Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
While ethylene is the simplest known olefin, JAs include its free acid and a number of conjugates. Both ethylene and JAs occur in almost all tissues of higher plants and regulate developmental and physiological processes (e.g., root development, accumulation of anthocyanins) in a complex manner.
- ethylene
- jasmonates
- biosynthesis
- signaling
- phytohormones
1. Ethylene Biosynthesis and Signaling
The biosynthetic and signaling pathways of ethylene have been excellently reviewed, such as in Johnson and Ecker[1] or Pattyn et al. [2]. Briefly, the ethylene biosynthetic pathway consists of three enzymatic reaction steps. In the first step, enzyme S-adenosylmethionine (SAM) synthetase converts methionine into S-adenosyl-methionine (S-AdoMet). In the following, enzyme 1-aminocyclopropane-1-carboxylate (ACC) synthase (ACS) converts S-AdoMet directly into ethylene precursor ACC. Finally, ACC oxidation leads to formation of ethylene via the enzyme ACC oxidase (ACO), which requires oxygen as a co-substrate and activator. Meanwhile, 5′-methylthioadenosine (MTA) is formed as a by-product of ACC synthesis and is then recycled to methionine via the Yang cycle. This maintains a methionine pool even when ethylene is being rapidly synthesized [3][4]. Most studies have focused on characterizing ACSs as key enzymes, since they have been considered the rate-limiting step in ethylene synthesis [5]. For instance, the Arabidopsis genome encodes nine ACS genes, while ACS2, ACS4-9, and ACS11 encode functional ACS; ACS1 encodes catalytically inactive enzymes or non-functional homodimers [6]. However, over the years, increasing numbers of studies have shown that, in certain specific processes of ethylene biosynthesis, ACO is the rate-limiting step [7], such as during flooding of tomato and Rumex palustris [8][9]. In rice, it was found that ACO genes ACO8 and ACO3 are strongly induced in rice shoots during flooding, while ACO1 is negatively regulated [10]. Although the biosynthetic pathway of ethylene is straightforward compared to other plant hormones, its production is tightly controlled at multiple levels to ensure optimized developmental and stress-induced ethylene synthesis [11][12][13][14].
Ethylene signaling includes the following main components: five ethylene receptors (Ethylene Response 1 (ETR1), ETR2, Ethylene Reticulum Sensor 1 (ERS1), ERS2, and Ethylene Insensitive 4 (EIN4)), a negative regulator Constitutive Triple Response 1 (CTR1), an ER-localized membrane protein EIN2, Ethylene Insensitive3-Binding F-Box Protein1 (EBF1) and EBF2, primary transcription factors EIN3/Ethylene Insensitive-Like Protein1 (EIL1), and ethylene response factors (ERFs) [15] (Figure 1A). As a gaseous plant hormone, ethylene appears to be able to diffuse freely across plant and plasma membranes until it binds to ethylene receptors anchored in the ER membrane to stimulate ethylene responses [16]. In the absence of ethylene, CTR1 is activated by the ethylene receptors and subsequently turns off EIN2 through phosphorylation of its C-terminal end (EIN2-CEND). Finally, involving F-box proteins EBF1 and EBF2, EIN3/EIL1 are degraded in the nucleus, preventing ethylene responses [17][18]. In the presence of ethylene, its binding to the receptors leads to inactivation of CTR1, while EIN2 is dephosphorylated and cleaved. The released EIN2-CEND represses translation of EB1 and EBF2 transcripts in the cytosol and subsequently enters the nucleus, where it directly or indirectly promotes activity of EIN3/EIL1 (Figure 1A) [19]. Thus, a transcriptional cascade is initiated, leading to activation and repression of hundreds of ethylene-responsive target genes, such as ERFs [20].
2. Jasmonates Biosynthesis and Signaling
JAs owe their name to Jasminum grandiflorum, where they were first discovered [21], but it was not until after several years that their functions in plants began to be elucidated [22][23]. Among the most active JAs, we find jasmonic acid (JA), methyl-jasmonate (MeJA), jasmonate-isoleucine (JA-Ile) [24][25], and 12-oxo-phytodienoic acid (OPDA) [26].
JAs biosynthesis has been extensively studied and is well-reviewed [27]. OPDA is the precursor of JAs and is formed in the chloroplast from the polyunsaturated fatty acid α-linolenic, which is released from membrane lipids [28]. This is the start of the 18:3 biosynthetic pathway and is catalyzed by lipases, such as 13-LOX (13-lipoxygenase). Then, allene oxide synthase (AOS) and allene oxide cyclase (AOC) yield OPDA through dehydration-cyclization. OPDA is transported to the peroxisome via the transporter JASSY [29] and partially by CTS ABC transporter (ATP-binding cassette COMATOSE) [30]. OPDA is reduced to OPC-8:0 (8-((1S,2S)-3-oxo-2-((Z)-pent-2-en-1-yl)cyclopentyl)octanoic acid) by OPDA reductase 3 (OPR3) and then activated to OPC-8-CoA. After three rounds of β-oxidation, the final compound obtained in the peroxisome is (+)-7-iso-JA, which is released into the cytosol and can be catalyzed into jasmonate-isoleucine (JA-Ile) by JAR1 (Jasmonate-amido synthetase 1), which will be involved in JA signaling acting on gene expression. Other derivatives of JA can also be formed in the cytosol, such as methyl-jasmonate (MeJA), glycosylated forms, and conjugated forms with amino acids, among others [31]. Recently, an alternative pathway of JA biosynthesis has been postulated and is independent of OPR3 [32]. Instead, once OPDA enters the peroxisome, it is β-oxidized to dnOPDA (2,3-Dinor-12-oxo-10,15(Z)-phytodienoic acid), tnOPDA (deuterated tetranor-OPDA), and finally to 4, 5-ddh-JA (4, 5-didehydro-JA). The latter is transported to the cytosol and reduced to JA by OPR2, an additional OPR enzyme. OPR3 is not present either in liverworts nor mosses [33][34], suggesting that the OPR3-independent pathway is more ancient in the plant lineage and that the OPR3 pathway is preferred in vascular plants [35].
JA-Ile is the most biologically active of the JAs, being crucial in JA signaling (Figure 1B). When JA-Ile levels increase, the conjugate form is transported into the nucleus by JAT1/ABCG16 (ATP binding cassette (ABC) transporter) [36]. Then, JA-Ile binds to the F-Box protein COI1 (CORANATINE INSENSITIVE 1 [37] of the SCF E3 ubiquitin ligase complex (SCFCOI1), and, later, they recruit JAZ ZIM-DOMAIN (JAZ), forming the temporary ternary complex of COI1–JA–JAZ [38][39]. JAZs, under no-stress conditions, are bonded to MYC TFs through NINJA adaptor protein (JAZ-bound NOVEL INTERACTOR OF JAZ) and recruit TOPLESS scaffolding protein, repressing JA-responsive genes [40]. With formation of the COI1–JA–JAZ complex, JAZs are degraded by the 26S proteasome; MYC2 (the master transcriptional factor), which is interacting with MED25 [41], is liberated, and gene expression is induced (Figure 1B) [42][43][44]. In lower plants, JA-Ile is not the ligand to COI1; in its place, dnOPDA from the OPR3-independent pathway binds to COI1 and JAZ proteins possess a single ortholog [45]. Even though the OPR3-independent pathway is characteristic of lower plants, such as liverworts, it is also present in higher plants, making OPDA (or its derivatives) also an active form of JAs that can trigger gene expression [46].
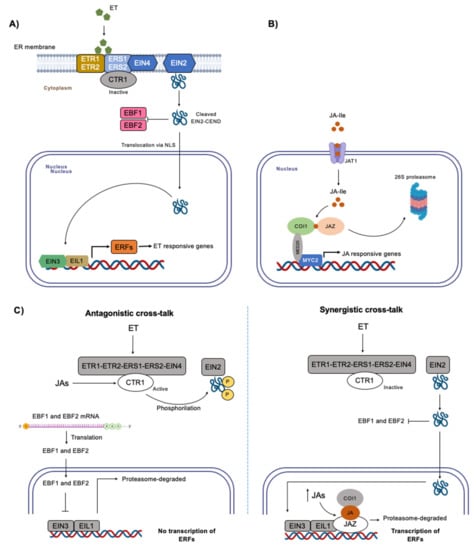
Figure 1. Ethylene and JAs signaling. (A) Ethylene signaling. In the presence of ethylene, receptors ETR1, ETR2, ERS1, ERS2, and EIN4 perceive ethylene and deactivate CTR1. EIN2-CEND is cleaved and released from EIN2 and inhibits translation of inhibitors EBF1 and EBF2. EIN2-CEND translocates to the nucleus and induces EIN3/EIL1, which in turn activates ERFs, and, finally, ERFs induce transcription of ethylene responsive genes. (B) JAs signaling. When JA-Ile accumulates in the cytosol, it enters the nucleus via JAT1. Then, it binds to protein COI1 from the SCFCOI1 complex, and, later, they recruit JAZ to form a temporary complex to promote degradation of JAZ. Upon this degradation, MYC2 that is interacting with MED25 is liberated and induces transcription of JA-responsive genes. (C) Proposed signaling pathway for ethylene and JAs cross-talk under abiotic stress. Two types of cross-talk have been described for ethylene and JAs. In an antagonistic cross-talk, JAs reactivate CTR1 after ET perception in the cytosol. CTR1 phosphorylates EIN2-CEND, inactivating it. In this manner, translation of EBF1 and EBF2 mRNA takes place and EBF1 and EBF2 enter the nucleus to repress EIN3/EIL1 by promoting its degradation. The result is no transcription of ERFs. In a synergistic cross-talk, both ethylene and JAs promote transcription of ERFs. ET does it through its usual pathway. The increase in JAs in the nucleus promotes formation of the COI1–JA–JAZ complex. JAZ repressing EIN3/EIL1 is degraded, and, hence, transcription of ERFs takes place. ET, ethylene; ETR1/2, Ethylene response 1/2; ERS1/2, Ethylene reticulum sensor 1/2; EIN4/2/3, Ethylene insensitive 4/2/3; CTR1, Constitutive triple response 1; EBF1/2, Ethylene insensitive3-Binding F-Box protein 1/2; EIN2-CEND, C-Terminal end of EIN2; EIL1, Ethylene Insensitive-Like Protein 1; ERFs, Ethylene transcription factors; JA-Ile, jasmonate-isoleucine; JAT1, jasmonate transporter 1; COI, CORANATINE INSENSITIVE 1; SCF, SCF E3 ubiquitin ligase complex; JAZ, JAZ ZIMDOMAIN; MYC2, basic- helix-loop-helix (bHLH) transcription factor; MED25, Mediator subunit 25. Part of this figure was created with Biorender.com (accessed on 20 February 2023).
This entry is adapted from the peer-reviewed paper 10.3390/ijms24065990
References
- Johnson, P.R.; Ecker, J.R.; The ethylene gas signal transduction pathway: a molecular perspective. The ethylene gas signal transduction pathway: a molecular perspective 1998, 32, 227–254, 10.1146/annurev.genet.32.1.227.
- Pattyn, J.; Vaughan-Hirsch, J.; Van De Poel, B.; The regulation of ethylene biosynthesis: a complex multilevel control circuitry. New Phytol. 2021, 229, 770–782, doi.org/10.1111/nph.16873.
- Yang, S.F.; Hoffman, N.E.; Ethylene Biosynthesis and its Regulation in Higher Plants.. Annu. Rev. Plant Physiol. 1984, 35, 155–189, .
- Kende, H.; Ethylene Biosynthesis. . Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 283–307, .
- Park, C.H.; Roh, J.; Youn, J.-H.; Son, S.-H.; Park, J.H.; Kim, S.Y.; Kim, T.-W.; Kim, S.-K.; Arabidopsis ACC Oxidase 1 Coordinated by Multiple Signals Mediates Ethylene Biosynthesis and Is Involved in Root Development.. Mol. Cells 2018, 41, 923–932, .
- Liang, X.; Abel, S.; Keller, J.A.; Shen, N.F.; Theologis, A.; The 1-aminocyclopropane-1-carboxylate synthase gene family of Arabidopsis thaliana.. Proc. Natl. Acad. Sci. USA 1992, 89, 11046–11050, .
- Houben, M.; Van de Poel, B.; 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene.. Front. Plant Sci. 2019, 10, 695, doi.org/10.3389/fpls.2019.00695.
- Vriezen, W.H.; Hulzink, R.; Mariani, C.; Voesenek, L.A.; 1-Aminocyclopropane-1-Carboxylate Oxidase Activity Limits Ethylene Biosynthesis in Rumex palustris during Submergence.. Plant Physiol. 1999, 121, 189–196, .
- English, P.J.; Lycett, G.; Roberts, J.A.; Jackson, M.B.; Increased 1-Aminocyclopropane-1-Carboxylic Acid Oxidase Activity in Shoots of Flooded Tomato Plants Raises Ethylene Production to Physiologically Active Levels.. Plant Physiol. 1995, 109, 1435–1440, .
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D.; Submergence Tolerant Rice: SUB1’s Journey from Landrace to Modern Cultivar.. Rice 2010, 3, 138–147, .
- Tsuchisaka, A.; Theologis, A.; Unique and Overlapping Expression Patterns among the Arabidopsis 1-Amino-Cyclopropane-1-Carboxylate Synthase Gene Family Members. . Plant Physiol. 2004, 136, 2982–3000, 10.1104/pp.104.049999..
- Tsuchisaka, A.; Theologis, A.; Heterodimeric interactions among the 1-amino-cyclopropane-1-carboxylate synthase polypeptides encoded by the Arabidopsis gene family.. Proc. Natl. Acad. Sci. USA 2004, 101, 2275–2280, .
- Skottke, K.R.; Yoon, G.M.; Kieber, J.J.; Delong, A.; Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms.. PLoS Genet. 2011, 7, e1001370, .
- Lyzenga, W.J.; Booth, J.K.; Stone, S.L.; The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7.. Plant J. 2012, 71, 23–34, .
- Müller, M.; Munné-Bosch, S.; Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling.. Plant Physiol. 2015, 169, 32–41, doi.org/10.1104/pp.15.00677.
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.; Ethylene and Sulfur Coordinately Modulate the Antioxidant System and ABA Accumulation in Mustard Plants Under Salt Stress. . Plants 2021, 10, 180, .
- Merchante, C.; Brumos, J.; Yun, J.; Hu, Q.; Spencer, K.R.; Enríquez, P.; Binder, B.M.; Heber, S.; Stepanova, A.N.; Alonso, J.M.; et al. Gene-Specific Translation Regulation Mediated by the Hormone-Signaling Molecule EIN2. Cell 2015, 163, 684–697, .
- Chen, H.; Bullock, D.A.; Alonso, J.M.; Stepanova, A.N.; To Fight or to Grow: The Balancing Role of Ethylene in Plant Abiotic Stress Responses. . Plants 2021, 11, 33, .
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V.; haping Ethylene Response: The Role of EIN3/EIL1 Transcription Factors.. Front. Plant Sci. 2019, 10, 1030, .
- Solano, R.; Stepanova, A.; Chao, Q.; Ecker, J.R.; Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1.. Genes Dev 1998, 12, 3703–3714, .
- Demole, E.; Lederer, E.; Mercier, D.; Isolement et détermination de la structure du jasmonate de méthyle, constituant odorant caractéristique de l’essence de jasmin. . Helv. Chim. Acta 1962, 45, 675–685, .
- Ueda, J.; Kato, J.; Isolation and Identification of a Senescence-promoting Substance from Wormwood (Artemisia absinthium L.). . Plant Physiol. 1980, 66, 246–249, .
- Dathe, W.; Rönsch, H.; Preiss, A.; Schade, W.; Sembdner, G.; Schreiber, K.; Endogenous plant hormones of the broad bean, Vicia faba L. (-)-jasmonic acid, a plant growth inhibitor in pericarp.. Planta 1981, 153, 530–535., .
- Poudel, A.N.; Holtsclaw, R.E.; Kimberlin, A.; Sen, S.; Zeng, S.; Joshi, T.; Lei, Z.; Sumner, L.W.; Singh, K.; Matsuura, H.; et al. 12-Hydroxy-Jasmonoyl-l-Isoleucine Is an Active Jasmonate That Signals through CORONATINE INSENSITIVE 1 and Contributes to the Wound Response in Arabidopsis. . Plant Cell Physiol. 2019, 60, 2152–2166, .
- Schuman, M.C.; Meldau, S.; Gaquerel, E.; Diezel, C.; McGale, E.; Greenfield, S.; Baldwin, I.T.; The Active Jasmonate JA-Ile Regulates a Specific Subset of Plant Jasmonate-Mediated Resistance to Herbivores in Nature. Front. Plant Sci. 2018, 9, 787, .
- Aleman, G.H.J.; Thirumalaikumar, V.P.; Jander, G.; Fernie, A.R.; Skirycz, A.; OPDA, more than just a jasmonate precursor.. Phytochemistry 2022, 204, 113432, .
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K.; Jasmonic Acid Signaling Pathway in Plants.. Int. J. Mol. Sci. 2019, 20, 2479, .
- Dave, A.; Graham, I.A.; Oxylipin Signaling: A Distinct Role for the Jasmonic Acid Precursor cis-(+)-12-Oxo-Phytodienoic Acid (cis-OPDA).. Front. Plant Sci. 2012, 3, 42, .
- Guan, L.; Denkert, N.; Eisa, A.; Lehmann, M.; Sjuts, I.; Weiberg, A.; Soll, J.; Meinecke, M.; Schwenkert, S.; JASSY, a chloroplast outer membrane protein required for jasmonate biosynthesis.. Proc. Natl. Acad. Sci. USA 2019, 116, 10568–10575, .
- Theodoulou, F.L.; Job, K.; Slocombe, S.P.; Footitt, S.; Holdsworth, M.; Baker, A.; Larson, T.R.; Graham, I.A.; Jasmonic Acid Levels Are Reduced in COMATOSE ATP-Binding Cassette Transporter Mutants. Implications for Transport of Jasmonate Precursors into Peroxisomes.. Plant Physiol. 2005, 137, 835–840, .
- Wasternack, C.; Hause, B.; Jasmonates: Biosynthesis, Perception, Signal Transduction and Action in Plant Stress Response, Growth and Development. . Ann. Bot. 2013, 111, 1021, .
- Chini, A.; Monte, I.; Zamarreño, A.M.; Hamberg, M.; Lassueur, S.; Reymond, P.; Weiss, S.; Stintzi, A.; Schaller, A.; Porzel, A.; et al. An OPR3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis.. Nat. Chem. Biol. 2018, 14, 171–178, .
- Stumpe, M.; Göbel, C.; Faltin, B.; Beike, A.K.; Hause, B.; Himmelsbach, K.; Bode, J.; Kramell, R.; Wasternack, C.; Frank, W.; et al. The moss Physcomitrella patens contains cyclopentenones but no jasmonates: Mutations in allene oxide cyclase lead to reduced fertility and altered sporophyte morphology.. New Phytol. 2010, 188, 740–749, .
- Yamamoto, Y.; Ohshika, J.; Takahashi, T.; Ishizaki, K.; Kohchi, T.; Matusuura, H.; Takahashi, K.; Functional analysis of allene oxide cyclase, MpAOC, in the liverwort Marchantia polymorpha. . Phytochemistry 2015, 116, 48–56, .
- Monte, I.; Ishida, S.; Zamarreño, A.M.; Hamberg, M.; Franco-Zorrilla, J.M.; García-Casado, G.; Gouhier-Darimont, C.; Reymond, P.; Takahashi, K.; García-Mina, J.M.; et al. Ligand-receptor co-evolution shaped the jasmonate pathway in land plants.. Nat. Chem. Biol. 2018, 14, 480–488, .
- Li, Q.; Zheng, J.; Li, S.; Huang, G.; Skilling, S.J.; Wang, L.; Li, L.; Li, M.; Yuan, L.; Liu, P.; et al. Transporter-Mediated Nuclear Entry of Jasmonoyl-Isoleucine Is Essential for Jasmonate Signaling. . Mol. Plant 2017, 10, 695–708, .
- Xie, D.-X.; Feys, B.F.; James, S.; Nieto-Rostro, M.; Turner, J.G.; COI1: An Arabidopsis Gene Required for Jasmonate-Regulated Defense and Fertility.. Science 1998, 280, 1091–1094, .
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.-F.; Sharon, M.; Browse, J.; et al. Jasmonate perception by inositol-phosphate-potentiated COI1–JAZ co-receptor. . Nature 2010, 468, 400–405, .
- Yan, J.; Yao, R.; Chen, L.; Li, S.; Gu, M.; Nan, F.; Xie, D.; Dynamic Perception of Jasmonates by the F-Box Protein COI1. . Mol. Plant 2018, 11, 1237–1247, .
- Pauwels, L.; Barbero, G.F.; Geerinck, J.; Tilleman, S.; Grunewald, W.; Pérez, A.C.; Chico, J.M.; Bossche, R.V.; Sewell, J.; Gil, E.; et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling.. Nature 2010, 464, 788–791, .
- Zhai, Q.; Deng, L.; Li, C.; Mediator subunit MED25: At the nexus of jasmonate signaling.. Curr. Opin. Plant Biol. 2020, 57, 78–86., .
- Boter, M.; Ruíz-Rivero, O.; Abdeen, A.; Prat, S.; Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis.. Genes Dev. 2004, 18, 1577–1591, .
- Lorenzo, O.; Chico, J.M.; Saénchez-Serrano, J.J.; Solano, R.; JASMONATE-INSENSITIVE1 Encodes a MYC Transcription Factor Essential to Discriminate between Different Jasmonate-Regulated Defense Responses in Arabidopsis. . Plant Cell 2004, 16, 1938–1950, .
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling.. Nature 2007, 448, 666–671, .
- Monte, I.; Franco-Zorrilla, J.M.; García-Casado, G.; Zamarreño, A.M.; García-Mina, J.M.; Nishihama, R.; Kohchi, T.; Solano, R.; A Single JAZ Repressor Controls the Jasmonate Pathway in Marchantia polymorpha.. Mol. Plant 2019, 12, 185–198, .
- Taki, N.; Sasaki-Sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K.; Ainai, T.; Yagi, K.; Sakurai, N.; Suzuki, H.; Masuda, T.; et al. 12-Oxo-Phytodienoic Acid Triggers Expression of a Distinct Set of Genes and Plays a Role in Wound-Induced Gene Expression in Arabidopsis. . Plant Physiol. 2005, 139, 1268–1283, .
- Taki, N.; Sasaki-Sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K.; Ainai, T.; Yagi, K.; Sakurai, N.; Suzuki, H.; Masuda, T.; et al. 12-Oxo-Phytodienoic Acid Triggers Expression of a Distinct Set of Genes and Plays a Role in Wound-Induced Gene Expression in Arabidopsis. . Plant Physiol. 2005, 139, 1268–1283, .
This entry is offline, you can click here to edit this entry!
