Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Microsatellite instability (MSI) occurs in a wide variety of tumor types and is one of the most important predictive biomarkers for immune checkpoint inhibitor therapy.
- microsatellite instability
- mismatch repair
- biomarker
1. Introduction
Microsatellite instability (MSI) results from impaired DNA mismatch repair (MMR) and causes an accumulation of mutations in microsatellites (MS), also called short tandem repeats (STRs). STRs consist of repeated sequences of 1–6 nucleotides and account for 3% of the genome, both coding and noncoding regions [1][2][3]. The nature of MS determines its outstanding tendency to accumulate errors. It happens due to DNA slippage in the process of DNA replication, which usually leads to a change in MS length [4][5].
The MMR system is highly conserved across species. MMR is responsible for the recognition and correction of mismatched nucleotides and plays a key role in maintaining genomic stability [6][7][8]. Four major proteins encoded by the MLH1 (mutL homologue 1) [9][10], MSH2 (mutS homologue 2) [11], MSH6 (mutS homologue 6), and PMS2 (postmeiotic segregation increased 2) [12] genes play a central role in this process [13]. The MMR system functions through the formation of heterodimers (hMutS and hMutL). hMutS consists of MSH2 and one of the secondary proteins, either MSH6 or MSH3, and recognizes mismatched nucleotides and small indels [7][8]. The hMutL heterodimers, consisting of MLH1 and one of the secondary proteins, PMS2, PMS1, or MLH3, participate in MMR reactions [14][15][16] via its endonuclease activity [17], 5′ nicking [18], modulation, and termination of exonuclease 1′s (Exo1) activity [18][19][20]. Thus, hMutL deficiency leads to Exo1 hyperactivity and increased DNA excision [19][20]. The inactivation of at least one of the following genes: MLH1, MSH2, MSH6, or PMS2, due to germline and/or somatic mutations or epigenetic silencing, results in the MMR system deficiency (dMMR) [21][22].
MSI occurs among various tumor types, and is most common in colorectal, small bowel, endometrial, and gastric cancers [3][23][24][25][26][27][28][29]. Most cases of MSI are sporadic, arising from epigenetic inactivation of MLH1 gene expression. However, the MSI can also be caused by Lynch syndrome, a hereditary condition resulting from germline pathogenic mutations in the MMR genes, coupled with inactivation of the second allele [30][31][32].
MSI is the one of the major biomarkers predictive of the immune checkpoint inhibitor (ICI) benefit across cancer types, both in Lynch syndrome-related and sporadic tumors. ICI therapy aims to overcome tumor immune escape through targeting immune inhibitory molecules (e.g., PD-1, PD-L1, LAG3, and CTLA4) expressed on the surfaces of tumor and immune cells [33][34][35]. Correct assessment of MSI is critical for adequate therapeutic decisions. According to ESMO recommendations [30], several methods are used in clinical practice to assess MSI status. The IHC method indirectly assesses MSI by detecting loss of staining for MLH1, MSH2, PMS2, and MSH6 proteins. An advantage of using IHC to detect MMR proteins is its convenience and ability to identify the target gene for future mutational confirmation [24]. PCR-based approaches directly determine MSI status via amplification of specific microsatellite repeats. ESMO suggests PCR in case of indeterminate IHC results, including disagreement or difficulties in interpreting IHC. The five poly-A mononucleotide repeats panel (BAT-25, BAT-26, NR-21, NR-24, NR-27) is considered the current standard [30], though the Bethesda panel, comprising of two mononucleotide (BAT-25 and BAT-26) and three dinucleotide (D5S346, D2S123, and D17S250) repeats, is also widely utilized in clinical practice [36][37]. In diagnostics, MSI (previously known as MSI-H) denotes alterations in the lengths of several MS (e.g., 2 of 5 loci in a standard PCR test with five poly-A mononucleotide repeats). In contrast, if the number of unstable loci does not exceed one, it is termed as MSS (microsatellite stable). NGS-based MSI detection is considered as one of the most promising, since its advantages include higher accuracy and an expanded spectrum of microsatellites analyzed, which is relevant for non-CRC tumors that can harbor a non-standard set of unstable microsatellites [38]. NGS also allows simultaneous analysis of a comprehensive spectrum of clinically significant biomarkers [14][30].
The first FDA-approved ICI drug was ipilimumab, which was initially approved in 2011 for the treatment of melanoma and is now used in a limited range of cancers. Pembrolizumab is a second FDA-approved ICI, which has the widest range of indications, including tissue-agnostic indications [39]. To date, more than 6 ICI have been approved for site-specific or tumor-agnostic indications. Overall, the objective response rate (ORR) for tumors with MSI varies between 34% and 69% depending on the line of therapy, while the rate of pathologic complete response (pCR) may reach 100% in neoadjuvant settings, which indicates a high efficacy of checkpoint inhibitors in MSI tumors [40][41][42]. At the same time, about a quarter of metastatic colorectal cancers and up to half of some other types of cancer are intrinsically resistant to ICI [43][44]. Several mechanisms of such an intrinsic resistance have been proposed, including the activating mutations in the RAS/RAF signaling pathway [45], mutations in the antigen presentation machinery and interferon pathway genes [46][47], establishment of an immunosuppressive microenvironment [48], as well as the influence of the gut microbiome [49] and immunoediting theory [50].
2. MSI: Molecular Epidemiology across Cancer Types
The MSI phenotype can be found in many cancer types. In a study analyzing more than 11,000 tissue samples from patients with 39 cancer types, MSI was found in 27 tumor types (overall in 3.8% of all samples) [51]. The tumor types where the MSI phenotype is observed include colon, gastric, endometrial, ovarian, hepatobiliary tract, urinary tract, brain, and skin cancers (Figure 1A). Of those, the highest prevalence of MSI is in colorectal cancer (10.2%, range 6.6–14.5%) [23][24][30][52][53][54][55][56][57][58][59], endometrial cancer (especially endometrioid histotype) (21.9%, range 15.1–29.6%) [25][26][27][52][60][61][62], gastric cancer (8.5%, range 6.4–10.9%) [3][27][28][30][52][63], and small bowel cancer (14.3%, range 5.4–26.3%) [52]. In gastric cancer, the frequency of MSI/dMMR varies significantly within histological subtypes: from 0.9% in the mixed-type and 2.9% in the diffuse-type, to 10.7% of the intestinal-type [28][64]. In other types of cancer, the MSI rate is relatively low [52], specifically 2–10% in ovarian [30][65][66][67] and only 1–2% in pancreatic cancer [30][68][69][70]. The assessments of the MSI rate in urothelial carcinoma are highly contradictory, with the reported values ranging from 1% to as high as 46% [71][72][73]. The MSI phenotype is also reported in Lynch syndrome-unrelated cancer types—in glioblastoma, cervical cancer, small intestine, melanoma, sarcoma, and others, but in these cancer types, MSI is much rarer [30][74][75].
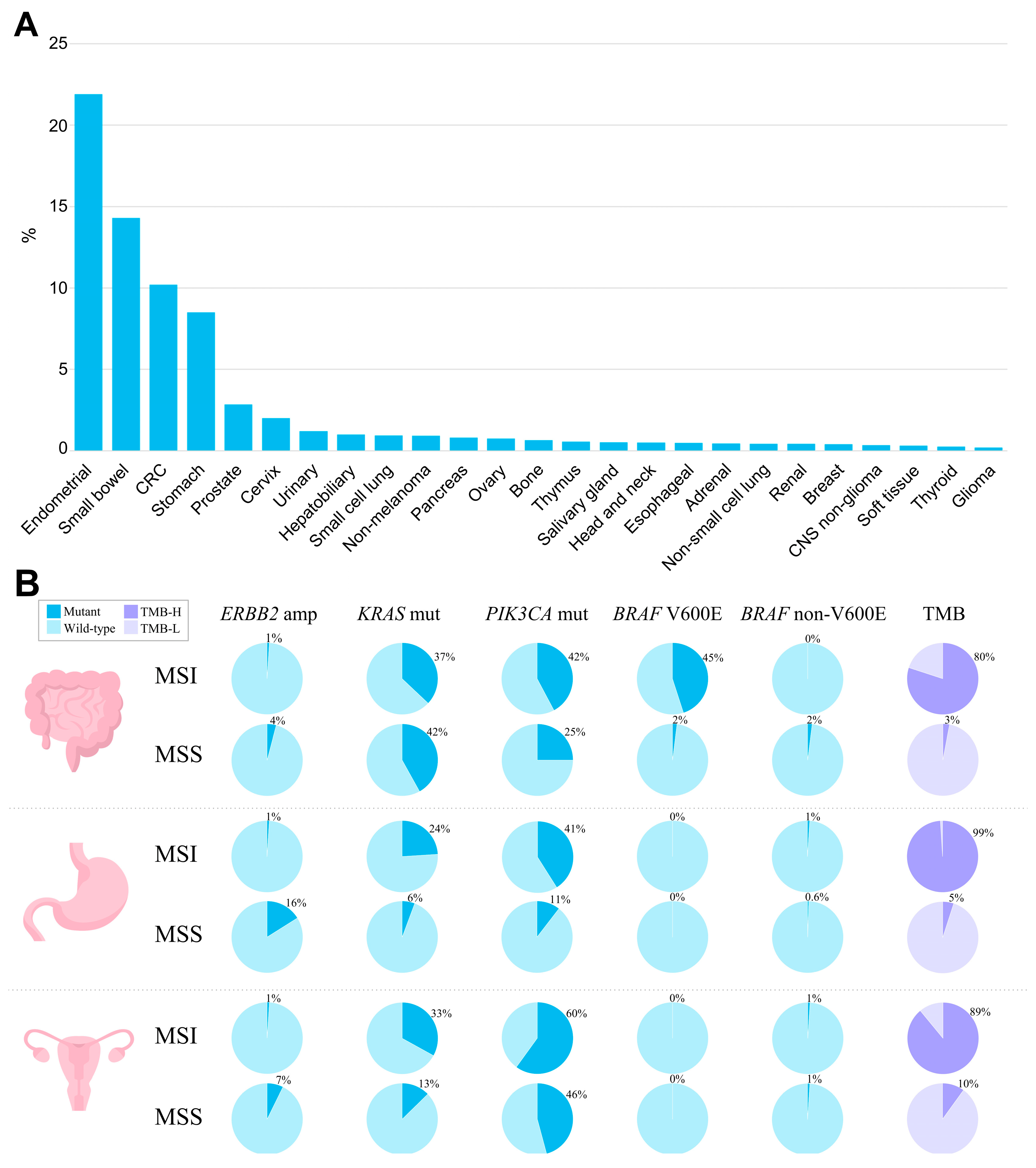
Figure 1. The landscape of MSI and genomic characteristics of MSI tumors across cancer types, according to TCGA. (A) Prevalence of MSI (%) across tumor types. (B) Prevalence of genomic alterations typically found in MSI and MSS tumors in colorectal adenocarcinoma, stomach adenocarcinoma, and uterine corpus endometrial carcinoma.
MSI is characteristic of both Lynch syndrome-associated cancer, where it is observed in nearly all cases, and sporadic cancer, where it reaches up to 10–15% of cases [59]. Conversely, Lynch syndrome comprises not more than 19% of MSI cancers, with the highest rate in MSI-positive colorectal cancer, followed by endometrial (5–10%), small bowel (12%), and gastric (4–15%) [64][75][76][77][78][79][80][81]. Respectively, most MSI cases (80% to 95%) arise sporadically [82]. The major cause of MSI is promoter hypermethylation of both MLH1 gene alleles, leading to a loss of MLH1, which is observed in ~90% of sporadic cancer [23][36][82][83][84][85].
When studying the molecular profiles of MSI tumors regardless of whether the tumor is Lynch syndrome-associated or sporadic, a number of oncogenic mutations can be found, including mutations in KRAS, NRAS, BRAF, PIK3CA, APC, TP53, etc. Specifically, KRAS is observed in MSI tumors with a frequency of 30–37% in endometrial, small bowel, and colorectal cancers, and at 15–28% in gastric cancer [29][54][64][86][87][88]. In colorectal cancer, the prevalence of KRAS mutations in MSI cases is lower than in MSS, where it reaches 46% [89]. Notably, MSS tumors harboring KRAS oncogenic mutations are characterized by more aggressive growth [85][90].
BRAF mutations are found with a high frequency (up to 45%) in MSI colorectal cancer, with mostly exclusive prevalence of p.V600E [91][92]. BRAF mutations have strong bias to sporadic cases, and mostly can never be observed in hereditary colorectal cancer [92][93][94][95]. According to Parsons et al.’s meta-analysis [96], BRAF V600E variants occur in only 1.4% of patients with Lynch syndrome. In sporadic colorectal carcinomas displaying the MSI phenotype, MLH1 hypermethylation and BRAF p.V600E mutations frequently co-occur, indicating a possible causal relationship between BRAF mutations and MLH1 loss [97][98][99]. However, the straight relationship between MLH1 hypermethylation and BRAF p.V600E mutation might be called into question by the fact that not all colorectal cancers with BRAF p.V600E mutations display silenced MLH1 with subsequent MSI. Such tumors remain microsatellite-stable, suggesting that other factors can influence the MSI phenotype in BRAF-positive cancer [100]. Indeed, only 20–30% of BRAF p.V600E-mutated metastatic colorectal cancer display MSI [101]. Additionally, the transition to the MSI phenotype via MLH1 hypermethylation is observed in approximately 75% of BRAF-mutated sessile serrated adenomas, with the remaining cases developing into MSS cancers. Among traditional serrated adenomas, BRAF mutations occur in two-thirds of the cases, but MLH1 silencing and the MSI phenotype are rare [102][103]. In other cancer types, the interplay between BRAF p.V600E and MSI/dMMR is not observed [3][27][62][104][105].
TP53 mutations are much less frequently observed in MSI as compared to MSS tumors, and their frequency in colorectal and gastric MSI cancer is 20–30%, as compared to 50–65% among MSS tumors [3][106]. Similarly, in the endometrial, pancreatic, and ovarian cancers, where TP53 mutations are common events, MSI tumors harboring TP53 can rarely be found [62][68][69][107]. This pattern of TP53 distribution among MSI and MSS tumors may indicate that TP53 mutations are unlikely to contribute to the MSI cancer tumorigenesis.
The prevalence of PIK3CA mutations in colorectal and gastric tumors is higher in MSI tumors (30–45%), as compared to MSS (10–25%). However, in endometrial cancer, PIK3CA mutations are equally common among both MSI and MSS tumors, accounting for 45–60% of all cases [62].
Other frequently altered genes in MSI tumors include RNF43, ATM, ARID1A, BRCA2, and PTEN. Mutations in these genes have been shown to have a several-fold increased mutation frequency in MSI colorectal carcinomas compared to MSS [108]. Approximately one in five cases of MSI tumors have important targetable fusions in NTRK1/2/3, ALK, or RET genes [108], but there is no significant relationship between MSI and HER2 amplification [109][110].
In the co-occurrence of MSI and other oncogenic alterations, the role of dMMR and its onset in Lynch syndrome colorectal carcinomas is described with three models [4]. In the first (classical) model, MMR deficiency is a secondary event that occurs after adenoma development, initially driven by somatic oncogenic mutations in APC and KRAS [111][112]. Distinguishing features of adenomas developing in accordance with this model are MMR proficiency and MSS. The classical model is typically observed in patients carrying germline MSH6 or PMS2 mutations [113]. It is known that in case of isolated loss of MSH6, MMR activity can be retained due to overlapping functions with MSH3, which explains the relatively low risk of cancer for MSH6 mutation carriers [114]. Notably, MLH1 and MSH2 mutation carriers rarely develop tumors via the classical model [111]. The prevalence of adenomas developing via this model of carcinogenesis can be roughly estimated at 25% [115][116][117]. In the second and third models, biallelic inactivation of MMR genes leading to dMMR is a driver event, and therefore MSI is observed in all such tumors [111][115]. The second model is mainly observed in MLH1 and MSH2 mutation carriers and is typically characterized by inactivation of tumor suppressors involved in the WNT pathway, predominantly TGFBR2 and RNF43, due to frameshift mutations within microsatellites in the corresponding genes [108][117]. The third model accounts for about 10% of LS-associated colorectal carcinomas and is exclusively observed in patients with MLH1 mutations. Here, dMMR is accomplished with mutations in CTNNB1 and TP53 [111].
The spectrum of potentially actionable alterations typically observed in MSI and MSS tumors based upon data generated by the TCGA Research Network [118] is summarized in Figure 1B.
Another important aspect worth mentioning is the relationship between MSI and tumor mutation burden (TMB). dMMR leads to a hypermutator phenotype and increased TMB, which is believed to make tumors more immunogenic. Considering all solid tumors, the simultaneous presence of MSI and TMB is quite rare and occurs only in about 3–7% of cases [30][119]. However, in tumors associated with Lynch syndrome, the overlap between TMB and MSI becomes more significant. The rate of TMB-H (≥10 mutations/megabase) patients among MSI cases is estimated at around 80–100% in colorectal cancer [52][120][121], 83–93% in endometrial cancer [52][122][123][124], and almost 100% in gastric and small bowel cancer [29][52][125]. In colorectal carcinoma, tumors with simultaneous presence of a high TMB and MSI/dMMR correspond to the CMS1 subtype [126], which is characterized by hypermutation, hypermethylation, enrichment in BRAF V600E mutations, as well as a strong infiltration of the tumor microenvironment with immune cells [56][127][128]. In this subtype, hypermethylation of the promoter regions of the MLH1 gene leads to its silencing, the accumulation of DNA mutations, and the expression of neoantigens that contribute to the high immunogenicity of the tumor [129]. The TMB levels in MSI tumors are likely dependent on certain MMR complex loss and tumor histology/primary site [130][131]. According to a study by Salem et al., evaluating colorectal, endometrial, and other tumors, overall, the loss of mutSα (MSH2/MSH6) leads to a more pronounced TMB than the loss of mutLα (MLH1/PMS2). However, in some types of tumor histology, secondary DNA repair pathways can better mitigate dMMR, resulting in a less pronounced TMB under the same IHC protein loss patterns. These findings support the diversity of gene- and histologically-specific heterogeneity of MSI/dMMR tumors [119]. Patients with MSI displaying a high TMB have a better prognosis and are also good candidates for checkpoint inhibitor therapy [129].
Three predictive biomarkers are currently used to select subgroups of patients eligible for ICI immunotherapy: PD-L1 expression, MSI/MMR status, and TMB.
Initially, it was considered that a common mechanism of tumor immune evasion is the aberrant expression of immune inhibitory molecules, PD-L1, on the surface of cancer cells [132]. However, over time, evidence has emerged that even in the absence of PD-L1 expression, tumors often remain sensitive to ICI [132][133]. Additionally, TMB-H and MSI tumors respond to the immunotherapy regardless of PD-L1 expression [134]. To some extent, it can be explained by the focal expression of PD-L1 [135], which can be missed during needle biopsy, or by the dynamic and inducible nature of PD-L1 expression [136]. This led to the PD-L1 expression-independent indications of ICI for many types of cancers. While anti-PD-L1 therapy acts to overcome local immune resistance, CTLA-4 is expressed in T-cells and acts non-locally, often causing autoimmune reactions and lymphocyte invasion in different unaffected organs [137][138]. For that reason, prescription of anti-CTLA4 therapy does not require determination of the CTLA4 expression status.
dMMR is the cause of the hypermutator phenotype and increased TMB, which likely leads to increased tumor immunogenicity. Indeed, the connection between MMR phenotype, TMB, and tumor immunogenicity is more sophisticated since some methods of TMB calculation may exclude truncating mutations in certain genes [139], while the dMMR phenotype is characterized by an increased frequency of frameshift mutations producing an abundance of highly immunogenic neoantigens [140][141][142]. Thus, dMMR/MSI status should be considered as an independent predictive biomarker of ICI effectiveness, and the presence of a TMB-H in this subgroup may be an additional factor indicating an increased immunogenicity of the tumor.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15082288
References
- Baretti, M.; Le, D.T. DNA Mismatch Repair in Cancer. Pharmacol. Ther. 2018, 189, 45–62.
- Ma, J.; Setton, J.; Lee, N.Y.; Riaz, N.; Powell, S.N. The Therapeutic Significance of Mutational Signatures from DNA Repair Deficiency in Cancer. Nat. Commun. 2018, 9, 3292.
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209.
- Lepore Signorile, M.; Disciglio, V.; di Carlo, G.; Pisani, A.; Simone, C.; Ingravallo, G. From Genetics to Histomolecular Characterization: An Insight into Colorectal Carcinogenesis in Lynch Syndrome. Int. J. Mol. Sci. 2021, 22, 6767.
- Lower, S.S.; McGurk, M.P.; Clark, A.G.; Barbash, D.A. Satellite DNA Evolution: Old Ideas, New Approaches. Curr. Opin. Genet. Dev. 2018, 49, 70–78.
- Kolodner, R.D.; Marsischky, G.T. Eukaryotic DNA Mismatch Repair. Curr. Opin. Genet. Dev. 1999, 9, 89–96.
- Kunkel, T.A.; Erie, D.A. DNA Mismatch Repair. Annu. Rev. Biochem. 2005, 74, 681–710.
- Modrich, P.; Lahue, R. Mismatch Repair in Replication Fidelity, Genetic Recombination, and Cancer Biology. Annu. Rev. Biochem. 1996, 65, 101–133.
- Bronner, C.E.; Baker, S.M.; Morrison, P.T.; Warren, G.; Smith, L.G.; Lescoe, M.K.; Kane, M.; Earabino, C.; Lipford, J.; Lindblom, A.; et al. Mutation in the DNA Mismatch Repair Gene Homologue HMLH1 Is Associated with Hereditary Non-Polyposis Colon Cancer. Nature 1994, 368, 258–261.
- Papadopoulos, N.; Nicolaides, N.C.; Wei, Y.F.; Ruben, S.M.; Carter, K.C.; Rosen, C.A.; Haseltine, W.A.; Fleischmann, R.D.; Fraser, C.M.; Adams, M.D.; et al. Mutation of a MutL Homolog in Hereditary Colon Cancer. Science 1994, 263, 1625–1629.
- Fishel, R.; Lescoe, M.K.; Rao, M.R.S.; Copeland, N.G.; Jenkins, N.A.; Garber, J.; Kane, M.; Kolodner, R. The Human Mutator Gene Homolog MSH2 and Its Association with Hereditary Nonpolyposis Colon Cancer. Cell 1993, 75, 1027–1038.
- Nicolaides, N.C.; Papadopoulos, N.; Liu, B.; Weit, Y.F.; Carter, K.C.; Ruben, S.M.; Rosen, C.A.; Haseltine, W.A.; Fleischmann, R.D.; Fraser, C.M.; et al. Mutations of Two PMS Homologues in Hereditary Nonpolyposis Colon Cancer. Nature 1994, 371, 75–80.
- Jiricny, J. Postreplicative Mismatch Repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012633.
- Gilson, P.; Merlin, J.L.; Harlé, A. Detection of Microsatellite Instability: State of the Art and Future Applications in Circulating Tumour DNA (CtDNA). Cancers 2021, 13, 1491.
- Iyer, R.R.; Pluciennik, A. DNA Mismatch Repair and Its Role in Huntington’s Disease. J. Huntingtons Dis. 2021, 10, 75–94.
- Li, Z.; Pearlman, A.H.; Hsieh, P. DNA Mismatch Repair and the DNA Damage Response. DNA Repair 2016, 38, 94–101.
- Kadyrov, F.A.; Dzantiev, L.; Constantin, N.; Modrich, P. Endonucleolytic Function of MutLalpha in Human Mismatch Repair. Cell 2006, 126, 297–308.
- Ortega, J.; Lee, G.S.; Gu, L.; Yang, W.; Li, G.M. Mispair-Bound Human MutS-MutL Complex Triggers DNA Incisions and Activates Mismatch Repair. Cell Res. 2021, 31, 542–553.
- Zhang, Y.; Yuan, F.; Presnell, S.R.; Tian, K.; Gao, Y.; Tomkinson, A.E.; Gu, L.; Li, G.M. Reconstitution of 5′-Directed Human Mismatch Repair in a Purified System. Cell 2005, 122, 693–705.
- Guan, J.; Lu, C.; Jin, Q.; Lu, H.; Chen, X.; Tian, L.; Zhang, Y.; Ortega, J.; Zhang, J.; Siteni, S.; et al. MLH1 Deficiency-Triggered DNA Hyperexcision by Exonuclease 1 Activates the CGAS-STING Pathway. Cancer Cell 2021, 39, 109–121.e5.
- Olave, M.C.; Graham, R.P. Mismatch Repair Deficiency: The What, How and Why It Is Important. Genes Chromosomes Cancer 2022, 61, 314–321.
- Li, G.M. Mechanisms and Functions of DNA Mismatch Repair. Cell Res. 2008, 18, 85–98.
- Diao, Z.; Han, Y.; Chen, Y.; Zhang, R.; Li, J. The Clinical Utility of Microsatellite Instability in Colorectal Cancer. Crit. Rev. Oncol. Hematol. 2021, 157, 103171.
- Cheah, P.-L.; Looi, L.-M.; Koh, C.-C.; Lau, T.-P.; Chang, S.-W.; Teoh, K.-H.; Mun, K.-S.; Nazarina, A.R. Screening for Microsatellite Instability in Colorectal Carcinoma: Practical Utility of Immunohistochemistry and PCR with Fragment Analysis in a Diagnostic Histopathology Setting. Malays. J. Pathol. 2019, 41, 91–100.
- Diaz-Padilla, I.; Romero, N.; Amir, E.; Matias-Guiu, X.; Vilar, E.; Muggia, F.; Garcia-Donas, J. Mismatch Repair Status and Clinical Outcome in Endometrial Cancer: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2013, 88, 154–167.
- Black, D.; Soslow, R.A.; Levine, D.A.; Tornos, C.; Chen, S.C.; Hummer, A.J.; Bogomolniy, F.; Olvera, N.; Barakat, R.R.; Boyd, J. Clinicopathologic Significance of Defective DNA Mismatch Repair in Endometrial Carcinoma. J. Clin. Oncol. 2006, 24, 1745–1753.
- Cortes-Ciriano, I.; Lee, S.; Park, W.Y.; Kim, T.M.; Park, P.J. A Molecular Portrait of Microsatellite Instability across Multiple Cancers. Nat. Commun. 2017, 8, 15180.
- Polom, K.; Marano, L.; Marrelli, D.; de Luca, R.; Roviello, G.; Savelli, V.; Tan, P.; Roviello, F. Meta-Analysis of Microsatellite Instability in Relation to Clinicopathological Characteristics and Overall Survival in Gastric Cancer. Br. J. Surg. 2018, 105, 159–167.
- Schrock, A.B.; Devoe, C.E.; McWilliams, R.; Sun, J.; Aparicio, T.; Stephens, P.J.; Ross, J.S.; Wilson, R.; Miller, V.A.; Ali, S.M.; et al. Genomic Profiling of Small-Bowel Adenocarcinoma. JAMA Oncol. 2017, 3, 1546–1553.
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.Y.; Andre, F.; et al. ESMO Recommendations on Microsatellite Instability Testing for Immunotherapy in Cancer, and Its Relationship with PD-1/PD-L1 Expression and Tumour Mutational Burden: A Systematic Review-Based Approach. Ann. Oncol. 2019, 30, 1232–1243.
- Porkka, N.; Valo, S.; Nieminen, T.T.; Olkinuora, A.; Mäki-Nevala, S.; Eldfors, S.; Peltomäki, P. Sequencing of Lynch Syndrome Tumors Reveals the Importance of Epigenetic Alterations. Oncotarget 2017, 8, 108020–108030.
- Lynch, H.T.; Lynch, P.M.; Lanspa, S.J.; Snyder, C.L.; Lynch, J.F.; Boland, C.R. Review of the Lynch Syndrome: History, Molecular Genetics, Screening, Differential Diagnosis, and Medicolegal Ramifications. Clin. Genet. 2009, 76, 1–18.
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Sadreddini, S.; Baradaran, B.; Aghebati-Maleki, A.; Sadreddini, S.; Shanehbandi, D.; Fotouhi, A.; Aghebati-Maleki, L. Immune Checkpoint Blockade Opens a New Way to Cancer Immunotherapy. J. Cell Physiol. 2019, 234, 8541–8549.
- de Giglio, A.; di Federico, A.; Nuvola, G.; Deiana, C.; Gelsomino, F. The Landscape of Immunotherapy in Advanced NSCLC: Driving beyond PD-1/PD-L1 Inhibitors (CTLA-4, LAG3, IDO, OX40, TIGIT, Vaccines). Curr. Oncol. Rep. 2021, 23, 126.
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J. Natl. Cancer Inst. 2004, 96, 261–268.
- Goel, A.; Nagasaka, T.; Hamelin, R.; Boland, C.R. An Optimized Pentaplex PCR for Detecting DNA Mismatch Repair-Deficient Colorectal Cancers. PLoS ONE 2010, 5, 9393.
- Ratovomanana, T.; Cohen, R.; Svrcek, M.; Renaud, F.; Cervera, P.; Siret, A.; Letourneur, Q.; Buhard, O.; Bourgoin, P.; Guillerm, E.; et al. Performance of Next-Generation Sequencing for the Detection of Microsatellite Instability in Colorectal Cancer with Deficient DNA Mismatch Repair. Gastroenterology 2021, 161, 814–826.e7.
- U.S. Food and Drug Administration Website. KEYTRUDA (Pembrolizumab). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125514s133lbl.pdf (accessed on 7 February 2023).
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10.
- Lenz, H.J.; van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170.
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; el Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376.
- Wang, R.; Lian, J.; Wang, X.; Pang, X.; Xu, B.; Tang, S.; Shao, J.; Lu, H. Intrinsic Resistance and Efficacy of Immunotherapy in Microsatellite Instability-High Colorectal Cancer: A Systematic Review and Meta-Analysis. Bosn. J. Basic Med. Sci. 2022, 23, 198–208.
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; de Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in Microsatellite Instability High or Mismatch Repair Deficient Cancers: Updated Analysis from the Phase II KEYNOTE-158 Study. Ann. Oncol. 2022, 33, 929–938.
- Taieb, J.; Svrcek, M.; Cohen, R.; Basile, D.; Tougeron, D.; Phelip, J.M. Deficient Mismatch Repair/Microsatellite Unstable Colorectal Cancer: Diagnosis, Prognosis and Treatment. Eur. J. Cancer 2022, 175, 136–157.
- Grasso, C.S.; Giannakis, M.; Wells, D.K.; Hamada, T.; Mu, X.J.; Quist, M.; Nowak, J.A.; Nishihara, R.; Qian, Z.R.; Inamura, K.; et al. Genetic Mechanisms of Immune Evasion in Colorectal Cancer. Cancer Discov. 2018, 8, 730–749.
- Zhang, C.; Li, D.; Xiao, B.; Zhou, C.; Jiang, W.; Tang, J.; Li, Y.; Zhang, R.; Han, K.; Hou, Z.; et al. B2M and JAK1/2-Mutated MSI-H Colorectal Carcinomas Can Benefit from Anti-PD-1 Therapy. J. Immunother. 2022, 45, 187–193.
- Abushukair, H.; Ababneh, O.; Zaitoun, S.; Saeed, A. Primary and Secondary Immune Checkpoint Inhibitors Resistance in Colorectal Cancer: Key Mechanisms and Ways to Overcome Resistance. Cancer Treat. Res. Commun. 2022, 33, 100643.
- Bouferraa, Y.; Chedid, A.; Amhaz, G.; el Lakkiss, A.; Mukherji, D.; Temraz, S.; Shamseddine, A. The Role of Gut Microbiota in Overcoming Resistance to Checkpoint Inhibitors in Cancer Patients: Mechanisms and Challenges. Int. J. Mol. Sci. 2021, 22, 8036.
- Ballhausen, A.; Przybilla, M.J.; Jendrusch, M.; Haupt, S.; Pfaffendorf, E.; Seidler, F.; Witt, J.; Hernandez Sanchez, A.; Urban, K.; Draxlbauer, M.; et al. The Shared Frameshift Mutation Landscape of Microsatellite-Unstable Cancers Suggests Immunoediting during Tumor Evolution. Nat. Commun. 2020, 11, 4740.
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.-Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 1, 1–15.
- Kang, Y.J.; O’Haire, S.; Franchini, F.; Ijzerman, M.; Zalcberg, J.; Macrae, F.; Canfell, K.; Steinberg, J. A Scoping Review and Meta-Analysis on the Prevalence of Pan-Tumour Biomarkers (DMMR, MSI, High TMB) in Different Solid Tumours. Sci. Rep. 2022, 12, 20495.
- Carr, P.R.; Alwers, E.; Bienert, S.; Weberpals, J.; Kloor, M.; Brenner, H.; Hoffmeister, M. Lifestyle Factors and Risk of Sporadic Colorectal Cancer by Microsatellite Instability Status: A Systematic Review and Meta-Analyses. Ann. Oncol. 2018, 29, 825–834.
- Ashktorab, H.; Ahuja, S.; Kannan, L.; Llor, X.; Nathan, E.; Xicola, R.M.; Laiyemo, A.O.; Carethers, J.M.; Brim, H.; Nouraie, M. A Meta-Analysis of MSI Frequency and Race in Colorectal Cancer. Oncotarget 2016, 7, 34546–34557.
- Gkekas, I.; Novotny, J.; Pecen, L.; Strigård, K.; Palmqvist, R.; Gunnarsson, U. Microsatellite Instability as a Prognostic Factor in Stage II Colon Cancer Patients, a Meta-Analysis of Published Literature. Anticancer Res. 2017, 37, 6563–6574.
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356.
- Petrelli, F.; Ghidini, M.; Cabiddu, M.; Pezzica, E.; Corti, D.; Turati, L.; Costanzo, A.; Varricchio, A.; Ghidini, A.; Barni, S.; et al. Microsatellite Instability and Survival in Stage II Colorectal Cancer: A Systematic Review and Meta-Analysis. Anticancer Res. 2019, 39, 6431–6441.
- Aaltonen, L.A.; Peltomäki, P.; Leach, F.S.; Sistonen, P.; Pylkkänen, L.; Mecklin, J.P.; Järvinen, H.; Powell, S.M.; Jen, J.; Hamilton, S.R.; et al. Clues to the Pathogenesis of Familial Colorectal Cancer. Science 1993, 260, 812–816.
- Mohamed, A.; Jiang, R.; Philip, P.A.; Diab, M.; Behera, M.; Wu, C.; Alese, O.; Shaib, W.L.; Gaines, T.M.; Balch, G.G.; et al. High-Risk Features Are Prognostic in DMMR/MSI-H Stage II Colon Cancer. Front. Oncol. 2021, 11, 755113.
- Hecht, J.L.; Mutter, G.L. Molecular and Pathologic Aspects of Endometrial Carcinogenesis. J. Clin. Oncol. 2006, 24, 4783–4791.
- Zighelboim, I.; Goodfellow, P.J.; Gao, F.; Gibb, R.K.; Powell, M.A.; Rader, J.S.; Mutch, D.G. Microsatellite Instability and Epigenetic Inactivation of MLH1 and Outcome of Patients with Endometrial Carcinomas of the Endometrioid Type. J. Clin. Oncol. 2007, 25, 2042–2048.
- Getz, G.; Gabriel, S.B.; Cibulskis, K.; Lander, E.; Sivachenko, A.; Sougnez, C.; Lawrence, M.; Kandoth, C.; Dooling, D.; Fulton, R.; et al. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73.
- Kwon, M.; An, M.; Klempner, S.J.; Lee, H.; Kim, K.M.; Sa, J.K.; Cho, H.J.; Hong, J.Y.; Lee, T.; Min, Y.W.; et al. Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability-High Gastric Cancer. Cancer Discov. 2021, 11, 2168–2185.
- Puliga, E.; Corso, S.; Pietrantonio, F.; Giordano, S. Microsatellite Instability in Gastric Cancer: Between Lights and Shadows. Cancer Treat. Rev. 2021, 95, 102175.
- Evrard, C.; Alexandre, J. Predictive and Prognostic Value of Microsatellite Instability in Gynecologic Cancer (Endometrial and Ovarian). Cancers 2021, 13, 2434.
- Murphy, M.A.; Wentzensen, N. Frequency of Mismatch Repair Deficiency in Ovarian Cancer: A Systematic Review This Article Is a US Government Work and, as Such, Is in the Public Domain of the United States of America. Int. J. Cancer 2011, 129, 1914–1922.
- Pal, T.; Permuth-Wey, J.; Kumar, A.; Sellers, T.A. Systematic Review and Meta-Analysis of Ovarian Cancers: Estimation of Microsatellite-High Frequency and Characterization of Mismatch Repair Deficient Tumor Histology. Clin. Cancer Res. 2008, 14, 6847–6854.
- Raphael, B.J.; Hruban, R.H.; Aguirre, A.J.; Moffitt, R.A.; Yeh, J.J.; Stewart, C.; Robertson, A.G.; Cherniack, A.D.; Gupta, M.; Getz, G.; et al. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e13.
- Luchini, C.; Brosens, L.A.A.; Wood, L.D.; Chatterjee, D.; Shin, J.I.; Sciammarella, C.; Fiadone, G.; Malleo, G.; Salvia, R.; Kryklyva, V.; et al. Comprehensive Characterisation of Pancreatic Ductal Adenocarcinoma with Microsatellite Instability: Histology, Molecular Pathology and Clinical Implications. Gut 2021, 70, 148–156.
- Luchini, C.; Scarpa, A. Microsatellite Instability in Pancreatic and Ampullary Carcinomas: Histology, Molecular Pathology, and Clinical Implications. Hum. Pathol. 2022, 132, 176–182.
- Kullmann, F.; Strissel, P.L.; Strick, R.; Stoehr, R.; Eckstein, M.; Bertz, S.; Wullich, B.; Sikic, D.; Wach, S.; Taubert, H.; et al. Frequency of Microsatellite Instability (MSI) in Upper Tract Urothelial Carcinoma: Comparison of the Bethesda Panel and the Idylla MSI Assay in a Consecutively Collected, Multi-Institutional Cohort. J. Clin. Pathol. 2023, 76, 126–132.
- Sobrino-Reig, E.; Meizoso, T.; García, J.; Varillas-Delgado, D.; Martin, Y.B. Morphological Predictors for Microsatellite Instability in Urothelial Carcinoma. Diagn. Pathol. 2021, 16, 106.
- Schneider, B.; Glass, Ä.; Jagdmann, S.; Hühns, M.; Claus, J.; Zettl, H.; Dräger, D.L.; Maruschke, M.; Hakenberg, O.W.; Erbersdobler, A.; et al. Loss of Mismatch-Repair Protein Expression and Microsatellite Instability in Upper Tract Urothelial Carcinoma and Clinicopathologic Implications. Clin. Genitourin. Cancer 2020, 18, e563–e572.
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413.
- Latham, A.; Srinivasan, P.; Kemel, Y.; Shia, J.; Bandlamudi, C.; Mandelker, D.; Middha, S.; Hechtman, J.; Zehir, A.; Dubard-Gault, M.; et al. Microsatellite Instability Is Associated with the Presence of Lynch Syndrome Pan-Cancer. J. Clin. Oncol. 2019, 37, 286.
- Hampel, H.; Frankel, W.; Panescu, J.; Lockman, J.; Sotamaa, K.; Fix, D.; Comeras, I.; la Jeunesse, J.; Nakagawa, H.; Westman, J.A.; et al. Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res. 2006, 66, 7810–7817.
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.T.H.B.M.; van Wezel, T.; et al. Practical Guidance for Mismatch Repair-Deficiency Testing in Endometrial Cancer. Ann. Oncol. 2017, 28, 96–102.
- Goodfellow, P.J.; Billingsley, C.C.; Lankes, H.A.; Ali, S.; Cohn, D.E.; Broaddus, R.J.; Ramirez, N.; Pritchard, C.C.; Hampel, H.; Chassen, A.S.; et al. Combined Microsatellite Instability, MLH1 Methylation Analysis, and Immunohistochemistry for Lynch Syndrome Screening in Endometrial Cancers from GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J. Clin. Oncol. 2015, 33, 4301–4308.
- Simpkins, S.B.; Bocker, T.; Swisher, E.M.; Mutch, D.G.; Gersell, D.J.; Kovatich, A.J.; Palazzo, J.P.; Fishel, R.; Goodfellow, P.J. MLH1 Promoter Methylation and Gene Silencing Is the Primary Cause of Microsatellite Instability in Sporadic Endometrial Cancers. Hum. Mol. Genet. 1999, 8, 661–666.
- Deshpande, M.; Romanski, P.A.; Rosenwaks, Z.; Gerhardt, J. Gynecological Cancers Caused by Deficient Mismatch Repair and Microsatellite Instability. Cancers 2020, 12, 3319.
- Leite, M.; Corso, G.; Sousa, S.; Milanezi, F.; Afonso, L.P.; Henrique, R.; Soares, J.M.; Castedo, S.; Carneiro, F.; Roviello, F.; et al. MSI Phenotype and MMR Alterations in Familial and Sporadic Gastric Cancer. Int. J. Cancer 2011, 128, 1606–1613.
- Gay, L.J.; Arends, M.J.; Mitrou, P.N.; Bowman, R.; Ibrahim, A.E.; Happerfield, L.; Luben, R.; McTaggart, A.; Ball, R.Y.; Rodwell, S.A. MLH1 Promoter Methylation, Diet, and Lifestyle Factors in Mismatch Repair Deficient Colorectal Cancer Patients from EPIC-Norfolk. Nutr. Cancer 2011, 63, 1000–1010.
- Poulogiannis, G.; Frayling, I.M.; Arends, M.J. DNA Mismatch Repair Deficiency in Sporadic Colorectal Cancer and Lynch Syndrome. Histopathology 2010, 56, 167–179.
- Meyer, L.A.; Broaddus, R.R.; Lu, K.H. Endometrial Cancer and Lynch Syndrome: Clinical and Pathologic Considerations. Cancer Control 2009, 16, 14–22.
- Ibrahim, A.E.K.; Arends, M.J. Molecular Typing of Colorectal Cancer: Applications in Diagnosis and Treatment. Diagn. Histopathol. 2012, 18, 70–80.
- Jin, J.; Shi, Y.; Zhang, S.; Yang, S. PIK3CA Mutation and Clinicopathological Features of Colorectal Cancer: A Systematic Review and Meta-Analysis. Acta Oncol. 2020, 59, 66–74.
- Brennetot, C.; Duval, A.; Hamelin, R.; Pinto, M.; Oliveira, C.; Seruca, R.; Schwartz, S. Frequent Ki-Ras Mutations in Gastric Tumors of the MSI Phenotype. Gastroenterology 2003, 125, 1282–1283.
- Polom, K.; Das, K.; Marrelli, D.; Roviello, G.; Pascale, V.; Voglino, C.; Rho, H.; Tan, P.; Roviello, F. KRAS Mutation in Gastric Cancer and Prognostication Associated with Microsatellite Instability Status. Pathol. Oncol. Res. 2019, 25, 333–340.
- Lin, E.I.; Tseng, L.H.; Gocke, C.D.; Reil, S.; Le, D.T.; Azad, N.S.; Eshleman, J.R. Mutational Profiling of Colorectal Cancers with Microsatellite Instability. Oncotarget 2015, 6, 42334–42344.
- Andreyev, H.J.N.; Norman, A.R.; Cunningham, D.; Oates, J.; Dix, B.R.; Iacopetta, B.J.; Young, J.; Walsh, T.; Ward, R.; Hawkins, N.; et al. Kirsten Ras Mutations in Patients with Colorectal Cancer: The “RASCAL II” Study. Br. J. Cancer 2001, 85, 692–696.
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch Repair Status and BRAF Mutation Status in Metastatic Colorectal Cancer Patients: A Pooled Analysis of the CAIRO, CAIRO2, COIN, and FOCUS Studies. Clin. Cancer Res. 2014, 20, 5322–5330.
- Salem, M.; Kopetz, S.; El-Refai, S.; Tabernero, J.; Sinicrope, F.; Tie, J.; George, T.; van Cutsem, E.; Mauer, E.; Lonardi, S.; et al. LBA SO-34 Impact of BRAF-V600E Mutation on Immunologic Characteristics of the Tumor Microenvironment (TME) and Associated Genomic Alterations in Patients with Microsatellite Instability-High (MSI-H) or Mismatch-Repair–Deficient (DMMR) Colorectal Cancer (CRC). Ann. Oncol. 2022, 33, S378.
- McGivern, A.; Wynter, C.V.A.; Whitehall, V.L.J.; Kambara, T.; Spring, K.J.; Walsh, M.D.; Barker, M.A.; Arnold, S.; Simms, L.A.; Leggett, B.A.; et al. Promoter Hypermethylation Frequency and BRAF Mutations Distinguish Hereditary Non-Polyposis Colon Cancer from Sporadic MSI-H Colon Cancer. Fam. Cancer 2004, 3, 101–107.
- Bessa, X.; Ballesté, B.; Andreu, M.; Castells, A.; Bellosillo, B.; Balaguer, F.; Castellví-bel, S.; Paya, A.; Jover, R.; Alenda, C.; et al. A Prospective, Multicenter, Population-Based Study of BRAF Mutational Analysis for Lynch Syndrome Screening. Clin. Gastroenterol. Hepatol. 2008, 6, 206–214.
- Porkka, N.; Lahtinen, L.; Ahtiainen, M.; Böhm, J.P.; Kuopio, T.; Eldfors, S.; Mecklin, J.P.; Seppälä, T.T.; Peltomäki, P. Epidemiological, Clinical and Molecular Characterization of Lynch-like Syndrome: A Population-Based Study. Int. J. Cancer 2019, 145, 87–98.
- Parsons, M.T.; Buchanan, D.D.; Thompson, B.; Young, J.P.; Spurdle, A.B. Correlation of Tumour BRAF Mutations and MLH1 Methylation with Germline Mismatch Repair (MMR) Gene Mutation Status: A Literature Review Assessing Utility of Tumour Features for MMR Variant Classification. J. Med. Genet. 2012, 49, 151–157.
- Kambara, T.; Simms, L.A.; Whitehall, V.L.J.; Spring, K.J.; Wynter, C.V.A.; Walsh, M.D.; Barker, M.A.; Arnold, S.; McGivern, A.; Matsubara, N.; et al. BRAF Mutation Is Associated with DNA Methylation in Serrated Polyps and Cancers of the Colorectum. Gut 2004, 53, 1137–1144.
- Weisenberger, D.J.; Siegmund, K.D.; Campan, M.; Young, J.; Long, T.I.; Faasse, M.A.; Kang, G.H.; Widschwendter, M.; Weener, D.; Buchanan, D.; et al. CpG Island Methylator Phenotype Underlies Sporadic Microsatellite Instability and Is Tightly Associated with BRAF Mutation in Colorectal Cancer. Nat. Genet. 2006, 38, 787–793.
- Alvi, M.A.; Loughrey, M.B.; Dunne, P.; McQuaid, S.; Turkington, R.; Fuchs, M.A.; McGready, C.; Bingham, V.; Pang, B.; Moore, W.; et al. Molecular Profiling of Signet Ring Cell Colorectal Cancer Provides a Strong Rationale for Genomic Targeted and Immune Checkpoint Inhibitor Therapies. Br. J. Cancer 2017, 117, 203–209.
- Fennell, L.J.; Jamieson, S.; McKeone, D.; Corish, T.; Rohdmann, M.; Furner, T.; Bettington, M.; Liu, C.; Kawamata, F.; Bond, C.; et al. MLH1-93 G/a Polymorphism Is Associated with MLH1 Promoter Methylation and Protein Loss in Dysplastic Sessile Serrated Adenomas with BRAFV600E Mutation. BMC Cancer 2018, 18, 35.
- Tabernero, J.; Ros, J.; Élez, E. The Evolving Treatment Landscape in BRAF-V600E-Mutated Metastatic Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 254–263.
- Bettington, M.; Walker, N.; Rosty, C.; Brown, I.; Clouston, A.; McKeone, D.; Pearson, S.A.; Leggett, B.; Whitehall, V. Clinicopathological and Molecular Features of Sessile Serrated Adenomas with Dysplasia or Carcinoma. Gut 2017, 66, 97–106.
- Bettington, M.L.; Walker, N.I.; Rosty, C.; Brown, I.S.; Clouston, A.D.; McKeone, D.M.; Pearson, S.A.; Klein, K.; Leggett, B.A.; Whitehall, V.L.J. A Clinicopathological and Molecular Analysis of 200 Traditional Serrated Adenomas. Mod. Pathol. 2015, 28, 414–427.
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; Disaia, P.; Gabra, H.; Glenn, P.; et al. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615.
- Weinstein, J.N.; Akbani, R.; Broom, B.M.; Wang, W.; Verhaak, R.G.W.; McConkey, D.; Lerner, S.; Morgan, M.; Creighton, C.J.; Smith, C.; et al. Comprehensive Molecular Characterization of Urothelial Bladder Carcinoma. Nature 2014, 507, 315–322.
- Muzny, D.M.; Bainbridge, M.N.; Chang, K.; Dinh, H.H.; Drummond, J.A.; Fowler, G.; Kovar, C.L.; Lewis, L.R.; Morgan, M.B.; Newsham, I.F.; et al. Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nature 2012, 487, 330–337.
- Wang, Y.K.; Bashashati, A.; Anglesio, M.S.; Cochrane, D.R.; Grewal, D.S.; Ha, G.; McPherson, A.; Horlings, H.M.; Senz, J.; Prentice, L.M.; et al. Genomic Consequences of Aberrant DNA Repair Mechanisms Stratify Ovarian Cancer Histotypes. Nat. Genet. 2017, 49, 856–864.
- Wang, J.; Li, R.; He, Y.; Yi, Y.; Wu, H.; Liang, Z. Next-Generation Sequencing Reveals Heterogeneous Genetic Alterations in Key Signaling Pathways of Mismatch Repair Deficient Colorectal Carcinomas. Mod. Pathol. 2020, 33, 2591–2601.
- Qiu, M.Z.; He, C.Y.; Yang, X.H.; Yang, L.Q.; Lin, J.Z.; Zhou, D.L.; Long, Y.K.; Guan, W.L.; Jin, Y.; Li, Y.H.; et al. Relationship of HER2 Alteration and Microsatellite Instability Status in Colorectal Adenocarcinoma. Oncologist 2021, 26, e1161–e1170.
- Saygin, I.; Cakir, E. The Status of HER2 in Colorectal Carcinoma and the Relation of HER2 with Prognostic Parameters and MSI. Indian J. Pathol. Microbiol. 2022, 65, 336–342.
- Engel, C.; Ahadova, A.; Seppälä, T.T.; Aretz, S.; Bigirwamungu-Bargeman, M.; Bläker, H.; Bucksch, K.; Büttner, R.; de Vos tot Nederveen Cappel, W.T.; Endris, V.; et al. Associations of Pathogenic Variants in MLH1, MSH2, and MSH6 With Risk of Colorectal Adenomas and Tumors and With Somatic Mutations in Patients with Lynch Syndrome. Gastroenterology 2020, 158, 1326–1333.
- Mäki-Nevala, S.; Valo, S.; Ristimäki, A.; Sarhadi, V.; Knuutila, S.; Nyström, M.; Renkonen-Sinisalo, L.; Lepistö, A.; Mecklin, J.P.; Peltomäki, P. DNA Methylation Changes and Somatic Mutations as Tumorigenic Events in Lynch Syndrome-Associated Adenomas Retaining Mismatch Repair Protein Expression. EBioMedicine 2019, 39, 280–291.
- Yurgelun, M.B.; Goel, A.; Hornick, J.L.; Sen, A.; Turgeon, D.K.; Ruffin IV, M.T.; Marcon, N.E.; Baron, J.A.; Bresalier, R.S.; Syngal, S.; et al. Microsatellite Instability and DNA Mismatch Repair Protein Deficiency in Lynch Syndrome Colorectal Polyps. Cancer Prev. Res. 2012, 5, 574–582.
- Chang, D.K.; Ricciardiello, L.; Goel, A.; Chang, C.L.; Boland, C.R. Steady-State Regulation of the Human DNA Mismatch Repair System. J. Biol. Chem. 2000, 275, 18424–18431.
- Ahadova, A.; Gallon, R.; Gebert, J.; Ballhausen, A.; Endris, V.; Kirchner, M.; Stenzinger, A.; Burn, J.; von Knebel Doeberitz, M.; Bläker, H.; et al. Three Molecular Pathways Model Colorectal Carcinogenesis in Lynch Syndrome. Int. J. Cancer 2018, 143, 139–150.
- Stormorken, A.T.; Clark, N.; Grindedal, E.; Mæhle, L.; Møller, P. Prevention of Colorectal Cancer by Colonoscopic Surveillance in Families with Hereditary Colorectal Cancer. Scand. J. Gastroenterol. 2007, 42, 611–617.
- Møller, P. The Prospective Lynch Syndrome Database Reports Enable Evidence-Based Personal Precision Health Care. Hered. Cancer Clin. Pract. 2020, 18, 6.
- The Cancer Genome Atlas Program—NCI. Available online: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (accessed on 10 January 2023).
- Salem, M.E.; Bodor, J.N.; Puccini, A.; Xiu, J.; Goldberg, R.M.; Grothey, A.; Korn, W.M.; Shields, A.F.; Worrilow, W.M.; Kim, E.S.; et al. Relationship between MLH1, PMS2, MSH2 and MSH6 Gene-Specific Alterations and Tumor Mutational Burden in 1057 Microsatellite Instability-High Solid Tumors. Int. J. Cancer 2020, 147, 2948–2956.
- Goodman, A.M.; Sokol, E.S.; Frampton, G.M.; Lippman, S.M.; Kurzrock, R. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer Immunol. Res. 2019, 7, 1570–1573.
- Fabrizio, D.A.; George, T.J.; Dunne, R.F.; Frampton, G.; Sun, J.; Gowen, K.; Kennedy, M.; Greenbowe, J.; Schrock, A.B.; Hezel, A.F.; et al. Beyond Microsatellite Testing: Assessment of Tumor Mutational Burden Identifies Subsets of Colorectal Cancer Who May Respond to Immune Checkpoint Inhibition. J. Gastrointest. Oncol. 2018, 9, 610–617.
- Lee, S.; Lara, O.; Karpel, H.; Pothuri, B. The Association of Tumor Mutational Burden, Microsatellite Stability, and Mismatch Repair Deficiency in an Endometrial Cancer Patient Cohort (194). Gynecol. Oncol. 2022, 166, S111.
- Oaknin, A.; Gilbert, L.; Tinker, A.V.; Brown, J.; Mathews, C.; Press, J.; Sabatier, R.; O’Malley, D.M.; Samouelian, V.; Boni, V.; et al. Safety and Antitumor Activity of Dostarlimab in Patients with Advanced or Recurrent DNA Mismatch Repair Deficient/Microsatellite Instability-High (DMMR/MSI-H) or Proficient/Stable (MMRp/MSS) Endometrial Cancer: Interim Results from GARNET-a Phase I, Single-Arm Study. J. Immunother. Cancer 2022, 10, e003777.
- Jones, N.L.; Xiu, J.; Rocconi, R.P.; Herzog, T.J.; Winer, I.S. Immune Checkpoint Expression, Microsatellite Instability, and Mutational Burden: Identifying Immune Biomarker Phenotypes in Uterine Cancer. Gynecol. Oncol. 2020, 156, 393–399.
- Cho, J.; Ahn, S.; Son, D.S.; Kim, N.K.D.; Lee, K.W.; Kim, S.; Lee, J.; Park, S.H.; Park, J.O.; Kang, W.K.; et al. Bridging Genomics and Phenomics of Gastric Carcinoma. Int. J. Cancer 2019, 145, 2407–2417.
- Müller, M.F.; Ibrahim, A.E.K.; Arends, M.J. Molecular Pathological Classification of Colorectal Cancer. Virchows Arch. 2016, 469, 125–134.
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus Molecular Subtypes and the Evolution of Precision Medicine in Colorectal Cancer. Nat. Rev. Cancer 2017, 17, 79–92.
- Włodarczyk, M.; Włodarczyk, J.; Siwiński, P.; Sobolewska-Włodarczyk, A.; Fichna, J. Genetic Molecular Subtypes in Optimizing Personalized Therapy for Metastatic Colorectal Cancer. Curr. Drug Targets 2018, 19, 1731–1737.
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of Colorectal Carcinogenesis. Gastroenterology 2020, 158, 291–302.
- Salem, M.E.; Xiu, J.; Lenz, H.-J.; Atkins, M.B.; Philip, P.A.; Hwang, J.J.; Gatalica, Z.; Xiao, N.; Gibney, G.T.; El-Deiry, W.S.; et al. Characterization of Tumor Mutation Load (TML) in Solid Tumors. J. Clin. Oncol. 2017, 35, 11517.
- Salem, M.E.; Puccini, A.; Grothey, A.; Raghavan, D.; Goldberg, R.M.; Xiu, J.; Michael Korn, W.; Weinberg, B.A.; Hwang, J.J.; Shields, A.F.; et al. Landscape of Tumor Mutation Load, Mismatch Repair Deficiency, and PD-L1 Expression in a Large Patient Cohort of Gastrointestinal Cancers. Mol. Cancer Res. 2018, 16, 805–812.
- Kumar, S. A Perfect Biomarker for Immune Checkpoint Inhibition—An Elusive Goal? Cancer Res. Stat. Treat. 2021, 4, 594–595.
- Grossman, J.E.; Vasudevan, D.; Joyce, C.E.; Hildago, M. Is PD-L1 a Consistent Biomarker for Anti-PD-1 Therapy? The Model of Balstilimab in a Virally-Driven Tumor. Oncogene 2021, 40, 1393–1395.
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365.
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat. Med. 2002, 8, 793–800.
- Kim, T.K.; Vandsemb, E.N.; Herbst, R.S.; Chen, L. Adaptive Immune Resistance at the Tumour Site: Mechanisms and Therapeutic Opportunities. Nat. Rev. Drug Discov. 2022, 21, 529–540.
- Weber, J.S.; Kähler, K.C.; Hauschild, A. Management of Immune-Related Adverse Events and Kinetics of Response with Ipilimumab. J. Clin. Oncol. 2012, 30, 2691–2697.
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 Leads to Massive Lymphoproliferation and Fatal Multiorgan Tissue Destruction, Revealing a Critical Negative Regulatory Role of CTLA-4. Immunity 1995, 3, 541–547.
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med. 2017, 9, 34.
- Mandal, R.; Samstein, R.M.; Lee, K.W.; Havel, J.J.; Wang, H.; Krishna, C.; Sabio, E.Y.; Makarov, V.; Kuo, F.; Blecua, P.; et al. Genetic Diversity of Tumors with Mismatch Repair Deficiency Influences Anti-PD-1 Immunotherapy Response. Science 2019, 364, 485–491.
- Turajlic, S.; Litchfield, K.; Xu, H.; Rosenthal, R.; McGranahan, N.; Reading, J.L.; Wong, Y.N.S.; Rowan, A.; Kanu, N.; al Bakir, M.; et al. Insertion-and-Deletion-Derived Tumour-Specific Neoantigens and the Immunogenic Phenotype: A Pan-Cancer Analysis. Lancet Oncol. 2017, 18, 1009–1021.
- Sena, L.A.; Fountain, J.; Isaacsson Velho, P.; Lim, S.J.; Wang, H.; Nizialek, E.; Rathi, N.; Nussenzveig, R.; Maughan, B.L.; Velez, M.G.; et al. Tumor Frameshift Mutation Proportion Predicts Response to Immunotherapy in Mismatch Repair-Deficient Prostate Cancer. Oncologist 2021, 26, e270–e278.
This entry is offline, you can click here to edit this entry!
