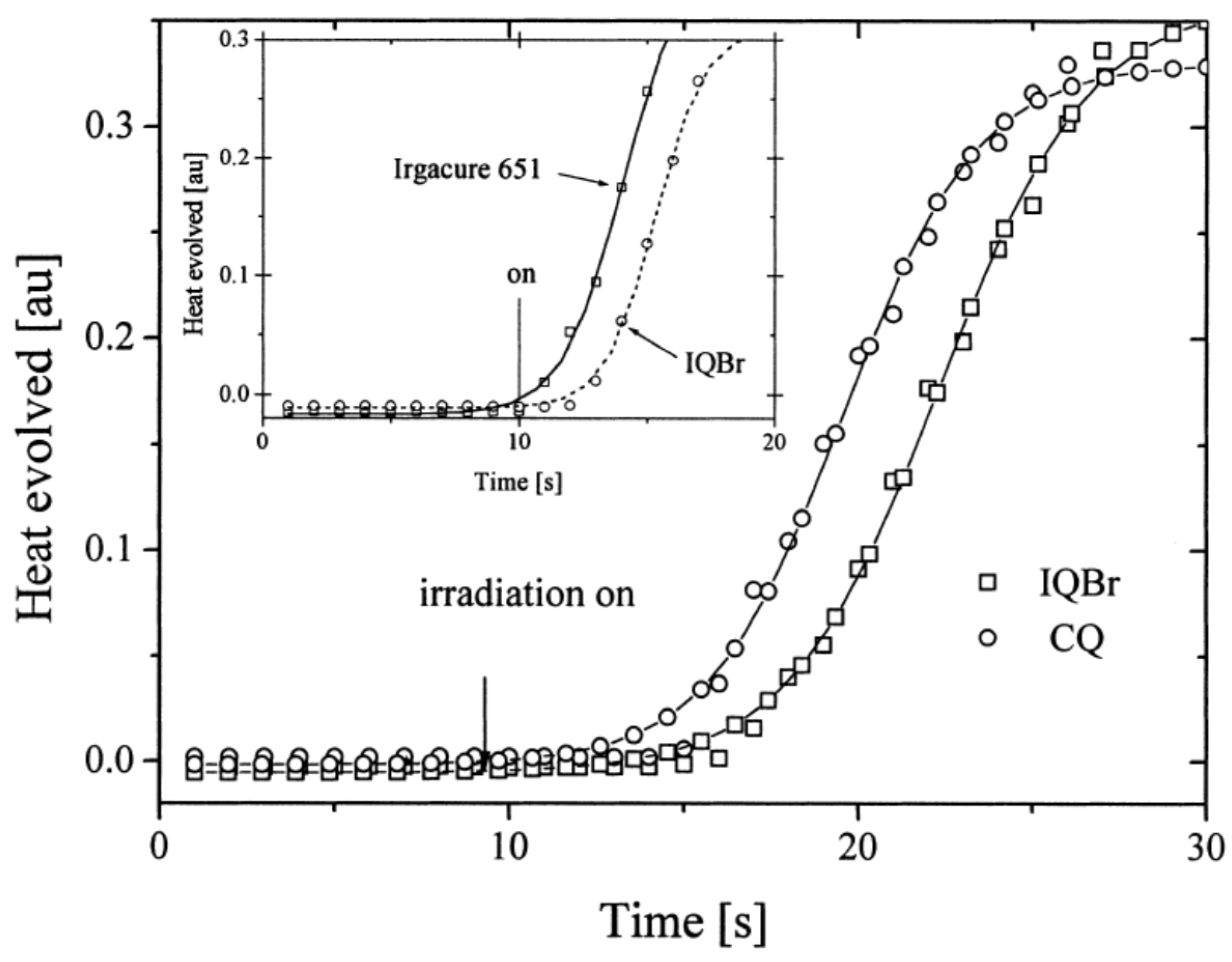
Figure 9. Polymerization profiles obtained during the FRP of TMPTA using IQBr, CQ and Irgacure 651 as the dyes and CN-NPG as the electron donor using a dental lamp as the light source.
In 2023, Jędrzejewska and coworkers revisited IQH in the context of a comparative study between indenono- and indoloquinoxaline derivatives IN1-IN5, IQH, IN7 and IN8 (See Figure 10) [219][220]. The different dyes were used as electron acceptors for (phenylthio)acetic acid (PTAA) [221], and the resulting photoredox pairs were used as photo-initiating systems for dental applications. The mechanism of radical generation is presented in Scheme 2.
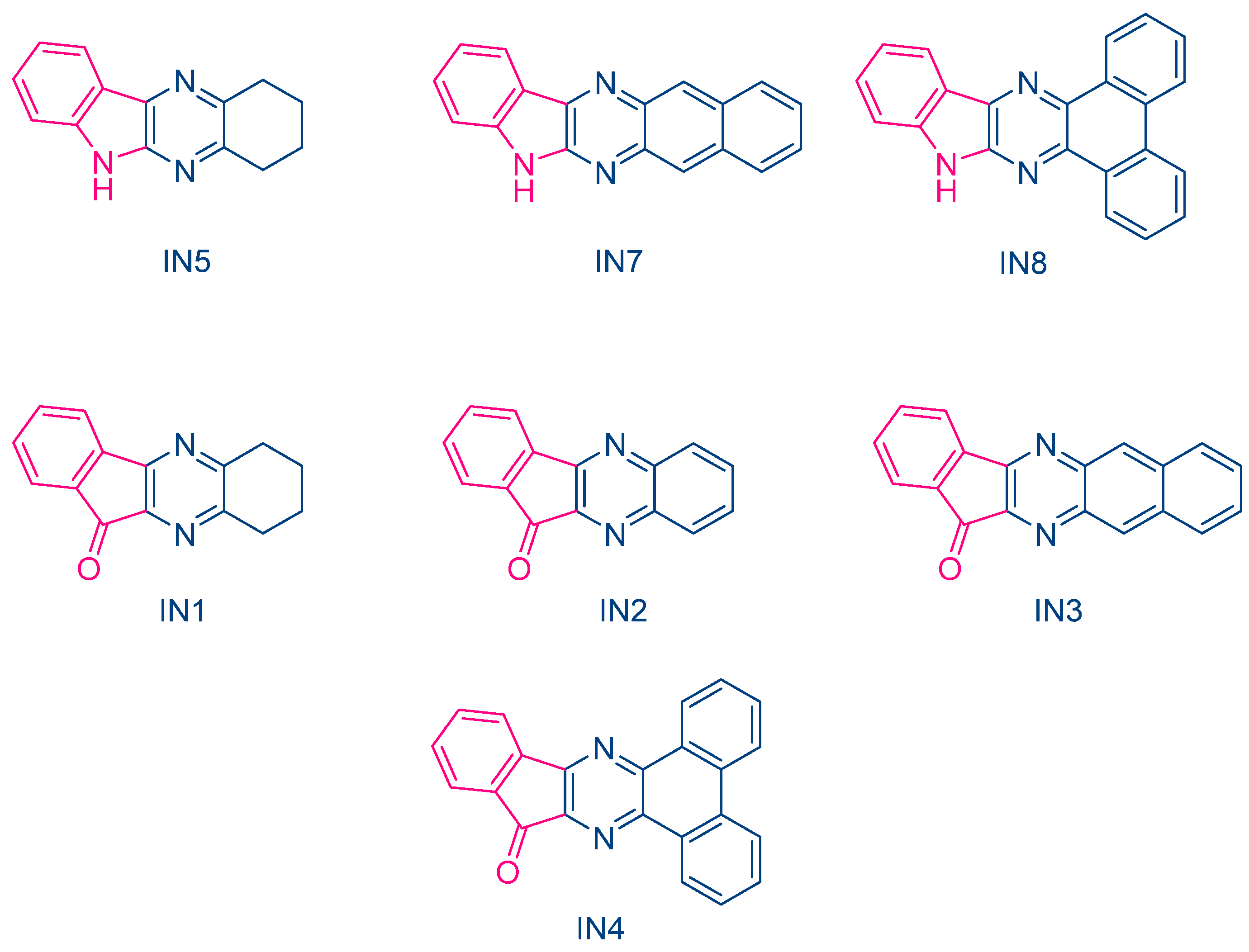
Figure 10. Chemical structures of indenono- and indoloquinoxaline derivatives.
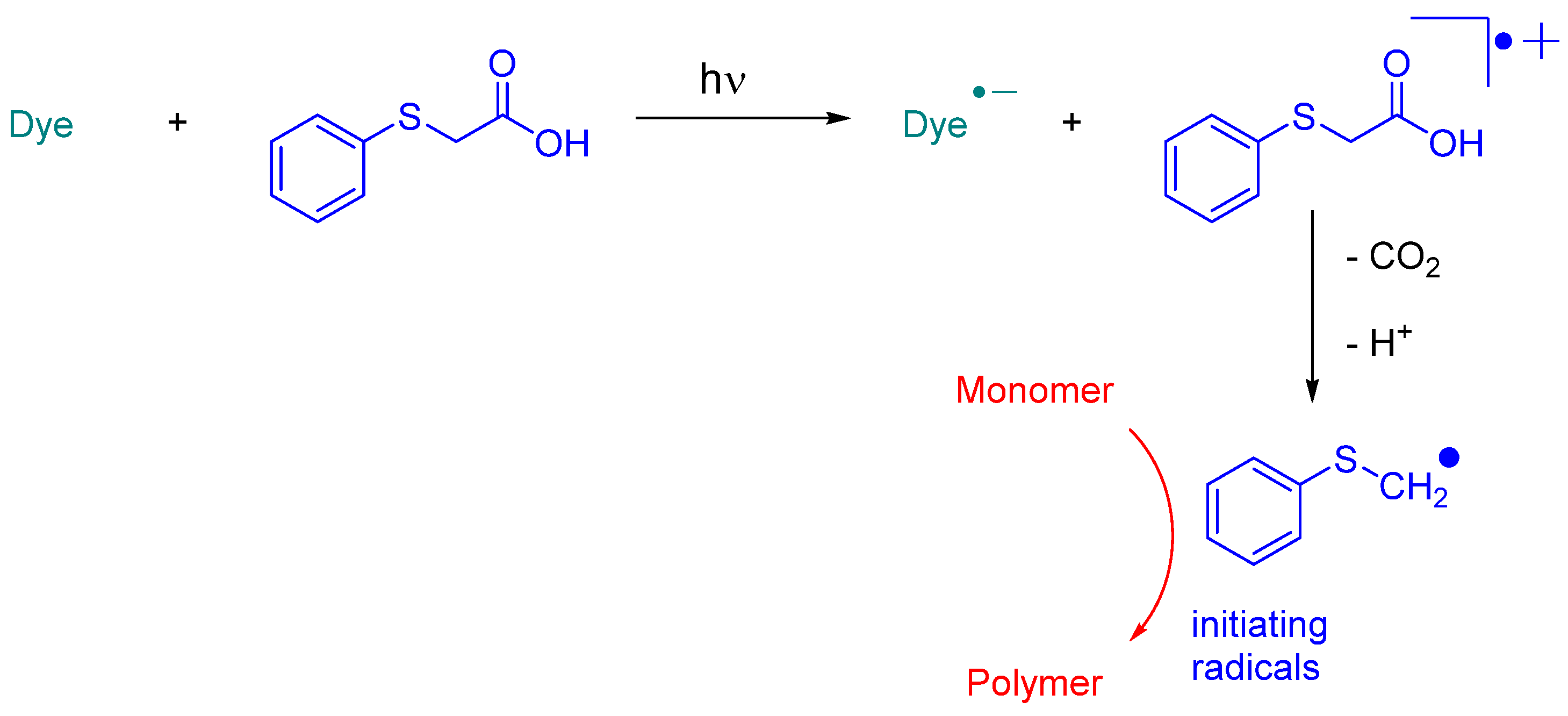
Scheme 2. Mechanism of photoinitiation with dye/phenylthioacetic acid system.
From the absorption viewpoint, all dyes IN1-IN5, IQH, IN7 and IN8 showed an intense absorption band centered in the near UV range. Only IN8 exhibited an absorption peak located in the visible range, peaking at 417 nm and attributable to the presence of the phenanthrene moiety extending the aromaticity of this dye. In fact, IN4 and IN8 exhibited the most red-shifted absorption for the two series of dyes, namely the indenono- and indoloquinoxaline series. This redshift was beneficial for the FRP experiments. Indeed, when paired with PTAA, the highest polymerization rates were obtained with these two dyes due to a better match between the emission of the dental lamp emitting at 400 nm and the absorption maxima of these chromophores. In fact, the photo-initiating abilities of these photoredox pairs were comparable to that of camphorquinone (CQ), a benchmark photoinitiator commonly used in dentistry. Interestingly, in the context of dental fillings, an increase of the temperature lower than 5 °C was evidenced, which did not exceed the temperature tolerance threshold for the tooth pulp.
The same performances were also obtained with another series of photoredox pairs based on acenaphthoquinoxalines and 2-mercaptobenzoxazole (MBX) used as the co-initiator (See Figure 11) [222]. In this series of dyes (QNX5, AN1, AN3-AN-8) and during the FRP of TMPTA, the lowest heat increase was obtained with the AN6/MBX combination. In addition, the temperature increase with the other dyes remained in the tolerance threshold for dental applications. Due to their strong absorption located in the UV range, colorless dental fillings could be obtained with these different acenaphthoquinoxalines, making these dyes suitable candidates for dental applications.
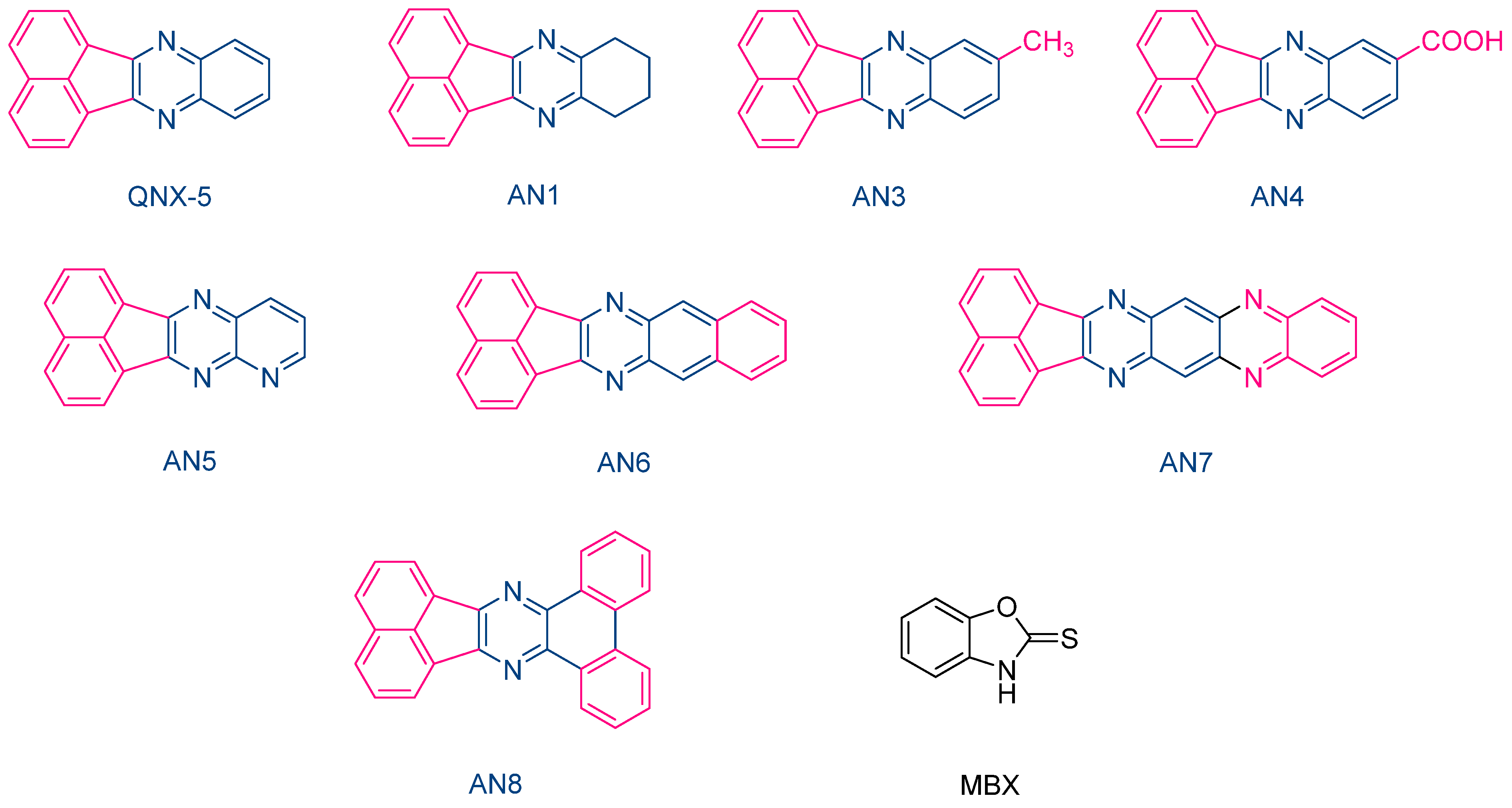
Figure 11. Chemical structures of various acenaphthoquinoxalines AN1, AN3-AN8 and 2-mercaptobenzoxazole used as the hydrogen donor.
While NPG was extensively used as an electron donor for quinoxaline derivatives, other compounds were also examined, as exemplified with N-methyldiethanolamine (MDEA) that was used as an electron donor for UV photopolymerization experiments (See Figure 12) [223]. 2,3-Diphenylquinoxaline (QNX-1), quinoxaline (QNX-3), 2,3-dimethylquinoxaline (QNX-4) and acenaphthoquinoxaline (QNX-5) were tested as UV photoinitiators.
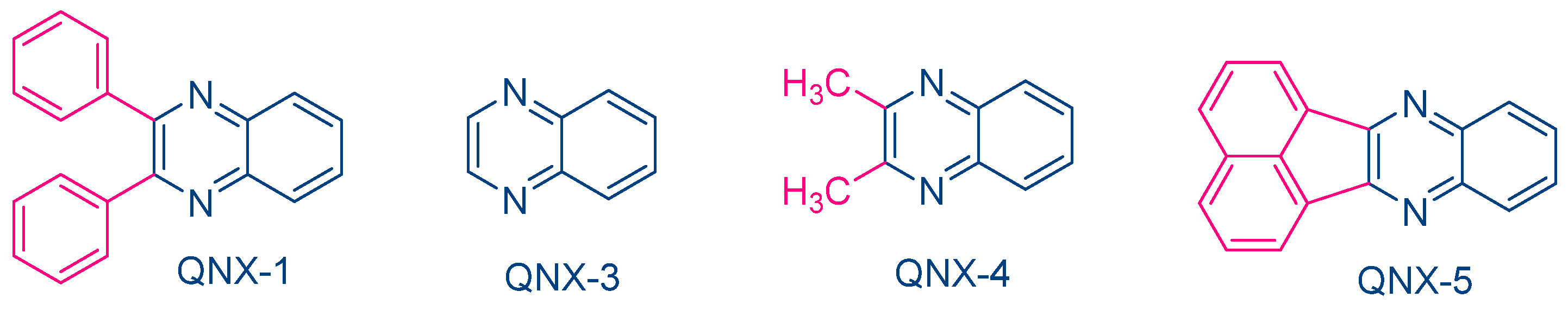
Figure 12. Quinoxalines investigated in combination with MDEA as the electron donor.
Interestingly, by using a polychromatic light, very fast polymerization processes were evidenced since a full curing of TMPTA was obtained within 4 s of irradiation (See Figure 13). The highest final monomer conversion was obtained for QNX-1, with a conversion of 60% after 4 s. Noticeably, the highest monomer conversions were obtained for the most polyaromatic structures (QNX-1, QNX-5), certainly attributable to higher molar extinction coefficients in the visible range. The TMPTA conversion could be monitored by Real-Time Fourier Transform Infrared (RT-FTIR) spectroscopy, giving direct access to the monomer conversion. To conduct this, modification of the IR peak at ca 6120 cm−1 was monitored, corresponding to a characteristic peak of TMPTA. The polymerization profiles were established from the difference between the initial peak area before irradiation and the peak area after irradiation for a given time t [224][225].
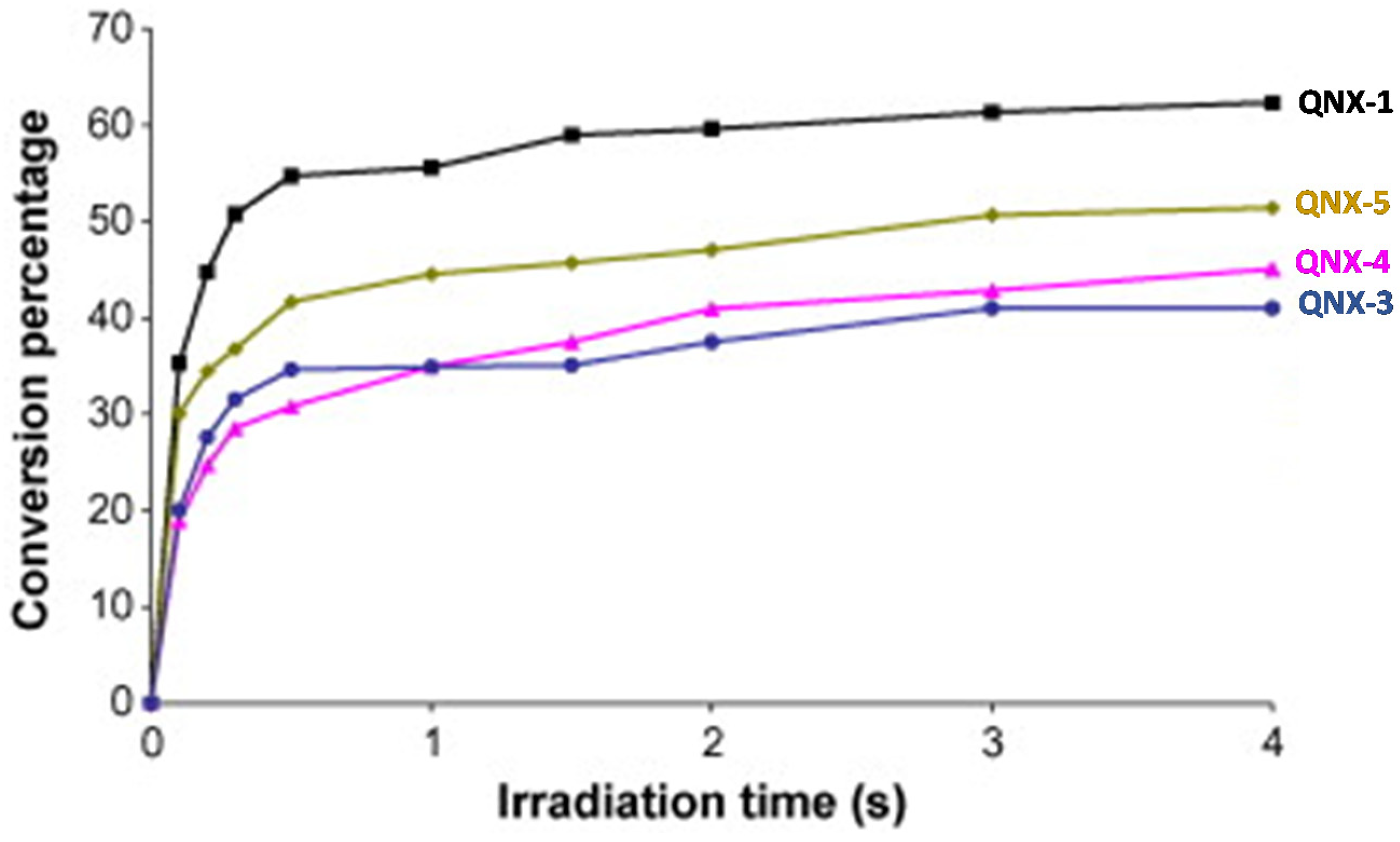
Figure 13. Polymerization profiles obtained during the FRP of TMPTA under air and using the two-component dyes/MDEA (1%/10% w/w).
This trend of reactivity was confirmed during the FRP of other monomers, such as a 75% P-3038 epoxyacrylate (EA) and 25% tripropyleneglycol diacrylate (TPGDA) (3/1 v/v) blend (See Figure 14). However, by elongating the irradiation time to 180 s, QNX-5 furnished a similar monomer conversion to QNX-1. Clearly, the difference in monomer conversions is directly related to differences in polymerization rates at early irradiation time.
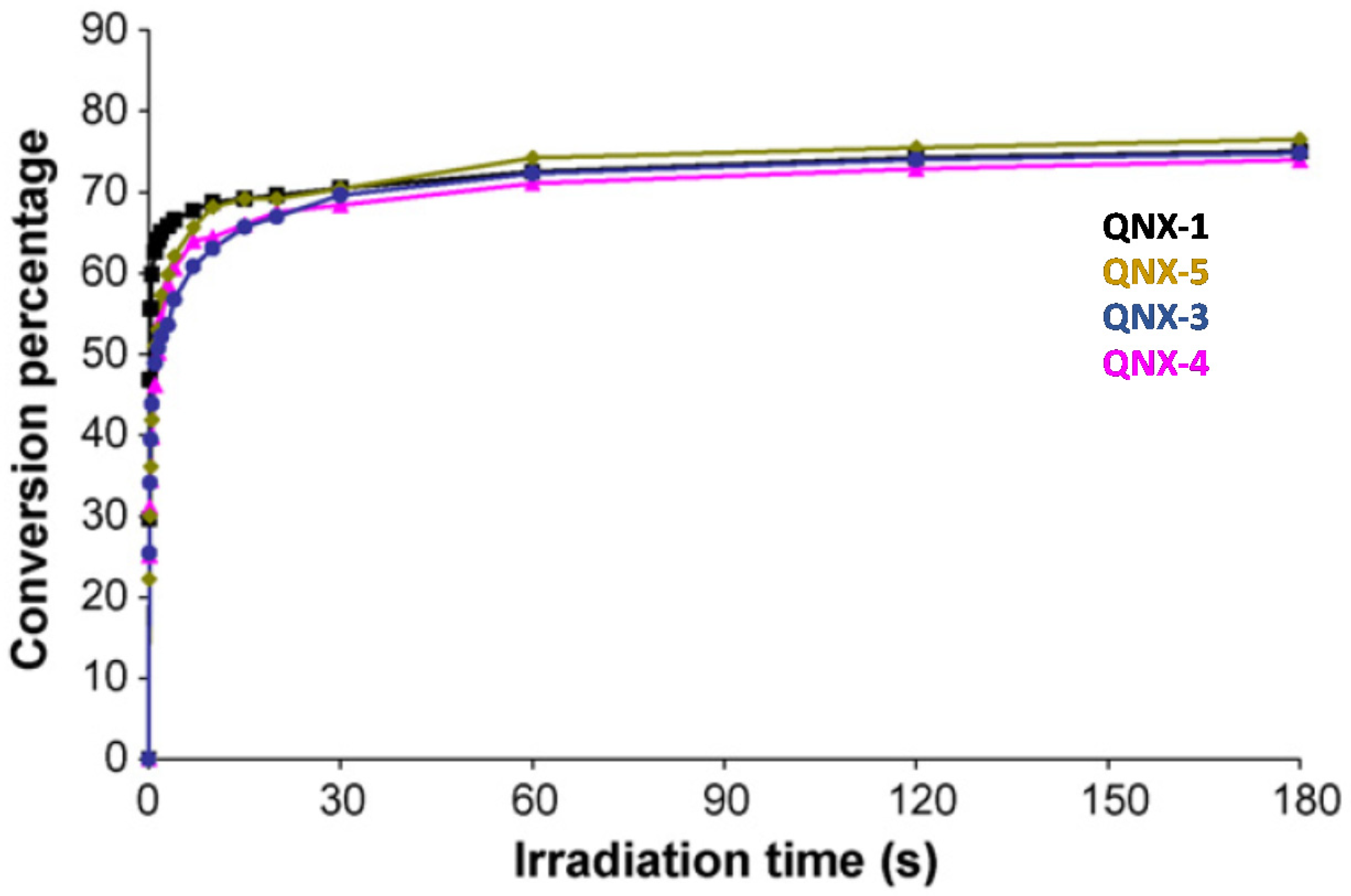
Figure 14. Polymerization profiles obtained during the FRP of an EA/TPGDA (3/1) blend under air and using the two-component dyes/MDEA (1%/10% w/w).
In the case of 5,12-dihydroquinoxalino[2,3b]quinoxalines (i.e., fluoflavins), these dyes were interesting candidates to induce the reductive decomposition of alkoxypyridinium salts (See Figure 15) [226]. The fluoflavin dyes/alkoxypyridinium salts combinations proved to be excellent two-component systems to initiate the FRP of TMPTA under visible light. It has to be noticed that previously to this work, cyanines [227], ketocoumarins [228] and acridinedione dyes [229] were also used as sensitizers capable of inducing the decomposition of alkoxypyridinium salts by photoinduced electron transfer. From the mechanistic viewpoint, upon excitation of the dye, a photoinduced electron transfer from fluoflavins towards the alkoxypyridinium salt can occur, generating an alkoxypyridinium radical that immediately decomposes, generating initiating alkoxy radicals (RO•) (See Scheme 3).
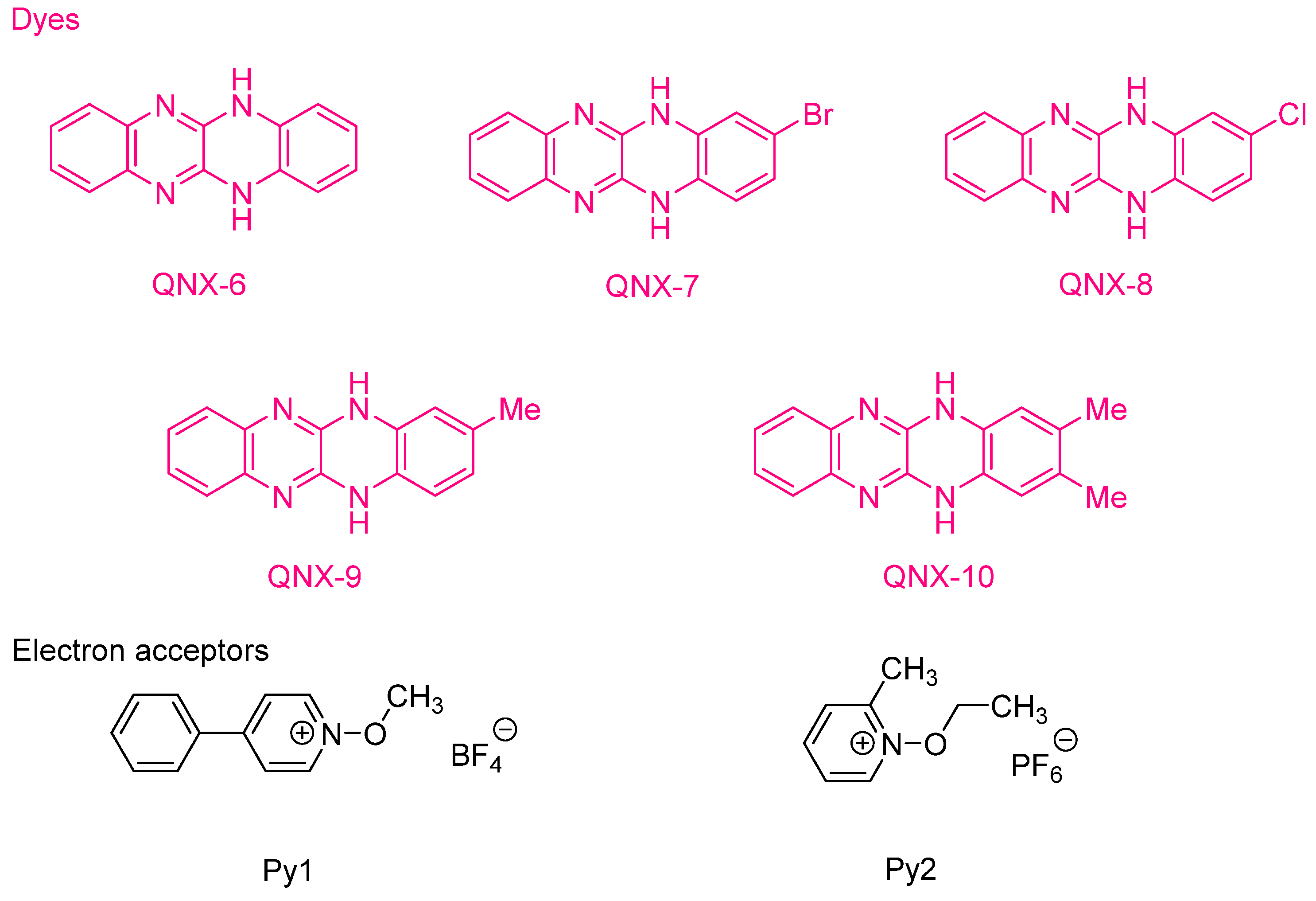
Figure 15. Chemical structures of 5,12-dihydroquinoxalino[2,3b]quinoxalines and alkoxypyridinium salts.
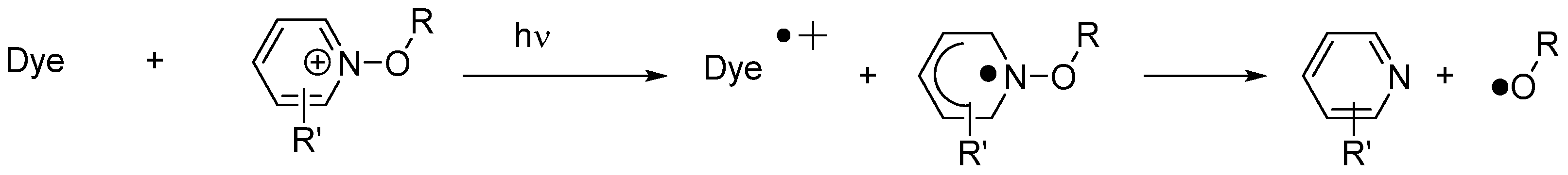
Scheme 3. Mechanism of photoinduced decomposition of alkoxypyridinium salts by electron transfer.
Examination of their photo-initiating abilities during the FRP of TMPTA upon irradiation with a visible light revealed QNX-7 and QNX-8 furnished the best polymerization rates (See Figure 16). More precisely, QNX-7 was determined as outperforming all the other dyes, irrespective of the alkoxypyridinium salt. Experiments carried out with Py2 also furnish higher monomer conversion than Py1, once again evidencing the necessity to screen both the electron donors and acceptors in order to obtain the best combination. Interestingly, an efficient photobleaching of the TMPTA resins was observed during polymerization, assigned to the addition of alkoxy radicals on fluoflavins, according to the mechanism depicted in Scheme 4. Precisely, a reaction between the fluoflavin radical cation and the ethoxyl radicals at the 9-position of the phenyl ring was proposed by analogy to previous works performed on anthracene [230][231]. After proton release, an ethoxy derivative of the fluoflavin dyes can be formed, enabling the ability to obtain an efficient bleaching of the final polymers.
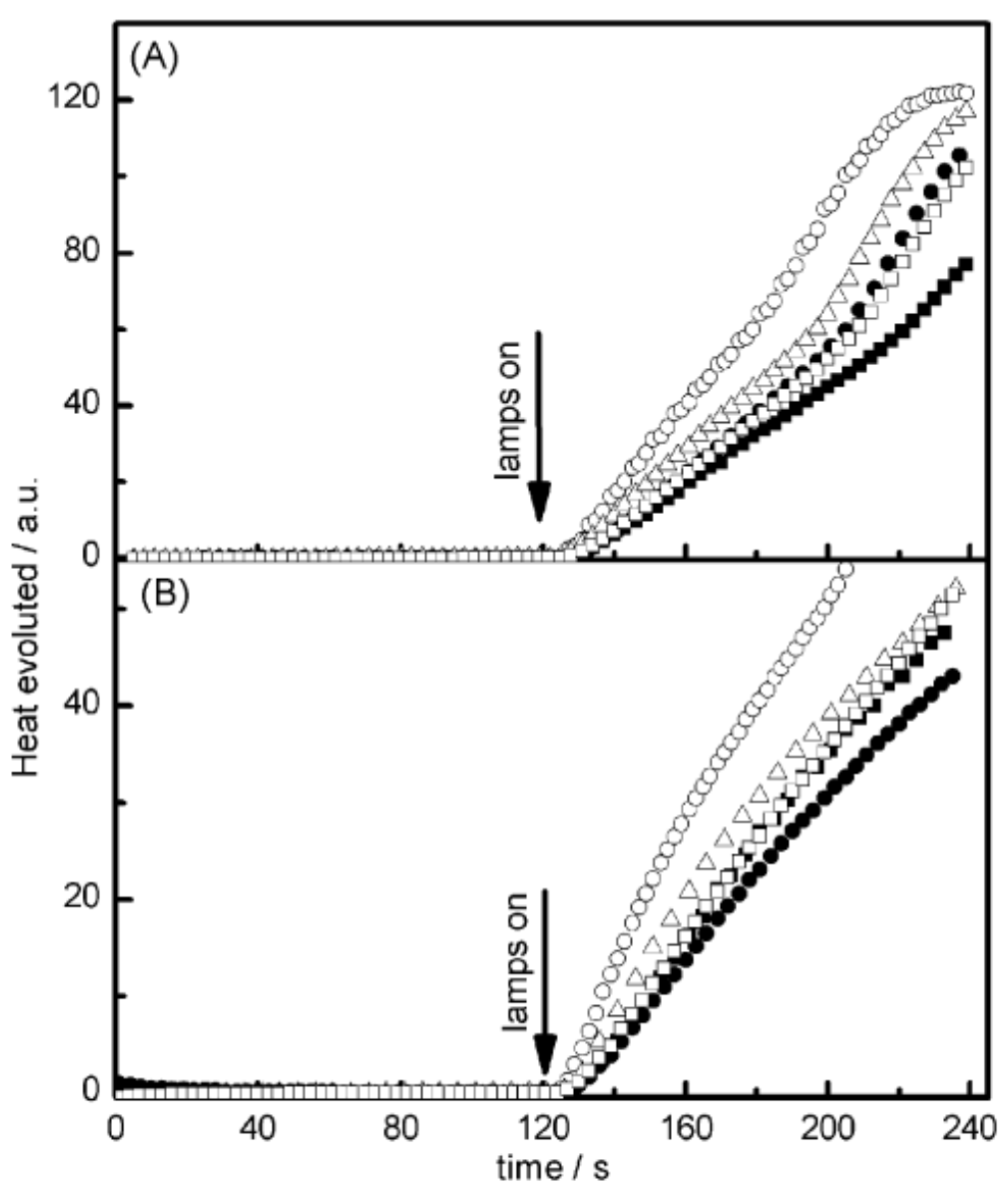
Figure 16. Photopolymerization profiles of TMPTA recorded for QNX-6 (▪); QNX-7 (O); QNX-8 (Δ); QNX-9 (•); QNX-10 (□) and (A) Py1 or (B) Py2.
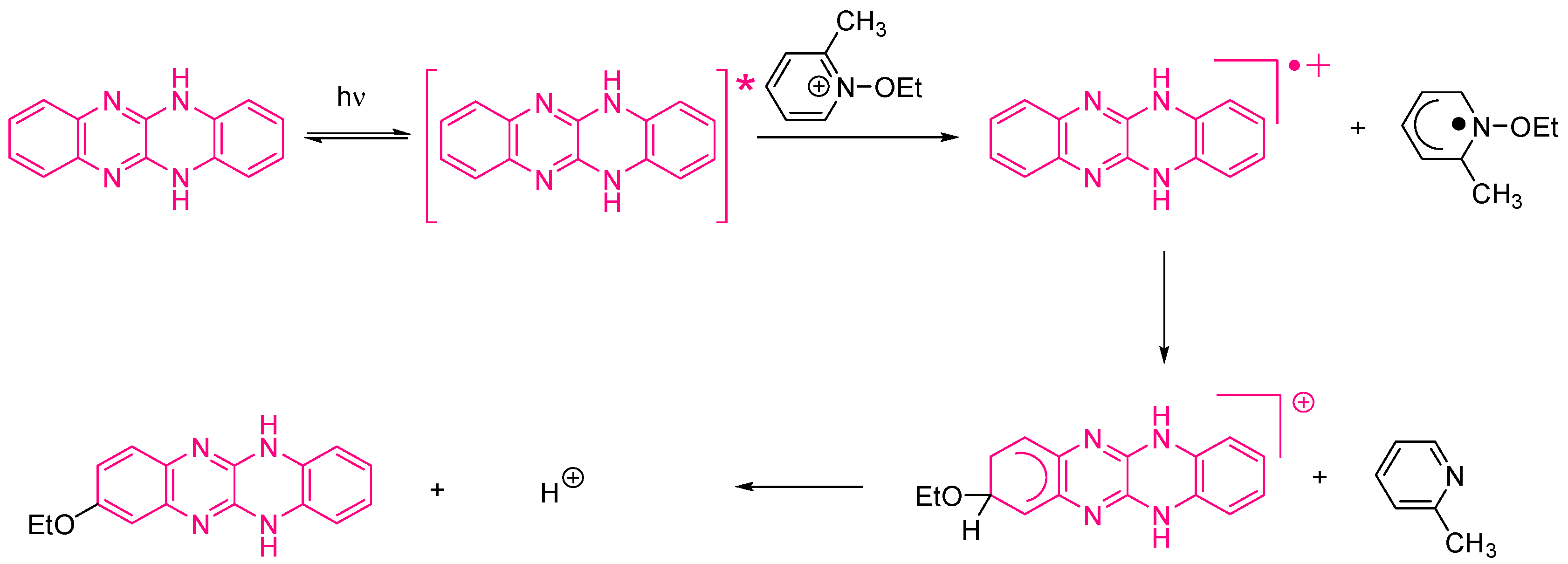
Scheme 4. Mechanism of photobleaching occurring with the fluoflavin dyes/alkoxypyridinium salts combinations.
The possibility to initiate free-radical/cationic hybrid polymerizations (concomitant polymerization of TMPTA and 3,4-epoxycyclohexylmethyl 3′,4′-epoxycyclohexanecarboxylate (EPOX)) with fluoflavin derivatives was also examined. Using QNX-6-QNX-8 as the photosensitizers for triarylsulfonium hexafluoroantimonates, epoxide conversions higher than that of acrylates were observed with all photo-initiating systems [232]. The lower acrylate conversion determined during hybrid photopolymerization was assigned to oxygen inhibition favoring the cationic polymerization of epoxides. Indeed, contrarily to the cationic polymerization, which is insensitive to oxygen inhibition, free radicals can react with molecular oxygen, generating unreactive peroxyl radicals. In 2009, the same authors examined a series of dyes analogues to fluoflavins, namely QNX-11-QNX-19, that were used as electron donors for the reductive photosensitization of N-methoxy-4-phenylpyridinium tetrafluoroborate (Py1) and N-ethoxy-2-methylpyridinium hexafluorophosphate (Py2) examined in the previous work and for diphenyliodonium hexafluorophosphate (Ph2I+PF6−) (See Figure 17) [233]. These photoinitiators were advantageously used for the FRP of acrylates under visible light and the cationic polymerization (CP) of cyclohexene oxide (CHO).
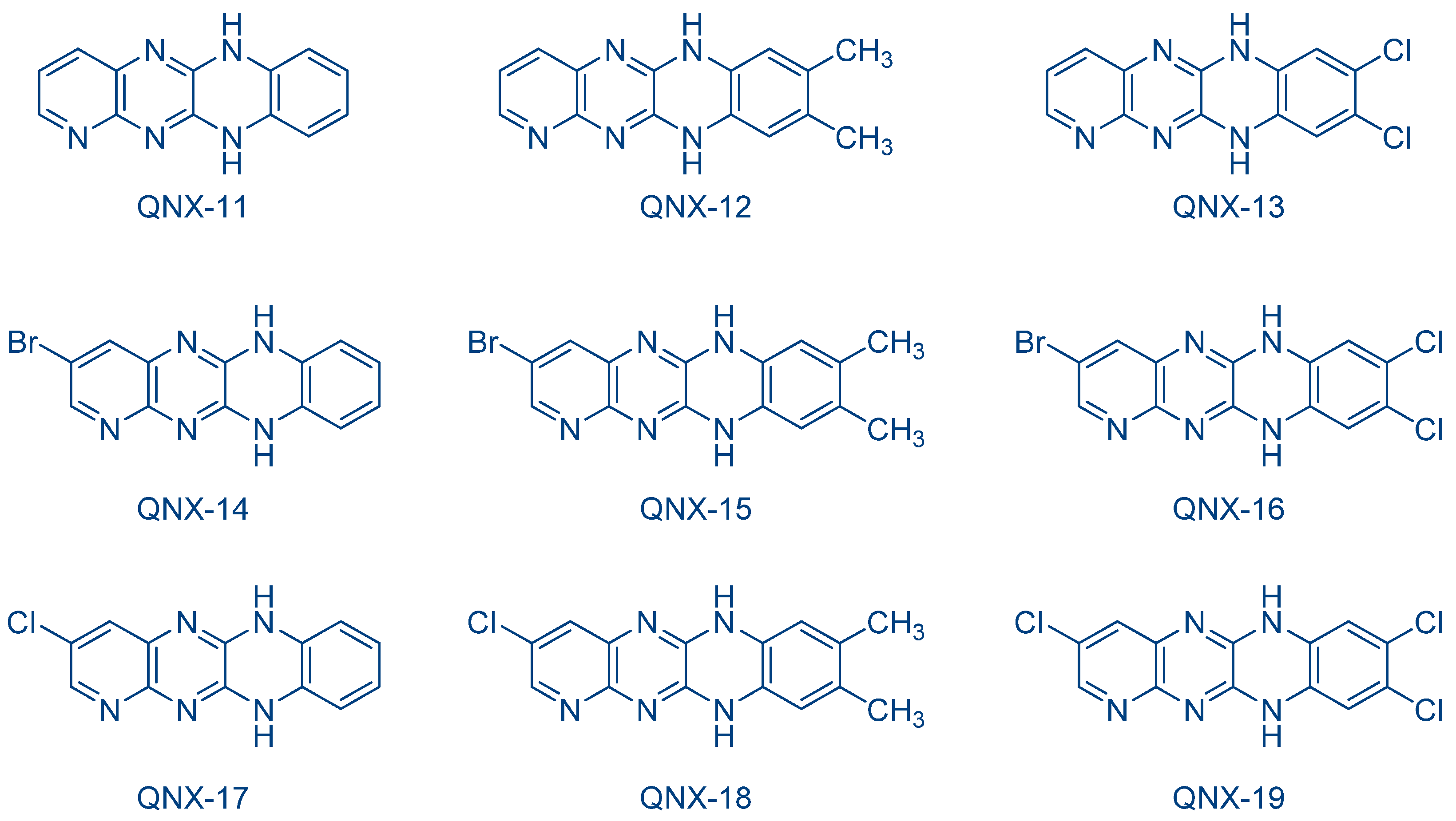
Figure 17. Chemical structures of QNX-11-QNX-19 examined as photosensitizers for iodonium salts.
This entry is adapted from the peer-reviewed paper 10.3390/catal13040718
References
- Jasinski, F.; Zetterlund, P.B.; Braun, A.M.; Chemtob, A. Photopolymerization in Dispersed Systems. Prog. Polym. Sci. 2018, 84, 47–88.
- Noè, C.; Hakkarainen, M.; Sangermano, M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymers 2021, 13, 89.
- Yuan, Y.; Li, C.; Zhang, R.; Liu, R.; Liu, J. Low Volume Shrinkage Photopolymerization System Using Hydrogen-Bond-Based Monomers. Prog. Org. Coat. 2019, 137, 105308.
- Khudyakov, I.V.; Legg, J.C.; Purvis, M.B.; Overton, B.J. Kinetics of Photopolymerization of Acrylates with Functionality of 1−6. Ind. Eng. Chem. Res. 1999, 38, 3353–3359.
- Dickens, S.H.; Stansbury, J.W.; Choi, K.M.; Floyd, C.J.E. Photopolymerization Kinetics of Methacrylate Dental Resins. Macromolecules 2003, 36, 6043–6053.
- Maffezzoli, A.; Pietra, A.D.; Rengo, S.; Nicolais, L.; Valletta, G. Photopolymerization of Dental Composite Matrices. Biomaterials 1994, 15, 1221–1228.
- Dikova, T.; Maximov, J.; Todorov, V.; Georgiev, G.; Panov, V. Optimization of Photopolymerization Process of Dental Composites. Processes 2021, 9, 779.
- Andreu, A.; Su, P.-C.; Kim, J.-H.; Ng, C.S.; Kim, S.; Kim, I.; Lee, J.; Noh, J.; Subramanian, A.S.; Yoon, Y.-J. 4D Printing Materials for Vat Photopolymerization. Addit. Manuf. 2021, 44, 102024.
- Chen, H.; Noirbent, G.; Zhang, Y.; Sun, K.; Liu, S.; Brunel, D.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Xiao, P.; et al. Photopolymerization and 3D/4D Applications Using Newly Developed Dyes: Search around the Natural Chalcone Scaffold in Photoinitiating Systems. Dye. Pigment. 2021, 188, 109213.
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611.
- Lalevée, J.; Fouassier, J.-P. Dyes and Chromophores in Polymer Science; ISTE Ltd. and John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; ISBN 978-1-84821-742-3.
- Armstrong, B.K.; Kricker, A. The Epidemiology of UV Induced Skin Cancer. Consequences Expo. Sunlightelements Assess Prot. 2001, 63, 8–18.
- de Gruijl, F.R. Skin Cancer and Solar UV Radiation. Eur. J. Cancer 1999, 35, 2003–2009.
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Review: Ultraviolet Radiation and Skin Cancer. Int. J. Dermatol. 2010, 49, 978–986.
- Shao, J.; Huang, Y.; Fan, Q. Visible Light Initiating Systems for Photopolymerization: Status, Development and Challenges. Polym. Chem. 2014, 5, 4195–4210.
- Kim, K.; Sinha, J.; Gao, G.; Childress, K.K.; Sartor, S.M.; Salazar, A.M.; Huang, S.; Musgrave, C.B.; Stansbury, J.W. High-Efficiency Radical Photopolymerization Enhanced by Autonomous Dark Cure. Macromolecules 2020, 53, 5034–5046.
- Shaukat, U.; Sölle, B.; Rossegger, E.; Rana, S.; Schlögl, S. Vat Photopolymerization 3D-Printing of Dynamic Thiol-Acrylate Photopolymers Using Bio-Derived Building Blocks. Polymers 2022, 14, 5377.
- Müller, S.M.; Schlögl, S.; Wiesner, T.; Haas, M.; Griesser, T. Recent Advances in Type I Photoinitiators for Visible Light Induced Photopolymerization. ChemPhotoChem 2022, 6, e202200091.
- Petko, F.; Świeży, A.; Ortyl, J. Photoinitiating Systems and Kinetics of Frontal Photopolymerization Processes—The Prospects for Efficient Preparation of Composites and Thick 3D Structures. Polym. Chem. 2021, 12, 4593–4612.
- Tehfe, M.A.; Louradour, F.; Lalevée, J.; Fouassier, J.-P. Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry. Appl. Sci. 2013, 3, 490–514.
- Awwad, N.; Bui, A.T.; Danilov, E.O.; Castellano, F.N. Visible-Light-Initiated Free-Radical Polymerization by Homomolecular Triplet-Triplet Annihilation. Chem 2020, 6, 3071–3085.
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073.
- Fiedor, P.; Pilch, M.; Szymaszek, P.; Chachaj-Brekiesz, A.; Galek, M.; Ortyl, J. Photochemical Study of a New Bimolecular Photoinitiating System for Vat Photopolymerization 3D Printing Techniques under Visible Light. Catalysts 2020, 11, 284.
- Hola, E.; Fiedor, P.; Dzienia, A.; Ortyl, J. Visible-Light Amine Thioxanthone Derivatives as Photoredox Catalysts for Photopolymerization Processes. ACS Appl. Polym. Mater. 2021, 3, 5547–5558.
- Hola, E.; Topa, M.; Chachaj-Brekiesz, A.; Pilch, M.; Fiedor, P.; Galek, M.; Ortyl, J. New, Highly Versatile Bimolecular Photoinitiating Systems for Free-Radical, Cationic and Thiol–Ene Photopolymerization Processes under Low Light Intensity UV and Visible LEDs for 3D Printing Application. RSC Adv. 2020, 10, 7509–7522.
- Xiao, P.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Vidal, L.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structural Effects in the Indanedione Skeleton for the Design of Low Intensity 300–500 Nm Light Sensitive Initiators. Macromolecules 2014, 47, 26–34.
- Fouassier, J.P.; Lalevée, J. Three-Component Photoinitiating Systems: Towards Innovative Tailor Made High Performance Combinations. RSC Adv. 2012, 2, 2621–2629.
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Fries, C.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. New Thioxanthone and Xanthone Photoinitiators Based on Silyl Radical Chemistry. Polym. Chem. 2011, 2, 1077–1084.
- Lalevée, J.; Telitel, S.; Tehfe, M.A.; Fouassier, J.P.; Curran, D.P.; Lacôte, E. N-Heterocyclic Carbene Boranes Accelerate Type I Radical Photopolymerizations and Overcome Oxygen Inhibition. Angew. Chem. Int. Ed. 2012, 51, 5958–5961.
- Oster, G. Dye-Sensitized Photopolymerization. Nature 1954, 173, 300–301.
- Bonardi, A.H.; Dumur, F.; Grant, T.M.; Noirbent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.-P.; Lalevée, J. High Performance Near-Infrared (NIR) Photoinitiating Systems Operating under Low Light Intensity and in the Presence of Oxygen. Macromolecules 2018, 51, 1314–1324.
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photopolymerization Processes of Thick Films and in Shadow Areas: A Review for the Access to Composites. Polym. Chem. 2017, 8, 7088–7101.
- Dumur, F. Recent Advances on Photobleachable Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2023, 186, 111874.
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. The Carbazole-Bound Ferrocenium Salt as a Specific Cationic Photoinitiator upon near-UV and Visible LEDs (365–405 Nm). Polym. Bull. 2016, 73, 493–507.
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Carbazole Scaffold Based Photoinitiator/Photoredox Catalysts: Toward New High Performance Photoinitiating Systems and Application in LED Projector 3D Printing Resins. Macromolecules 2017, 50, 2747–2758.
- Al Mousawi, A.; Lara, D.M.; Noirbent, G.; Dumur, F.; Toufaily, J.; Hamieh, T.; Bui, T.-T.; Goubard, F.; Graff, B.; Gigmes, D.; et al. Carbazole Derivatives with Thermally Activated Delayed Fluorescence Property as Photoinitiators/Photoredox Catalysts for LED 3D Printing Technology. Macromolecules 2017, 50, 4913–4926.
- Al Mousawi, A.; Garra, P.; Dumur, F.; Bui, T.-T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; et al. Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing. Molecules 2017, 22, 2143.
- Mousawi, A.A.; Arar, A.; Ibrahim-Ouali, M.; Duval, S.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; et al. Carbazole-Based Compounds as Photoinitiators for Free Radical and Cationic Polymerization upon near Visible Light Illumination. Photochem. Photobiol. Sci. 2018, 17, 578–585.
- Abdallah, M.; Magaldi, D.; Hijazi, A.; Graff, B.; Dumur, F.; Fouassier, J.-P.; Bui, T.-T.; Goubard, F.; Lalevée, J. Development of New High-Performance Visible Light Photoinitiators Based on Carbazole Scaffold and Their Applications in 3d Printing and Photocomposite Synthesis. J. Polym. Sci. Part Polym. Chem. 2019, 57, 2081–2092.
- Dumur, F. Recent Advances on Carbazole-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 125, 109503.
- Liu, S.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Nitro-Carbazole Based Oxime Esters as Dual Photo/Thermal Initiators for 3D Printing and Composite Preparation. Macromol. Rapid Commun. 2021, 42, 2100207.
- Hammoud, F.; Hijazi, A.; Duval, S.; Lalevée, J.; Dumur, F. 5,12-DihydroindoloCarbazole: A Promising Scaffold for the Design of Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2022, 162, 110880.
- Liu, S.; Giacoletto, N.; Schmitt, M.; Nechab, M.; Graff, B.; Morlet-Savary, F.; Xiao, P.; Dumur, F.; Lalevée, J. Effect of Decarboxylation on the Photoinitiation Behavior of Nitrocarbazole-Based Oxime Esters. Macromolecules 2022, 55, 2475–2485.
- Hammoud, F.; Hijazi, A.; Ibrahim-Ouali, M.; Lalevée, J.; Dumur, F. Chemical Engineering around the 5,12-DihydroindoloCarbazole Scaffold: Fine Tuning of the Optical Properties of Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2022, 172, 111218.
- Xu, C.; Gong, S.; Wu, X.; Wu, Y.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. High-Efficient Carbazole-Based Photo-Bleachable Dyes as Free Radical Initiators for Visible Light Polymerization. Dye. Pigment. 2022, 198, 110039.
- Dumur, F. Recent Advances on Carbazole-Based Oxime Esters as Photoinitiators of Polymerization. Eur. Polym. J. 2022, 175, 111330.
- Hammoud, F.; Giacoletto, N.; Nechab, M.; Graff, B.; Hijazi, A.; Dumur, F.; Lalevée, J. 5,12-Dialkyl-5,12-DihydroindoloCarbazole-Based Oxime-Esters for LED Photoinitiating Systems and Application on 3D Printing. Macromol. Mater. Eng. 2022, 307, 2200082.
- Zhang, J.; Lalevée, J.; Zhao, J.; Graff, B.; Stenzel, M.H.; Xiao, P. Dihydroxyanthraquinone Derivatives: Natural Dyes as Blue-Light-Sensitive Versatile Photoinitiators of Photopolymerization. Polym. Chem. 2016, 7, 7316–7324.
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated Camphorquinone as Visible-Light Photoinitiator for Biomedical Application: Synthesis, Characterization, and Application. Arab. J. Chem. 2016, 9, 745–754.
- Santini, A.; Gallegos, I.T.; Felix, C.M. Photoinitiators in Dentistry: A Review. Prim. Dent. J. 2013, 2, 30–33.
- Chen, H.; Noirbent, G.; Sun, K.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Liu, S.; Xiao, P.; Dumur, F.; et al. Photoinitiators Derived from Natural Product Scaffolds: Monochalcones in Three-Component Photoinitiating Systems and Their Applications in 3D Printing. Polym. Chem. 2020, 11, 4647–4659.
- Tang, L.; Nie, J.; Zhu, X. A High Performance Phenyl-Free LED Photoinitiator for Cationic or Hybrid Photopolymerization and Its Application in LED Cationic 3D Printing. Polym. Chem. 2020, 11, 2855–2863.
- Xu, Y.; Noirbent, G.; Brunel, D.; Ding, Z.; Gigmes, D.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Allyloxy Ketones as Efficient Photoinitiators with High Migration Stability in Free Radical Polymerization and 3D Printing. Dye. Pigment. 2021, 185, 108900.
- Xu, Y.; Ding, Z.; Zhu, H.; Graff, B.; Knopf, S.; Xiao, P.; Dumur, F.; Lalevée, J. Design of Ketone Derivatives as Highly Efficient Photoinitiators for Free Radical and Cationic Photopolymerizations and Application in 3D Printing of Composites. J. Polym. Sci. 2020, 58, 3432–3445.
- Chen, H.; Noirbent, G.; Liu, S.; Brunel, D.; Graff, B.; Gigmes, D.; Zhang, Y.; Sun, K.; Morlet-Savary, F.; Xiao, P.; et al. Bis-Chalcone Derivatives Derived from Natural Products as near-UV/Visible Light Sensitive Photoinitiators for 3D/4D Printing. Mater. Chem. Front. 2021, 5, 901–916.
- Liu, S.; Zhang, Y.; Sun, K.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Design of Photoinitiating Systems Based on the Chalcone-Anthracene Scaffold for LED Cationic Photopolymerization and Application in 3D Printing. Eur. Polym. J. 2021, 147, 110300.
- Giacoletto, N.; Dumur, F. Recent Advances in Bis-Chalcone-Based Photoinitiators of Polymerization: From Mechanistic Investigations to Applications. Molecules 2021, 26, 3192.
- Ibrahim-Ouali, M.; Dumur, F. Recent Advances on Chalcone-Based Photoinitiators of Polymerization. Eur. Polym. J. 2021, 158, 110688.
- Chen, H.; Noirbent, G.; Liu, S.; Zhang, Y.; Sun, K.; Morlet-Savary, F.; Gigmes, D.; Xiao, P.; Dumur, F.; Lalevée, J. In Situ Generation of Ag Nanoparticles during Photopolymerization by Using Newly Developed Dyes-Based Three-Component Photoinitiating Systems and the Related 3D Printing Applications and Their Shape Change Behavior. J. Polym. Sci. 2021, 59, 843–859.
- Chen, H.; Vahdati, M.; Xiao, P.; Dumur, F.; Lalevée, J. Water-Soluble Visible Light Sensitive Photoinitiating System Based on Charge Transfer Complexes for the 3D Printing of Hydrogels. Polymers 2021, 13, 3195.
- Tehfe, M.-A.; Dumur, F.; Xiao, P.; Delgove, M.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Chalcone Derivatives as Highly Versatile Photoinitiators for Radical, Cationic, Thiol–Ene and IPN Polymerization Reactions upon Exposure to Visible Light. Polym. Chem. 2014, 5, 382–390.
- Sun, K.; Xu, Y.; Dumur, F.; Morlet-Savary, F.; Chen, H.; Dietlin, C.; Graff, B.; Lalevée, J.; Xiao, P. In Silico Rational Design by Molecular Modeling of New Ketones as Photoinitiators in Three-Component Photoinitiating Systems: Application in 3D Printing. Polym. Chem. 2020, 11, 2230–2242.
- Chen, H.; Regeard, C.; Salmi, H.; Morlet-Savary, F.; Giacoletto, N.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. Interpenetrating Polymer Network Hydrogels Using Natural Based Dyes Initiating Systems: Antibacterial Activity and 3D/4D Performance. Eur. Polym. J. 2022, 166, 111042.
- Zivic, N.; Bouzrati-Zerrelli, M.; Villotte, S.; Morlet-Savary, F.; Dietlin, C.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A Novel Naphthalimide Scaffold Based Iodonium Salt as a One-Component Photoacid/Photoinitiator for Cationic and Radical Polymerization under LED Exposure. Polym. Chem. 2016, 7, 5873–5879.
- Bonardi, A.-H.; Zahouily, S.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Ibrahim-Ouali, M.; Gigmes, D.; Hoffmann, N.; Dumur, F.; Lalevée, J. New 1,8-Naphthalimide Derivatives as Photoinitiators for Free-Radical Polymerization Upon Visible Light. Catalysts 2019, 9, 637.
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Naphthalimide-Tertiary Amine Derivatives as Blue-Light-Sensitive Photoinitiators. ChemPhotoChem 2018, 2, 481–489.
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Naphthalimide Derivatives: Substituent Effects on the Photoinitiating Ability in Polymerizations under Near UV, Purple, White and Blue LEDs (385, 395, 405, 455, or 470 Nm). Macromol. Chem. Phys. 2015, 216, 1782–1790.
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Naphthalimide-Phthalimide Derivative Based Photoinitiating Systems for Polymerization Reactions under Blue Lights. J. Polym. Sci. Part Polym. Chem. 2015, 53, 665–674.
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A Benzophenone-Naphthalimide Derivative as Versatile Photoinitiator of Polymerization under near UV and Visible Lights. J. Polym. Sci. Part Polym. Chem. 2015, 53, 445–451.
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. N--1,8-Naphthalimide Derivatives as Photoinitiators under LEDs. Polym. Chem. 2018, 9, 994–1003.
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structure Design of Naphthalimide Derivatives: Toward Versatile Photoinitiators for Near-UV/Visible LEDs, 3D Printing, and Water-Soluble Photoinitiating Systems. Macromolecules 2015, 48, 2054–2063.
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. UV-Violet-Blue LED Induced Polymerizations: Specific Photoinitiating Systems at 365, 385, 395 and 405 Nm. Polymer 2014, 55, 6641–6648.
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Blue Light Sensitive Dyes for Various Photopolymerization Reactions: Naphthalimide and Naphthalic Anhydride Derivatives. Macromolecules 2014, 47, 601–608.
- Xiao, P.; Dumur, F.; Frigoli, M.; Tehfe, M.-A.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Naphthalimide Based Methacrylated Photoinitiators in Radical and Cationic Photopolymerization under Visible Light. Polym. Chem. 2013, 4, 5440–5448.
- Noirbent, G.; Dumur, F. Recent Advances on Naphthalic Anhydrides and 1,8-Naphthalimide-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 132, 109702.
- Rahal, M.; Mokbel, H.; Graff, B.; Pertici, V.; Gigmes, D.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Naphthalimide-Based Dyes as Photoinitiators under Visible Light Irradiation and Their Applications: Photocomposite Synthesis, 3D Printing and Polymerization in Water. ChemPhotoChem 2021, 5, 476–490.
- Rahal, M.; Graff, B.; Toufaily, J.; Hamieh, T.; Ibrahim-Ouali, M.; Dumur, F.; Lalevée, J. Naphthyl-Naphthalimides as High-Performance Visible Light Photoinitiators for 3D Printing and Photocomposites Synthesis. Catalysts 2021, 11, 1269.
- Zivic, N.; Zhang, J.; Bardelang, D.; Dumur, F.; Xiao, P.; Jet, T.; Versace, D.-L.; Dietlin, C.; Morlet-Savary, F.; Graff, B.; et al. Novel Naphthalimide–Amine Based Photoinitiators Operating under Violet and Blue LEDs and Usable for Various Polymerization Reactions and Synthesis of Hydrogels. Polym. Chem. 2015, 7, 418–429.
- Xiao, P.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Design of High Performance Photoinitiators at 385–405 Nm: Search around the Naphthalene Scaffold. Macromolecules 2014, 47, 973–978.
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Amino and Nitro Substituted 2-Amino-1H-BenzoIsoquinoline-1,3(2H)-Diones: As Versatile Photoinitiators of Polymerization from Violet-Blue LED Absorption to a Panchromatic Behavior. Polym. Chem. 2015, 6, 1171–1179.
- Chen, H.; Pieuchot, L.; Xiao, P.; Dumur, F.; Lalevée, J. Water-Soluble/Visible-Light-Sensitive Naphthalimide Derivative-Based Photoinitiating Systems: 3D Printing of Antibacterial Hydrogels. Polym. Chem. 2022, 13, 2918–2932.
- Liu, S.; Giacoletto, N.; Graff, B.; Morlet-Savary, F.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-Naphthalimide Ester Derivatives as Type I; Photoinitiators for LED Photopolymerization. Mater. Today Chem. 2022, 26, 101137.
- Liu, S.; Chen, H.; Zhang, Y.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Noirbent, G.; Pigot, C.; Brunel, D.; et al. Monocomponent Photoinitiators Based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing. Polymers 2020, 12, 1394.
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Variations on the Benzophenone Skeleton: Novel High Performance Blue Light Sensitive Photoinitiating Systems. Macromolecules 2013, 46, 7661–7667.
- Zhang, J.; Frigoli, M.; Dumur, F.; Xiao, P.; Ronchi, L.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Design of Novel Photoinitiators for Radical and Cationic Photopolymerizations under Near UV and Visible LEDs (385, 395, and 405 Nm). Macromolecules 2014, 47, 2811–2819.
- Liu, S.; Brunel, D.; Noirbent, G.; Mau, A.; Chen, H.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Xiao, P.; Dumur, F.; et al. New Multifunctional Benzophenone-Based Photoinitiators with High Migration Stability and Their Applications in 3D Printing. Mater. Chem. Front. 2021, 5, 1982–1994.
- Liu, S.; Brunel, D.; Sun, K.; Zhang, Y.; Chen, H.; Xiao, P.; Dumur, F.; Lalevée, J. Novel Photoinitiators Based on Benzophenone-Triphenylamine Hybrid Structure for LED Photopolymerization. Macromol. Rapid Commun. 2020, 41, 2000460.
- Liu, S.; Brunel, D.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. A Monocomponent Bifunctional Benzophenone–Carbazole Type II Photoinitiator for LED Photoinitiating Systems. Polym. Chem. 2020, 11, 3551–3556.
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Trifunctional Photoinitiators Based on a Triazine Skeleton for Visible Light Source and UV LED Induced Polymerizations. Macromolecules 2012, 45, 8639–8647.
- Lin, J.-T.; Lalevee, J. Efficacy Modeling of New Multi-Functional Benzophenone-Based System for Free-Radical/Cationic Hybrid Photopolymerization Using 405 Nm LED. J. Polym. Res. 2022, 29, 100.
- Kirschner, J.; Baralle, A.; Paillard, J.; Graff, B.; Becht, J.-M.; Klee, J.E.; Lalevée, J. Silyl Glyoximides: Toward a New Class of Visible Light Photoinitiators. Macromol. Chem. Phys. 2022, 223, 2100500.
- Dumur, F. Recent Advances on Visible Light Phenothiazine-Based Photoinitiators of Polymerization. Eur. Polym. J. 2022, 165, 110999.
- Deng, L.; Tang, L.; Qu, J. Novel Chalcone-Based Phenothiazine Derivative Photoinitiators for Visible Light Induced Photopolymerization with Photobleaching and Good Biocompatibility. Prog. Org. Coat. 2022, 167, 106859.
- Rahal, M.; Abdallah, M.; Bui, T.-T.; Goubard, F.; Graff, B.; Dumur, F.; Toufaily, J.; Hamieh, T.; Lalevée, J. Design of New Phenothiazine Derivatives as Visible Light Photoinitiators. Polym. Chem. 2020, 11, 3349–3359.
- Li, S.; Hu, J.; Zhang, S.; Feng, C.; Zhang, L.; Wang, C.; He, Z.; Zhang, L. Novel A-π-D-π-A Structure Two-Photon Polymerization Initiators Based on Phenothiazine and Carbazole Derivatives. Chem. Pap. 2021, 75, 5249–5256.
- Grishin, D.F.; Lizyakina, O.S.; Vaganova, L.B.; Kaltenberg, A.A.; Grishin, I.D. Radical Polymerization of Methyl Methacrylate in the Presence of Methylene Blue and Organobromides under Visible Light Irradiation. Iran. Polym. J. 2021, 30, 1117–1126.
- Xu, D.; Zou, X.; Zhu, Y.; Yu, Z.; Jin, M.; Liu, R. Phenothiazine-Based Charge-Transfer Complexes as Visible-Light Photoinitiating Systems for Acrylate and Thiol-Ene Photopolymerization. Prog. Org. Coat. 2022, 166, 106772.
- Zhu, Y.; Xu, D.; Zhang, Y.; Zhou, Y.; Yagci, Y.; Liu, R. Phenacyl Phenothiazinium Salt as a New Broad-Wavelength-Absorbing Photoinitiator for Cationic and Free Radical Polymerizations. Angew. Chem. Int. Ed. 2021, 60, 16917–16921.
- Chao, P.; Gu, R.; Ma, X.; Wang, T.; Zhao, Y. Thiophene-Substituted Phenothiazine-Based Photosensitisers for Radical and Cationic Photopolymerization Reactions under Visible Laser Beams (405 and 455 Nm). Polym. Chem. 2016, 7, 5147–5156.
- Zhou, T.F.; Ma, X.Y.; Han, W.X.; Guo, X.P.; Gu, R.Q.; Yu, L.J.; Li, J.; Zhao, Y.M.; Wang, T. D–D–A Dyes with Phenothiazine–Carbazole/Triphenylamine as Double Donors in Photopolymerization under 455 Nm and 532 Nm Laser Beams. Polym. Chem. 2016, 7, 5039–5049.
- Wang, M.; Ma, X.; Yu, J.; Jia, X.; Han, D.; Zhou, T.; Yang, J.; Nie, J.; Wang, T. Aromatic Amine–Sulfone/Sulfoxide Conjugated D–π-A–π-D-Type Dyes in Photopolymerization under 405 Nm and 455 Nm Laser Beams. Polym. Chem. 2015, 6, 4424–4435.
- Hao, F.; Liu, Z.; Zhang, M.; Liu, J.; Zhang, S.; Wu, J.; Zhou, H.; Tian, Y. Four New Two-Photon Polymerization Initiators with Varying Donor and Conjugated Bridge: Synthesis and Two-Photon Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 538–542.
- Wang, Y.; Chen, R.; Liu, D.; Peng, C.; Wang, J.; Dong, X. New Functionalized Thioxanthone Derivatives as Type I Photoinitiators for Polymerization under UV-Vis LEDs. New J. Chem. 2023, 47, 5330–5337.
- Karaca, N.; Ocal, N.; Arsu, N.; Jockusch, S. Thioxanthone-Benzothiophenes as Photoinitiator for Free Radical Polymerization. J. Photochem. Photobiol. Chem. 2016, 331, 22–28.
- Balta, D.K.; Cetiner, N.; Temel, G.; Turgut, Z.; Arsu, N. An Annelated Thioxanthone as a New Type II Initiator. J. Photochem. Photobiol. Chem. 2008, 199, 316–321.
- Balta, D.K.; Temel, G.; Goksu, G.; Ocal, N.; Arsu, N. Thioxanthone–Diphenyl Anthracene: Visible Light Photoinitiator. Macromolecules 2012, 45, 119–125.
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a Light on an Adaptable Photoinitiator: Advances in Photopolymerizations Initiated by Thioxanthones. Polym. Chem. 2015, 6, 6595–6615.
- Eren, T.N.; Yasar, N.; Aviyente, V.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevee, J.; Avci, D. Photophysical and Photochemical Studies of Novel Thioxanthone-Functionalized Methacrylates through LED Excitation. Macromol. Chem. Phys. 2016, 217, 1501–1512.
- Qiu, J.; Wei, J. Thioxanthone Photoinitiator Containing Polymerizable N-Aromatic Maleimide for Photopolymerization. J. Polym. Res. 2014, 21, 559.
- Tar, H.; Sevinc Esen, D.; Aydin, M.; Ley, C.; Arsu, N.; Allonas, X. Panchromatic Type II Photoinitiator for Free Radical Polymerization Based on Thioxanthone Derivative. Macromolecules 2013, 46, 3266–3272.
- Wu, Q.; Wang, X.; Xiong, Y.; Yang, J.; Tang, H. Thioxanthone Based One-Component Polymerizable Visible Light Photoinitiator for Free Radical Polymerization. RSC Adv. 2016, 6, 66098–66107.
- Wu, Q.; Tang, K.; Xiong, Y.; Wang, X.; Yang, J.; Tang, H. High-Performance and Low Migration One-Component Thioxanthone Visible Light Photoinitiators. Macromol. Chem. Phys. 2017, 218, 1600484.
- Wu, X.; Jin, M.; Malval, J.-P.; Wan, D.; Pu, H. Visible Light-Emitting Diode-Sensitive Thioxanthone Derivatives Used in Versatile Photoinitiating Systems for Photopolymerizations. J. Polym. Sci. Part Polym. Chem. 2017, 55, 4037–4045.
- Lalevée, J.; Tehfe, M.-A.; Dumur, F.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P. Light-Harvesting Organic Photoinitiators of Polymerization. Macromol. Rapid Commun. 2013, 34, 239–245.
- Esen, D.S.; Karasu, F.; Arsu, N. The Investigation of Photoinitiated Polymerization of Multifunctional Acrylates with TX-BT by Photo-DSC and RT-FTIR. Prog. Org. Coat. 2011, 70, 102–107.
- Gencoglu, T.; Eren, T.N.; Lalevée, J.; Avci, D. A Water Soluble, Low Migration, and Visible Light Photoinitiator by Thioxanthone-Functionalization of Poly(Ethylene Glycol)-Containing Poly(β-Amino Ester). Macromol. Chem. Phys. 2022, 223, 2100450.
- Zhao, J.; Lalevée, J.; Lu, H.; MacQueen, R.; Kable, S.H.; Schmidt, T.W.; Stenzel, M.H.; Xiao, P. A New Role of Curcumin: As a Multicolor Photoinitiator for Polymer Fabrication under Household UV to Red LED Bulbs. Polym. Chem. 2015, 6, 5053–5061.
- Crivello, J.V.; Bulut, U. Curcumin: A Naturally Occurring Long-Wavelength Photosensitizer for Diaryliodonium Salts. J. Polym. Sci. Part Polym. Chem. 2005, 43, 5217–5231.
- Han, W.; Fu, H.; Xue, T.; Liu, T.; Wang, Y.; Wang, T. Facilely Prepared Blue-Green Light Sensitive Curcuminoids with Excellent Bleaching Properties as High Performance Photosensitizers in Cationic and Free Radical Photopolymerization. Polym. Chem. 2018, 9, 1787–1798.
- Mishra, A.; Daswal, S. Curcumin, A Novel Natural Photoinitiator for the Copolymerization of Styrene and Methylmethacrylate. J. Macromol. Sci. Part A 2005, 42, 1667–1678.
- Tehfe, M.-A.; Dumur, F.; Contal, E.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New Insights into Radical and Cationic Polymerizations upon Visible Light Exposure: Role of Novel Photoinitiator Systems Based on the Pyrene Chromophore. Polym. Chem. 2013, 4, 1625–1634.
- Telitel, S.; Dumur, F.; Faury, T.; Graff, B.; Tehfe, M.-A.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New Core-Pyrene π Structure Organophotocatalysts Usable as Highly Efficient Photoinitiators. Beilstein J. Org. Chem. 2013, 9, 877–890.
- Uchida, N.; Nakano, H.; Igarashi, T.; Sakurai, T. Nonsalt 1-(Arylmethyloxy)Pyrene Photoinitiators Capable of Initiating Cationic Polymerization. J. Appl. Polym. Sci. 2014, 131, 40510.
- Mishra, A.; Daswal, S. 1-(Bromoacetyl)Pyrene, a Novel Photoinitiator for the Copolymerization of Styrene and Methylmethacrylate. Radiat. Phys. Chem. 2006, 75, 1093–1100.
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Design of New Type I and Type II Photoinitiators Possessing Highly Coupled Pyrene–Ketone Moieties. Polym. Chem. 2013, 4, 2313–2324.
- Dumur, F. Recent Advances on Pyrene-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 126, 109564.
- Tehfe, M.-A.; Dumur, F.; Vilà, N.; Graff, B.; Mayer, C.R.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. A Multicolor Photoinitiator for Cationic Polymerization and Interpenetrated Polymer Network Synthesis: 2,7-Di-Tert-Butyldimethyldihydropyrene. Macromol. Rapid Commun. 2013, 34, 1104–1109.
- Telitel, S.; Dumur, F.; Gigmes, D.; Graff, B.; Fouassier, J.P.; Lalevée, J. New Functionalized Aromatic Ketones as Photoinitiating Systems for near Visible and Visible Light Induced Polymerizations. Polymer 2013, 54, 2857–2864.
- Tehfe, M.-A.; Lalevée, J.; Telitel, S.; Contal, E.; Dumur, F.; Gigmes, D.; Bertin, D.; Nechab, M.; Graff, B.; Morlet-Savary, F.; et al. Polyaromatic Structures as Organo-Photoinitiator Catalysts for Efficient Visible Light Induced Dual Radical/Cationic Photopolymerization and Interpenetrated Polymer Networks Synthesis. Macromolecules 2012, 45, 4454–4460.
- Mokbel, H.; Toufaily, J.; Hamieh, T.; Dumur, F.; Campolo, D.; Gigmes, D.; Fouassier, J.P.; Ortyl, J.; Lalevée, J. Specific Cationic Photoinitiators for near UV and Visible LEDs: Iodonium versus Ferrocenium Structures. J. Appl. Polym. Sci. 2015, 132, 42759.
- Villotte, S.; Gigmes, D.; Dumur, F.; Lalevée, J. Design of Iodonium Salts for UV or Near-UV LEDs for Photoacid Generator and Polymerization Purposes. Molecules 2020, 25, 149.
- Tasdelen, M.A.; Kumbaraci, V.; Jockusch, S.; Turro, N.J.; Talinli, N.; Yagci, Y. Photoacid Generation by Stepwise Two-Photon Absorption: Photoinitiated Cationic Polymerization of Cyclohexene Oxide by Using Benzodioxinone in the Presence of Iodonium Salt. Macromolecules 2008, 41, 295–297.
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. Macromolecules 1977, 10, 1307–1315.
- He, Y.; Zhou, W.; Wu, F.; Li, M.; Wang, E. Photoreaction and Photopolymerization Studies on Squaraine Dyes/Iodonium Salts Combination. J. Photochem. Photobiol. Chem. 2004, 162, 463–471.
- Jun, L.I.; Miaozhen, L.I.; Huaihai, S.; Yongyuan, Y.; Erjian, W. Photopolymerization Initiated by Dimethylaminochalcone/Diphenyliodonium Salt Combination System Sensitive to Visible Light. Chin. J Polym Sci 1993, 11, 163–170.
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent Advances and Challenges in the Design of Organic Photoacid and Photobase Generators for Polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422.
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent Advances on Push–Pull Organic Dyes as Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2020, 133, 109797.
- Pigot, C.; Brunel, D.; Dumur, F. Indane-1,3-Dione: From Synthetic Strategies to Applications. Molecules 2022, 27, 5976.
- Dumur, F. Recent Advances on Visible Light Photoinitiators of Polymerization Based on Indane-1,3-Dione and Related Derivatives. Eur. Polym. J. 2021, 143, 110178.
- Garra, P.; Dumur, F.; Gigmes, D.; Al Mousawi, A.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. Copper (Photo)Redox Catalyst for Radical Photopolymerization in Shadowed Areas and Access to Thick and Filled Samples. Macromolecules 2017, 50, 3761–3771.
- Xiao, P.; Zhang, J.; Campolo, D.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper and Iron Complexes as Visible-Light-Sensitive Photoinitiators of Polymerization. J. Polym. Sci. Part Polym. Chem. 2015, 53, 2673–2684.
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper Photoredox Catalyst “G1”: A New High Performance Photoinitiator for near-UV and Visible LEDs. Polym. Chem. 2017, 8, 5580–5592.
- Xiao, P.; Dumur, F.; Zhang, J.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper Complexes: The Effect of Ligands on Their Photoinitiation Efficiencies in Radical Polymerization Reactions under Visible Light. Polym. Chem. 2014, 5, 6350–6357.
- Telitel, S.; Dumur, F.; Campolo, D.; Poly, J.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Iron Complexes as Potential Photocatalysts for Controlled Radical Photopolymerizations: A Tool for Modifications and Patterning of Surfaces. J. Polym. Sci. Part Polym. Chem. 2016, 54, 702–713.
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Iron Complexes as Photoinitiators for Radical and Cationic Polymerization through Photoredox Catalysis Processes. J. Polym. Sci. Part Polym. Chem. 2015, 53, 42–49.
- Tehfe, M.-A.; Dumur, F.; Telitel, S.; Gigmes, D.; Contal, E.; Bertin, D.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Zinc-Based Metal Complexes as New Photocatalysts in Polymerization Initiating Systems. Eur. Polym. J. 2013, 49, 1040–1049.
- Dumur, F.; Bertin, D.; Gigmes, D. Iridium (III) Complexes as Promising Emitters for Solid–State Light–Emitting Electrochemical Cells (LECs). Int. J. Nanotechnol. 2012, 9, 377–395.
- Tehfe, M.-A.; Lepeltier, M.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structural Effects in the Iridium Complex Series: Photoredox Catalysis and Photoinitiation of Polymerization Reactions under Visible Lights. Macromol. Chem. Phys. 2017, 218, 1700192.
- Akhigbe, J.; Luciano, M.; Zeller, M.; Brückner, C. Mono- and Bisquinoline-Annulated Porphyrins from Porphyrin β,Β′-Dione Oximes. J. Org. Chem. 2015, 80, 499–511.
- Allonas, X.; Lalevée, J.; Fouassier, J.P.; Tachi, H.; Shirai, M.; Tsunooka, M. Triplet State of O-Acyloximes Studied by Time-Resolved Absorption Spectroscopy. Chem. Lett. 2000, 29, 1090–1091.
- Chen, S.; Jin, M.; Malval, J.-P.; Fu, J.; Morlet-Savary, F.; Pan, H.; Wan, D. Substituted Stilbene-Based Oxime Esters Used as Highly Reactive Wavelength-Dependent Photoinitiators for LED Photopolymerization. Polym. Chem. 2019, 10, 6609–6621.
- Fast, D.E.; Lauer, A.; Menzel, J.P.; Kelterer, A.-M.; Gescheidt, G.; Barner-Kowollik, C. Wavelength-Dependent Photochemistry of Oxime Ester Photoinitiators. Macromolecules 2017, 50, 1815–1823.
- Hu, P.; Qiu, W.; Naumov, S.; Scherzer, T.; Hu, Z.; Chen, Q.; Knolle, W.; Li, Z. Conjugated Bifunctional Carbazole-Based Oxime Esters: Efficient and Versatile Photoinitiators for 3D Printing under One- and Two-Photon Excitation. ChemPhotoChem 2020, 4, 224–232.
- Lee, W.J.; Kwak, H.S.; Lee, D.; Oh, C.; Yum, E.K.; An, Y.; Halls, M.D.; Lee, C.-W. Design and Synthesis of Novel Oxime Ester Photoinitiators Augmented by Automated Machine Learning. Chem. Mater. 2022, 34, 116–127.
- Ma, X.; Cao, D.; Fu, H.; You, J.; Gu, R.; Fan, B.; Nie, J.; Wang, T. Multicomponent Photoinitiating Systems Containing Arylamino Oxime Ester for Visible Light Photopolymerization. Prog. Org. Coat. 2019, 135, 517–524.
- Pang, Y.; Fan, S.; Wang, Q.; Oprych, D.; Feilen, A.; Reiner, K.; Keil, D.; Slominsky, Y.L.; Popov, S.; Zou, Y.; et al. NIR-Sensitized Activated Photoreaction between Cyanines and Oxime Esters: Free-Radical Photopolymerization. Angew. Chem. Int. Ed. 2020, 59, 11440–11447.
- Sameshima, K.; Kura, H.; Matsuoka, Y.; Sotome, H.; Miyasaka, H. Improvement of the Photopolymerization and Bottom-Curing Performance of Benzocarbazole Oxime Ester Photoinitiators with Red-Shifted Absorption. Jpn. J. Appl. Phys. 2022, 61, 035504.
- Qiu, W.; Li, M.; Yang, Y.; Li, Z.; Dietliker, K. Cleavable Coumarin-Based Oxime Esters with Terminal Heterocyclic Moieties: Photobleachable Initiators for Deep Photocuring under Visible LED Light Irradiation. Polym. Chem. 2020, 11, 1356–1363.
- Breloy, L.; Negrell, C.; Mora, A.-S.; Li, W.S.J.; Brezová, V.; Caillol, S.; Versace, D.-L. Vanillin Derivative as Performing Type I Photoinitiator. Eur. Polym. J. 2020, 132, 109727.
- Hammoud, F.; Hijazi, A.; Schmitt, M.; Dumur, F.; Lalevée, J. A Review on Recently Proposed Oxime Ester Photoinitiators. Eur. Polym. J. 2023, 188, 111901.
- Ding, Y.; Jiang, S.; Gao, Y.; Nie, J.; Du, H.; Sun, F. Photochromic Polymers Based on Fluorophenyl Oxime Ester Photoinitiators as Photoswitchable Molecules. Macromolecules 2020, 53, 5701–5710.
- Yang, F.; Zhong, M.; Zhao, X.; Li, J.; Sun, F.; Nie, J.; Zhu, X. High Efficiency Photoinitiators with Extremely Low Concentration Based on Furans Derivative. J. Photochem. Photobiol. Chem. 2021, 406, 112994.
- Kabatc, J.; Iwińska, K.; Balcerak, A.; Kwiatkowska, D.; Skotnicka, A.; Czech, Z.; Bartkowiak, M. Onium Salts Improve the Kinetics of Photopolymerization of Acrylate Activated with Visible Light. RSC Adv. 2020, 10, 24817–24829.
- Kocaarslan, A.; Kütahya, C.; Keil, D.; Yagci, Y.; Strehmel, B. Near-IR and UV-LED Sensitized Photopolymerization with Onium Salts Comprising Anions of Different Nucleophilicities. ChemPhotoChem 2019, 3, 1127–1132.
- Fouassier, J.-P.; Morlet-Savary, F.; Lalevée, J.; Allonas, X.; Ley, C. Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions. Materials 2010, 3, 5130–5142.
- Kabatc, J.; Ortyl, J.; Kostrzewska, K. New Kinetic and Mechanistic Aspects of Photosensitization of Iodonium Salts in Photopolymerization of Acrylates. RSC Adv. 2017, 7, 41619–41629.
- Fujiwara, T.; Nomura, K.; Inagaki, A. Cu–Pd Dinuclear Complexes with Earth-Abundant Cu Photosensitizer: Synthesis and Photopolymerization. Organometallics 2020, 39, 2464–2469.
- Wang, J.; Mathias, L.J. A Polymerizable Photosensitizer and Its Photopolymerization Kinetics. Polym. Int. 2005, 54, 1537–1542.
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II Photoinitiators as Activators for Photoinduced Metal-Free Atom Transfer Radical Polymerization. Polym. Chem. 2017, 8, 1972–1977.
- Chen, Y.-C.; Kuo, Y.-T. Photocuring Kinetic Studies of TMPTMA Monomer by Type II Photoinitiators of Different Weight Ratios of 2-Chlorohexaaryl Biimidazole (o-Cl-HABI) and N-Phenylglycine (NPG). J. Photopolym. Sci. Technol. 2018, 31, 487–492.
- Li, J.; Zhang, X.; Ali, S.; Akram, M.Y.; Nie, J.; Zhu, X. The Effect of Polyethylene Glycoldiacrylate Complexation on Type II Photoinitiator and Promotion for Visible Light Initiation System. J. Photochem. Photobiol. Chem. 2019, 384, 112037.
- Kirschner, J.; Baralle, A.; Graff, B.; Becht, J.-M.; Klee, J.E.; Lalevée, J. 1-Aryl-2-(Triisopropylsilyl)Ethane-1,2-Diones: Toward a New Class of Visible Type I Photoinitiators for Free Radical Polymerization of Methacrylates. Macromol. Rapid Commun. 2019, 40, 1900319.
- Christmann, J.; Allonas, X.; Ley, C.; Ibrahim, A.; Croutxé-Barghorn, C. Triazine-Based Type-II Photoinitiating System for Free Radical Photopolymerization: Mechanism, Efficiency, and Modeling. Macromol. Chem. Phys. 2017, 218, 1600597.
- Kreutzer, J.; Kaya, K.; Yagci, Y. Poly(Propylene Oxide)-Thioxanthone as One-Component Type II Polymeric Photoinitiator for Free Radical Polymerization with Low Migration Behavior. Eur. Polym. J. 2017, 95, 71–81.
- Allegrezza, M.L.; DeMartini, Z.M.; Kloster, A.J.; Digby, Z.A.; Konkolewicz, D. Visible and Sunlight Driven RAFT Photopolymerization Accelerated by Amines: Kinetics and Mechanism. Polym. Chem. 2016, 7, 6626–6636.
- Ciftci, M.; Tasdelen, M.A.; Yagci, Y. Sunlight Induced Atom Transfer Radical Polymerization by Using Dimanganese Decacarbonyl. Polym. Chem. 2014, 5, 600–606.
- Decker, C.; Bendaikha, T. Interpenetrating Polymer Networks. II. Sunlight-Induced Polymerization of Multifunctional Acrylates. J. Appl. Polym. Sci. 1998, 70, 2269–2282.
- Konkolewicz, D.; Schröder, K.; Buback, J.; Bernhard, S.; Matyjaszewski, K. Visible Light and Sunlight Photoinduced ATRP with Ppm of Cu Catalyst. ACS Macro Lett. 2012, 1, 1219–1223.
- Lalevée, J.; Fouassier, J.P. Recent Advances in Sunlight Induced Polymerization: Role of New Photoinitiating Systems Based on the Silyl Radical Chemistry. Polym. Chem. 2011, 2, 1107–1113.
- Li, J.; Lu, H.; Zheng, H.; Zhou, X.; Nie, J.; Zhu, X. Thermally Activated Pyrrole Chalcone Free Radical Photoinitiator with Excellent Stability to Sunlight. Eur. Polym. J. 2022, 162, 110884.
- Li, J.; Lu, H.; Zheng, H.; Li, J.; Yao, L.; Wang, Y.; Zhou, X.; Fu, Z.; Nie, J.; Zhu, X. Improvement in the Storage Stability of Free Radical Photocurable Materials under Sunlight Based on the Cis → Trans Photoisomerization of Pyrrole Chalcone Photoinitiator. Prog. Org. Coat. 2022, 171, 107025.
- Tehfe, M.-A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Green Chemistry: Sunlight-Induced Cationic Polymerization of Renewable Epoxy Monomers Under Air. Macromolecules 2010, 43, 1364–1370.
- Wang, J.; Rivero, M.; Muñoz Bonilla, A.; Sanchez-Marcos, J.; Xue, W.; Chen, G.; Zhang, W.; Zhu, X. Natural RAFT Polymerization: Recyclable-Catalyst-Aided, Opened-to-Air, and Sunlight-Photolyzed RAFT Polymerizations. ACS Macro Lett. 2016, 5, 1278–1282.
- Yu, J.; Gao, Y.; Jiang, S.; Sun, F. Naphthalimide Aryl Sulfide Derivative Norrish Type I Photoinitiators with Excellent Stability to Sunlight under Near-UV LED. Macromolecules 2019, 52, 1707–1717.
- Li, J.; Zhang, X.; Nie, J.; Zhu, X. Visible Light and Water-Soluble Photoinitiating System Based on the Charge Transfer Complex for Free Radical Photopolymerization. J. Photochem. Photobiol. Chem. 2020, 402, 112803.
- Allen, N.S.; Chen, W.; Catalina, F.; Green, P.N.; Green, A. Photochemistry of Novel Water-Soluble Para-Substituted Benzophenone Photoinitiators: A Polymerization, Spectroscopic and Flash Photolysis Study. J. Photochem. Photobiol. Chem. 1988, 44, 349–360.
- Alupei, I.C.; Alupei, V.; Ritter, H. Cyclodextrins in Polymer Synthesis: Photoinitiated Free-Radical Polymerization of N-Isopropylacrylamide in Water Initiated by a Methylated β-Cyclodextrin/2-Hydroxy-2-Methyl-1-Phenylpropan-1-One Host/Guest Complex. Macromol. Rapid Commun. 2002, 23, 55–58.
- Balta, D.K.; Temel, G.; Aydin, M.; Arsu, N. Thioxanthone Based Water-Soluble Photoinitiators for Acrylamide Photopolymerization. Eur. Polym. J. 2010, 46, 1374–1379.
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grützmacher, H.; Liska, R. Highly Efficient Water-Soluble Visible Light Photoinitiators. J. Polym. Sci. Part Polym. Chem. 2016, 54, 473–479.
- Bibaut-Renauld, C.; Burget, D.; Fouassier, J.P.; Varelas, C.G.; Thomatos, J.; Tsagaropoulos, G.; Ryrfors, L.O.; Karlsson, O.J. Use of α-Diketones as Visible Photoinitiators for the Photocrosslinking of Waterborne Latex Paints. J. Polym. Sci. Part Polym. Chem. 2002, 40, 3171–3181.
- Guo, L.; Yang, D.; Xia, L.; Qu, F.; Dou, Y.; Qu, F.; Kong, R.; You, J. A Highly Water-Soluble, Sensitive, Coumarin-Based Fluorescent Probe for Detecting Thiols, and Its Application in Bioimaging. New J. Chem. 2017, 41, 15277–15282.
- Eren, T.N.; Lalevée, J.; Avci, D. Bisphosphonic Acid-Functionalized Water-Soluble Photoinitiators. Macromol. Chem. Phys. 2019, 220, 1900268.
- Eren, T.N.; Lalevée, J.; Avci, D. Water Soluble Polymeric Photoinitiator for Dual-Curing of Acrylates and Methacrylates. J. Photochem. Photobiol. Chem. 2020, 389, 112288.
- Dietliker, K. Chapter 13 Water-Soluble Photoinitiators: Present and Future. In Photopolymerisation Initiating Systems; The Royal Society of Chemistry: London, UK, 2018; pp. 358–430. ISBN 978-1-78262-962-7.
- Huang, X.; Wang, X.; Zhao, Y. Study on a Series of Water-Soluble Photoinitiators for Fabrication of 3D Hydrogels by Two-Photon Polymerization. Dye. Pigment. 2017, 141, 413–419.
- Li, Z.; Torgersen, J.; Ajami, A.; Mühleder, S.; Qin, X.; Husinsky, W.; Holnthoner, W.; Ovsianikov, A.; Stampfl, J.; Liska, R. Initiation Efficiency and Cytotoxicity of Novel Water-Soluble Two-Photon Photoinitiators for Direct 3D Microfabrication of Hydrogels. RSC Adv. 2013, 3, 15939–15946.
- Knaus, S.; Gruber, H.F. Photoinitiators with Functional Groups. III. Water-Soluble Photoinitiators Containing Carbohydrate Residues. J. Polym. Sci. Part Polym. Chem. 1995, 33, 929–939.
- Kminek, I.; Yagci, Y.; Schnabel, W. A Water-Soluble Poly(Methylphenylsilylene) Derivative as a Photoinitiator of Radical Polymerization of Hydrophilic Vinyl Monomers. Polym. Bull. 1992, 29, 277–282.
- Le, C.M.Q.; Petitory, T.; Wu, X.; Spangenberg, A.; Ortyl, J.; Galek, M.; Infante, L.; Thérien-Aubin, H.; Chemtob, A. Water-Soluble Photoinitiators from Dimethylamino-Substituted Monoacylphosphine Oxide for Hydrogel and Latex Preparation. Macromol. Chem. Phys. 2021, 222, 2100217.
- Dumur, F. Recent Advances on Water-Soluble Photoinitiators of Polymerization. Eur. Polym. J. 2023, 189, 111942.
- Pereira, J.A.; Pessoa, A.M.; Cordeiro, M.N.D.S.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, Its Derivatives and Applications: A State of the Art Review. Eur. J. Med. Chem. 2015, 97, 664–672.
- Soleymani, M.; Chegeni, M. The Chemistry and Applications of the Quinoxaline Compounds. Curr. Org. Chem. 2019, 23, 1789–1827.
- Payne, A.-J.; McCahill, J.S.J.; Welch, G.C. Indoloquinoxaline as a Terminal Building Block for the Construction of π-Conjugated Small Molecules Relevant to Organic Electronics. Dye. Pigment. 2015, 123, 139–146.
- Yuan, J.; Ouyang, J.; Cimrová, V.; Leclerc, M.; Najari, A.; Zou, Y. Development of Quinoxaline Based Polymers for Photovoltaic Applications. J. Mater. Chem. C 2017, 5, 1858–1879.
- Sun, C.; Zhu, C.; Meng, L.; Li, Y. Quinoxaline-Based D–A Copolymers for the Applications as Polymer Donor and Hole Transport Material in Polymer/Perovskite Solar Cells. Adv. Mater. 2022, 34, 2104161.
- Wardani, R.P.; Jin, H.C.; Kim, J.H.; Chang, D.W. Synthesis of Quinoxaline-Based D-A Type Conjugated Polymers for Photovoltaic Applications. Mol. Cryst. Liq. Cryst. 2020, 705, 15–21.
- Ashraf, R.S.; Hoppe, H.; Shahid, M.; Gobsch, G.; Sensfuss, S.; Klemm, E. Synthesis and Properties of Fluorene-Based Polyheteroarylenes for Photovoltaic Devices. J. Polym. Sci. Part Polym. Chem. 2006, 44, 6952–6961.
- Kuehl, V.A.; Duong, P.H.H.; Sadrieva, D.; Amin, S.A.; She, Y.; Li-Oakey, K.D.; Yarger, J.L.; Parkinson, B.A.; Hoberg, J.O. Synthesis, Postsynthetic Modifications, and Applications of the First Quinoxaline-Based Covalent Organic Framework. ACS Appl. Mater. Interfaces 2021, 13, 37494–37499.
- He, K.; Chen, S.; Xu, W.; Tai, X.; Chen, Y.; Sun, P.; Fan, Q.; Huang, W. High-Stability NIR-II Fluorescence Polymer Synthesized by Atom Transfer Radical Polymerization for Application in High-Resolution NIR-II Imaging. Biomater. Sci. 2021, 9, 6434–6443.
- Kucybała, Z.; Pączkowski, J. 3-Benzoyl-7-Diethylamino-5-Methyl-1-Phenyl-1H-Quinoxalin-2-One: An Effective Dyeing Photoinitiator for Free Radical Polymerization. J. Photochem. Photobiol. Chem. 1999, 128, 135–138.
- Kolehmainen, E.; Kucybała, Z.; Gawinecki, R.; Pączkowski, J.; Kacała, A. Unequivocal Determination of Isomeric Products of Reaction between 3-Methyl-1-Phenyl-2-Pyrazoline-4,5-Dione and Aromatic 1,2-Diamines. Tetrahedron 1999, 55, 8475–8480.
- Arsu, N.; Aydın, M. Photoinduced Free Radical Polymerization Initiated with Quinoxalines. Angew. Makromol. Chem. 1999, 270, 1–4.
- Aydın, M.; Arsu, N. Photoinitiated Free Radical Polymerization of Methylmethacrylate by Using of Quinoxalines in the Presence of Aldehydes. Prog. Org. Coat. 2006, 56, 338–342.
- Cao, X.; Jin, F.; Li, Y.-F.; Chen, W.-Q.; Duan, X.-M.; Yang, L.-M. Triphenylamine-Modified Quinoxaline Derivatives as Two-Photon Photoinitiators. New J. Chem. 2009, 33, 1578–1582.
- Kucybala, Z.; Kosobucka, A.; Paczkowski, J. Development of New Dyeing Photoinitiators for Free Radical Polymerization Based on 3-Methyl-1-Phenyl-1H-PentaazacyclopentaNaphthalene Skeleton III. J. Photochem. Photobiol. Chem. 2000, 136, 227–234.
- Kucybała, Z.; Pyszka, I.; Pączkowski, J. Development of New Dyeing Photoinitiators for Free Radical Polymerization Based on the 1H-PyrazoloQuinoxaline Skeleton. Part 2. J. Chem. Soc. Perkin Trans. 2 2000, 7, 1559–1567.
- Przyjazna, B.; Kucybała, Z.; Pączkowski, J. Development of New Dyeing Photoinitiators Based on 6H-IndoloQuinoxaline Skeleton. Polymer 2004, 45, 2559–2566.
- Qu, B.; Xu, Y.; Shi, W.; Raanby, B. Photoinitiated Crosslinking of Low-Density Polyethylene. 6. Spin-Trapping ESR Studies on Radical Intermediates. Macromolecules 1992, 25, 5215–5219.
- Pyszka, I.; Jędrzejewska, B. Photoinitiation Abilities of Indeno- and Indoloquinoxaline Derivatives and Mechanical Properties of Dental Fillings Based on Multifunctional Acrylic Monomers and Glass Ionomer. Polymer 2023, 266, 125625.
- Pyszka, I.; Skowroński, Ł.; Jędrzejewska, B. Study on New Dental Materials Containing Quinoxaline-Based Photoinitiators in Terms of Exothermicity of the Photopolymerization Process. Int. J. Mol. Sci. 2023, 24, 2752.
- Pyszka, I.; Kucybała, Z.; Pączkowski, J. Reinvestigation of the Mechanism of the Free Radical Polymerization Photoinitiation Process by Camphorquinone–Coinitiator Systems: New Results. Macromol. Chem. Phys. 2004, 205, 2371–2375.
- Pyszka, I.; Jędrzejewska, B. Acenaphthoquinoxaline Derivatives as Dental Photoinitiators of Acrylates Polymerization. Materials 2021, 14, 4881.
- Karaca Balta, D.; Keskin, S.; Karasu, F.; Arsu, N. Quinoxaline Derivatives as Photoinitiators in UV-Cured Coatings. Prog. Org. Coat. 2007, 60, 207–210.
- Lalevée, J.; Blanchard, N.; Tehfe, M.-A.; Peter, M.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Efficient Dual Radical/Cationic Photoinitiator under Visible Light: A New Concept. Polym. Chem. 2011, 2, 1986–1991.
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New Photoinitiating Systems and Strategies. Polym. Chem. 2015, 6, 3895–3912.
- Podsiadły, R. Photoreaction and Photopolymerization Studies on Fluoflavin Dye–Pyridinium Salt Systems. J. Photochem. Photobiol. Chem. 2008, 198, 60–68.
- Gould, I.R.; Shukla, D.; Giesen, D.; Farid, S. Energetics of Electron-Transfer Reactions of Photoinitiated Polymerization: Dye-Sensitized Fragmentation of N-Alkoxypyridinium Salts. Helv. Chim. Acta 2001, 84, 2796–2812.
- Timpe, H.-J.; Kronfeld, K.-P.; Lammel, U.; Fouassier, J.-P.; Lougnot, D.-J. Excited States of Ketones as Electron Donors—Ketone—Iodonium Salt Systems as Photoinitiators for Radical Polymerization. J. Photochem. Photobiol. Chem. 1990, 52, 111–122.
- Timpe, H.J.; Ulrich, S.; Decker, C.; Fouassier, J.P. Photoinitiated Polymerization of Acrylates and Methacrylates with Decahydroacridine-1,8-Dione/Onium Salt Initiator Systems. Macromolecules 1993, 26, 4560–4566.
- Kura, H.; Fujihara, K.; Kimura, A.; Ohno, T.; Matsumura, M.; Hirata, Y.; Okada, T. Initial Step of Anthracene-Sensitized Photoacid Generation from Diphenyliodonium Hexafluorophosphate in an Epoxy Matrix Studied by Steady-State and Laser-Flash Photolyses. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2937–2946.
- Dossow, D.; Zhu, Q.Q.; Hizal, G.; Yag, Y.; Schnabel, W. Photosensitized Cationic Polymerization of Cyclohexene Oxide: A Mechanistic Study Concerning the Use of Pyridinium-Type Salts. Polymer 1996, 37, 2821–2826.
- Podsiadły, R.; Podemska, K.; Szymczak, A.M. Novel Visible Photoinitiators Systems for Free-Radical/Cationic Hybrid Photopolymerization. Dye. Pigment. 2011, 91, 422–426.
- Podsiadły, R. The Synthesis of Novel, Visible-Wavelength Oxidizable Polymerization Sensitizers Based on the 5,12-DihydroquinoxalinoPyridopyrazine Skeleton. Dye. Pigment. 2009, 80, 86–92.
This entry is offline, you can click here to edit this entry!
