1. Intrahepatic Lymphatic Vessels
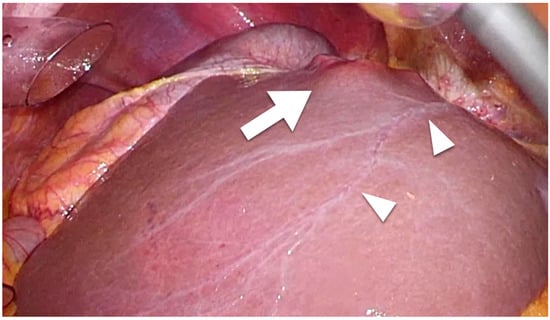
Intrahepatic lymphatic vessels such as sublobular or portal vessels are difficult to detect macroscopically during surgery. However, capsular lymphatic vessels can be detected macroscopically using laparoscope-enhanced views (
Figure 1). Regarding microscopic findings, although the lymphatic vessels and portal veins can be difficult to distinguish from each other using standard histochemical techniques, the anti-podoplanin antibody D2-40 is specific for lymphatic vessel endothelium and enables the detection of intrahepatic lymphatic vessels [
60]. Regarding lymphangiogenesis, lymphatic vessel counts from microscopic findings were used to determine lymphatic vessel density (LVD) [
53,
61,
62]. In most studies describing methods for the assessment and quantification of LVD, the lymphatic vessel numbers counted manually at 200× magnification (a 0.25-mm
2 field) in several areas of highest vascular density identified in low-magnification (×25–40) views were used for assessing LVD [
60,
61]. Researchers found that D2-40 was consistently used for detecting lymphatic vessels in studies published since 2007.
Figure 1. Laparoscopic findings of colorectal liver metastasis. Arrowheads show the capsular (superficial) lymphatic vessels. Tumor (arrow) that has invaded a lymphatic vessel can easily metastasize.
2. Intrahepatic Lymphatic Vessels and Liver Cirrhosis
Yokomori et al. also reported that not only capsular lymphatic vessels, but also non-capsular lymphatic vessels, are enlarged in cirrhotic patients due to the high portal pressure [
60]. The high portal pressure with increased sinusoidal blood flow is caused by architectural deformations around the portal and central veins [
3]. Lymph fluid production in the liver is increased up to 30-fold in cirrhotic patients [
3]. This represents the main cause of ascites, which comprises fluid that leaked from lymphatic vessels [
2,
56]. Furthermore, the number of lymphatic vessels also increased in cirrhotic liver [
4]. These changes are due to the expression of lymphangiogenic growth factors such as vascular endothelial growth factor (VEGF)-C or VEGF-D due to the processes of tissue repair, inflammation, and tumor-related factors [
4].
3. HCC
The long-term prognostic impact of intrahepatic LVI in HCC was not investigated previously. The incidence and prognostic impact of intrahepatic LVI in HCC are thus unclear. Two patients who underwent liver transplantation and showed lymphangiosis carcinomatosa in the resected specimens showed relatively good prognosis, with both surviving without recurrence (39 months and 16 months) after transplant [
63]. In contrast, tumor-associated lymphangiogenesis reportedly correlated with prognosis after the resection of HCC [
53]. Thelen et al. quantified peritumoral intrahepatic LVD using D2-40 for patients who underwent hepatectomy for HCC, revealing that tumors with high LVD were associated with poor OS (
Table 1) [
53]. High LVD was defined as >22.9 vessels in a 200× view (0.25-mm
2 field) [
53]. The high-LVD group showed a poorer disease-free survival (DFS) rate than the low-LVD group (18% vs. 40%, respectively;
p = 0.047) and LVD was selected as an independent predictor of DFS (but not of OS) in multivariate analysis [
53]. Lymphatic vessels were detected in the liver stroma in both healthy and cirrhotic livers, whereas HCC exhibited lymphatic vessels in both the tumor parenchyma and intratumoral septa [
53]. Cioca et al. reported that high expression of podoplanin in tumor cells was associated with higher frequency of poorly differentiated histopathological type than that of low in HCC (59% vs. 41%, respectively;
p = 0.040), suggesting a role of podoplanin in hepatocarcinogenesis [
64]. Furthermore, high peritumoral LVD correlated with both cirrhosis and vascular invasion (
p = 0.006 and
p = 0.018, respectively) [
64]. In another study, lymphangiogenesis-related long non-coding RNAs were able to be used to estimate HCC prognosis (median survival time [MST]: approximately 2 years for long non-coding RNA pairs with high-risk group vs. 7 years for those with low-risk group,
p < 0.001) and may be useful to select candidates for anti-tumor immunotherapy and chemotherapy [
65].
Several molecular and genetic mechanisms, such as HCC-associated long noncoding RNA (HANR), VEGF-C and -D, VEGF receptor (VEGFR)-3, heparanase-1, lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), hypoxia-inducible factor (HIF)-2a and homeobox prospero-like protein-1 (Prox-1), showed correlations with peritumoral lymphangiogenesis in HCC and associations with a greater risk of metastasis [
1,
66,
67,
68,
69,
70,
71].
4. ICC
The prognostic impact of intrahepatic LVI was reported in much greater detail for ICC than for HCC (
Table 2) [
38,
61,
72,
73,
74]. Nakajima et al. reported on 16 patients with LVI among 102 ICC patients in 1988 [
75]. While they identified LNM in 12 (75%) of the 16 LVI-positive patients [
67], the incidence of LNM was similar to that of LVI-negative patients (70%) and no significant correlation between LVI and LNM was detected [
75]. Fisher et al. reported not only LVI, but also perineural invasion as independent prognosticators of OS [
38]. Lang et al. reported that although LVI was associated with significantly poorer OS in univariate analyses, R0 resection and cancer stage, but not LVI, remained as independent predictors of OS in multivariate analyses [
74]. Conversely, Cho et al. reported significant differences in MST, at 9 months in LVI-positive patients and 23 months in LVI-negative patients (
p = 0.008) and selected LVI as an independent predictor of OS [
73]. Recently, Lurje et al. reported that LVI-positive patients after ICC resection showed shorter MST than LVI-negative patients in a univariate analysis (
Table 2) [
72]. However, although multivariate analysis in their study showed LVI as an independent predictor of OS in the perihilar cholangiocarcinoma cohort, LVI was not selected as an independent predictor of OS in the ICC cohort [
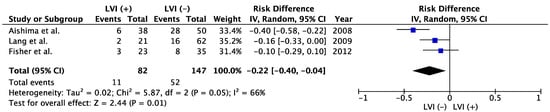
72]. A meta-analysis showed that positive LVI in patients with resected ICC was associated with poorer OS than negative LVI (
Figure 2). Five-year OS was 35.3% in LVI (−) group and 13.4% in LVI (+) group (risk difference −0.22; 95% confidence interval −0.40 to −0.04;
p = 0.01,
Figure 2). Shirabe et al. and Patel et al. also reported the poor prognostic impact of LVI on ICC, but those studies were excluded from the meta-analyses because the patient cohorts might have represented duplicates of those in studies by Aishima et al. and Fisher et al., respectively [
33,
76]. Furthermore, another report that included 26 patients with ICC and 14 patients with perihilar cholangiocarcinoma who underwent liver transplant found that pathological LVI was associated with poorer recurrence-free survival (hazard ratio, 2.1) [
77]. In contrast, Yoshikawa et al. reported that LVI had no significant impact on prognosis in ICC [
78]. They reported that epidermal growth factor receptor (EGFR) was associated with poor prognosis and represented an independent prognosticator in multivariate analyses (5-year OS 17.7% in EGFR-positive patients vs. 47.1% in EGFR-negative patients,
p = 0.0008) [
78].
Table 1. Studies evaluating lymphangiogenesis in liver cancer.
Figure 2. Meta-analysis of studies evaluating the impact of lymphatic vessel invasion on 5-year overall survival for intrahepatic cholangiocarcinoma. CI, confidence interval; IV, inverse variance; LVI, lymph vessel invasion [
38,
61,
74].
Table 2. Studies evaluating lymphatic vessel invasion in liver cancer.
Lymphangiogenesis was reported as a possible prognostic marker for HCC, and also as a predictor of unfavorable prognosis for ICC (
Table 1) [
62,
79]. Both studies also reported that high LVD was associated with the presence of LNM and poorer OS [
62,
79]. The cut-off value between high and low LVD was set at a count of 13 vessels in a 400× view for the study of Sha et al. [
79] and 12.66 vessels in a 200× view for that of Thelen et al. [
62]. Although the report by Sha et al. [
79] concluded that high LVD represented an independent predictor of OS in multivariate analyses, the report by Thelen et al. [
62] selected vascular invasion, not high LVD, as an independent predictor of OS. A different study by Sha et al. [
80] reported that expression of VEGFR-3, which promotes lymphangiogenesis, correlated with dismal prognosis in ICC. The 5-year OS was 14.6% in VEGFR-3-positive patients, compared to 53.2% in VEGFR-3-negative patients (
p < 0.001). Aishima et al. also reported that 5-year OS was significantly poorer in VEGF-C-positive patients (0%) than in VEGF-C-negative patients (49%,
p = 0.0007) [
61]. Several molecular mechanisms promoting lymphangiogenesis were reported, such as thrombospondin 1 and 2, C-X-C motif chemokine receptor 2 (CXCR2)-CXC ligand 5 (CXCL5) signaling and platelet-derived growth factor-D [
81,
82,
83,
84]. Liu et al. reported that the herbal medicine oxyresveratrol can prevent LNM by inhibiting lymphangiogenesis [
85]. Intratumoral lymphangiogenesis was correlated with LNM and prognosis in hilar cholangiocarcinoma [
82]. They reported that the frequency of a LNM-positive state was 68% in high-LVD patients and 12% in low-LVD patients (
p < 0.001), while 5-year OS rates were 7.0% and 76.4%, respectively (
p < 0.001) [
86].
5. CRLM
In terms of CRLM, several studies investigated the intrahepatic lymphatic system and clinical outcomes (
Table 2) [
34,
35,
36,
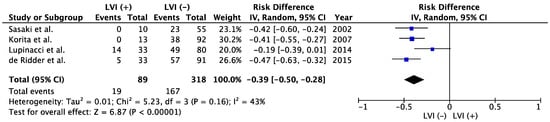
37]. Because of the rarity of candidates for resection of liver metastases from other origins, the present study did not identify any other reports evaluating LVI as a prognostic factor besides those involving CRLM. Among cases with CRLM, LVI represented a poor prognosticator in the meta-analysis (
Figure 3). The meta-analysis showed significantly better 5-year OS in LVI (−) group than that of LVI (+) (52.5% vs. 21.3%; risk difference −0.39; 95% confidence interval −0.50 to −0.28;
p < 0.00001
Figure 3). Sasaki et al. and Korita et al. reported dismal prognosis among LVI-positive patients, with 5-year OS rates of 0% [
35,
37]. Korita et al. also reported that LVI was significantly associated with LNM [
37]. While Lupinacci et al. reported that vascular invasions other than LVI were unrelated to recurrence or survival [
36], de Ridder et al. reported that LVI in combination with other vascular invasions is an important sign of adverse prognosis [
34].
Figure 3. Meta-analysis of studies evaluating the impact of lymphatic vessel invasion on 5-year overall survival for colorectal liver metastasis. CI, confidence interval; IV, inverse variance; LVI, lymph vessel invasion [
34,
35,
36,
37].
Schoppmann et al. reported significant correlations between LVI and lymphangiogenesis at both primary colorectal sites and liver metastatic sites [
87]. LVD was greater in LVI-positive patients than in LVI-negative patients (
p = 0.0001) [
87]. They concluded that the lymphatic pathway represents a key route for the metastasis of colorectal cancer to the liver, along with the hematogenous pathway [
87]. They also reported that high tumor expression of VEGF-C, which promotes lymphangiogenesis and metastasis [
41,
87,
88,
89], was associated with poor prognosis in patients with CRLM (MST: approximately 1 year in VEGF-C-positive patients vs. 2 years in VEGF-C-negative patients,
p = 0.010) [
87]. Various lymphangiogenic gene expressions and molecular mechanisms (VEGF-C, neuropilin-2 [Nrp-2], podoplanin, LYVE-1, mannose receptor-C type 1 (MRC1), chemokine (C-C) ligand 21 [CCL-21]) were associated with poor prognosis in CRLM [
90]. High expression of VEGF-C and Nrp-2 was also associated with lymph node recurrence following CRLM resection [
90].
6. Association between LVI and LNM
Two main routes of cancer metastasis were reported: hematogenous spread and lymphogenous spread [
56]. The route most likely to result in metastasis can depend on tumor factors, the peritumoral microenvironment and patient status [
56]. With hematogenous spread, tumor cells directly enter blood vessels and disseminate to distant sites. With lymphogenous routes, tumor cells penetrate into lymphatic vessels and disseminate to regional or distant lymph nodes. Tumor cells in lymph nodes then enter the thoracic duct and move from there to the subclavian vein to metastasize to distant sites [
56]. Although the mechanisms from LVI to LNM are known, little work was carried out on correlations between LVI and LNM in liver cancer. Korita et al. revealed an association between LVI and hepatic nodes in CRLM, with a higher incidence of hepatic node involvement in LVI-positive patients (23%) than in LVI-negative patients (4%,
p = 0.039) [
37]. They suggested that LVI in patients with liver metastasis spread to regional lymph nodes via the hepatic networks of portal, sublobular and capsular lymphatic vessels [
37]. They termed this phenomenon “remetastasis”, since liver metastases are mainly spread hematogenously, but liver metastases with LVI may spread to LNs via lymphogenous routes [
37].
High-level lymphangiogenesis was also associated with a significantly higher incidence of LNM than low-level lymphangiogenesis in ICC in studies by Sha et al. and Thelen et al. (
Table 1: 64.0% vs. 28.6%,
p < 0.001 and 59% vs. 38%,
p < 0.001, respectively) [
79]. Tumor cells can easily migrate along tumor-associated lymphatic vessels into lymph nodes [
79]. As mentioned earlier, lymphangiogenesis also correlates with LNM in hilar cholangiocarcinoma [
86]. Nevertheless, LNM is widely known as a strong prognosticator of OS in both HCC and ICC [
44]. In pooled analyses of HCC from a systematic review by Amini et al., 3- and 5-year OS rates were 27.5% and 20.8% in LNM-positive patients and 60.2% and 42.6% in LNM-negative patients [
44]. With ICC, 3- and 5-year OS rates were 0.2% and 0% in LNM-positive patients and 55.6% and 45.1% in LNM-negative patients [
44].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15072142